Key Points
ODSH counters the inhibitory effect of PF4 on megakaryopoiesis in murine and human cell cultures.
ODSH improves platelet count recovery in murine models of both CIT and RIT.
Abstract
Thrombocytopenia is a significant complication of chemotherapy and radiation therapy. Platelet factor 4 (PF4; CXCL4) is a negative paracrine of megakaryopoiesis. We have shown that PF4 levels are inversely related to steady-state platelet counts, and to the duration and severity of chemotherapy- and radiation-induced thrombocytopenia (CIT and RIT, respectively). Murine studies suggest that blocking the effect of PF4 improves megakaryopoiesis, raising nadir platelet counts and shortening the time to platelet count recovery. We examined the ability of 2-O, 3-O desulfated heparin (ODSH), a heparin variant with little anticoagulant effects, to neutralize PF4’s effects on megakaryopoiesis. Using megakaryocyte colony assays and liquid cultures, we show that ODSH restored megakaryocyte proliferation in PF4-treated Cxcl4−/− murine and human CD34+-derived megakaryocyte cultures (17.4% megakaryocyte colonies, P < .01 compared with PF4). In murine CIT and RIT models, ODSH, started 24 hours after injury, was examined for the effect on hematopoietic recovery demonstrating higher platelet count nadirs (9% ± 5% treated vs 4% ± 4% control) and significantly improved survival in treated animals (73% treated vs 36% control survival). Treatment with ODSH was able to reduce intramedullary free PF4 concentrations by immunohistochemical analysis. In summary, ODSH mitigated CIT and RIT in mice by neutralizing the intramedullary negative paracrine PF4. ODSH, already in clinical trials in humans as an adjuvant to chemotherapy, may be an important, clinically relevant therapeutic for CIT and RIT.
Introduction
Radiation-induced thrombocytopenia (RIT) is a significant cause of morbidity and mortality1 and is particularly important in the hematopoietic form of acute radiation syndrome.2-4 Bleeding and thrombocytopenia account for significant morbidity and mortality in the setting of radiation injury,4-5 and some studies have found that platelet count, more than any other hematologic marker, correlates with survival after total body irradiation.2,6-8 Our previous studies have shown that endogenous levels of a platelet-specific chemokine, platelet factor 4 (PF4; CXCL4) are inversely correlated with bone marrow recovery after radiation injury.9 PF4 is a known mediator of megakaryopoiesis, plays an important role in the negative regulation of megakaryopoiesis,10-12 and is found at high concentrations intramedullary, allowing it to act locally within the bone marrow niche in a negative paracrine fashion to regulate local megakaryocytes13 and stem cells.14 We have also previously shown that this effect on megakaryopoiesis by PF4 can be blocked by preventing PF4 binding to cell surfaces using anti-PF4 antibodies or unfractionated heparin (UFH) in vitro10,12 and F(ab′)2 fragments of these antibodies in vivo, likely due to their ability to penetrate into the marrow.9,12 Delivery of UFH at effective doses in vivo is problematic because of its anticoagulant effects. The PF4 effect on megakaryopoiesis is mediated by interaction of PF4 with the low-density lipoprotein receptor–related protein 1 (LRP1),12 an interaction that can be disrupted by addition of receptor-associated protein or anti-LRP1 antibodies.12
2-O, 3-O desulfated heparin (ODSH; Figure 1A) retains interactions with many positively charged proteins, but loses its ability to interact with antithrombin and factor Xa with 10-fold less anticoagulant activity compared with UFH.15 ODSH dosing can thus be significantly higher than heparin. Importantly, ODSH retains its ability to bind to PF4,16-17 making it a potentially attractive compound for neutralizing PF4’s intramedullary effect on megakaryocyte development without compounding the bleeding diathesis in settings of thrombocytopenia.
Chemical activity of ODSH. (A) Chemical structure of the ODSH polysaccharide showing replacement of 2 sulfhydryl groups with hydroxyl groups at the 2-O and 3-O positions. (B) ELISA performed with an LRP1-coated plate showing dose-dependent inhibition of PF4 binding with increasing ODSH concentrations. (C) Uptake of PF4 by cultured megakaryocytes. N = 3 experiments performed in duplicate. P < .001 for PF4 vs PF4+ODSH for WT murine megakaryocytes, and P = .002 for PF4 vs PF4+ODSH for Cxcl4−/− murine megakaryocytes.
Chemical activity of ODSH. (A) Chemical structure of the ODSH polysaccharide showing replacement of 2 sulfhydryl groups with hydroxyl groups at the 2-O and 3-O positions. (B) ELISA performed with an LRP1-coated plate showing dose-dependent inhibition of PF4 binding with increasing ODSH concentrations. (C) Uptake of PF4 by cultured megakaryocytes. N = 3 experiments performed in duplicate. P < .001 for PF4 vs PF4+ODSH for WT murine megakaryocytes, and P = .002 for PF4 vs PF4+ODSH for Cxcl4−/− murine megakaryocytes.
We now demonstrate that ODSH is effective in vitro in blocking PF4’s effect on megakaryopoiesis. We also demonstrate that ODSH administered 24 hours after injury improved survival in a murine model of hematopoietic form of acute radiation syndrome, established in both wild-type (WT) and human PF4-overexpressing (hPF4+) mice. The improved in vivo survival effect of ODSH on platelet counts appears to be due to removal of intramedullary PF4. The clinical implications of these findings are discussed.
Materials and methods
Murine cell lines
Several previously described cell lines from mice with different levels of PF4 were studied, and include homozygous PF4 knockout mice (Cxcl4−/−)18 and mice transgenic for hPF4 expression specifically in megakaryocytes (hPF4+)19 as well as WT mice (The Jackson Laboratory), all on a C57BL6 background. The transgenic hPF4+ mice were on a Cxcl4−/− background, so that studies were done using hPF4+Cxcl4−/− mice in comparison with Cxcl4−/− littermate control mice.
The genomic type of all animals was determined by polymerase chain reaction as described.18-19 All of the animals were housed at the Children’s Hospital of Philadelphia (CHOP) animal facility, and procedures were performed after approval by CHOP’s animal care and use committee.
ODSH manufacture and dose determination
ODSH was produced under good manufacturing practice conditions by Scientific Protein Laboratories. Sodium ODSH was provided in 10-mL sterile vials at a concentration of 50 mg/mL (Cantex Pharmaceuticals). ODSH was further diluted to necessary concentrations using sterile water for dilution. Using previously published data on translation dosing between animal and human trials,20 we calculated a starting murine dose of 50 mg/kg per day in 2 divided doses based on human clinical trial dosing of ODSH at 0.25 mg/kg per hour for 24 hours (6 mg/kg per day) where a murine dose of 50 mg/kg per day translates to a human dose of roughly 5 mg/kg per day.
In vitro anti-PF4 activity
The ability of ODSH (Figure 1A) to block PF4’s binding to LRP1 was assessed by enzyme-linked immunosorbent assay (ELISA). Briefly, a 96-well polyvinyl chloride plate (Corning Costar) was coated with 0.5 µg of recombinant human LRP1 (rhLRP1) cluster II Fc or rhLRP1 cluster III Fc (both R&D Systems) to provide the initial binding surface for PF4. Both cluster II and cluster III being used as the LRP1-binding site for PF4 is not well defined, but nearly all LRP1 ligands bind in these 2 sites. ODSH was dissolved in water to obtain a 5 mg/mL starting solution for serial dilutions in phosphate-buffered saline (PBS) Tween 20 (PBST; Sigma), 0.1% bovine serum albumin (Pierce). These dilutions (ODSH in PBST and bovine serum albumin) were mixed with PF4 (1 µg/mL), and incubated overnight prior to provide ODSH-PF4 mixtures. For measurement of anti-PF4 activity, 50 µL of the ODSH-PF4 mixtures were added to the LRP-coated plates and incubated at 37°C for 1 hour. Plates were washed 4 times with PBST, and 50 µL of anti-PF4 monoclonal antibody (clone MAB7951; R&D Systems) was added to the plate and incubated for 1 hour at room temperature. Plates were decanted and washed 4 times with PBST, and 50 µL of horseradish peroxidase–conjugated donkey anti-mouse IgG (R&D Systems) was added for a 1-hour incubation. Plates were again decanted and washed 4 times with PBST, and 3,3′,5,5′-tetramethylbenzidine single-solution chromogen (Life Technologies) was added to develop the plate followed by 1 N HCl to stop the reaction. Solutions were transferred to polystyrene plates and read at 450 nm and plotted against concentration of inhibitor on semilog paper and graphed using the 4-parameter formula in SOFTMaxPRO 4.3 (Molecular Devices).
Culture of marrow-derived megakaryocytes and megakaryocyte colony assays
Deidentified, human CD34+ bone marrow cells were purchased from the University of Pennsylvania Stem Cell Center Core (derived from healthy donors under informed consent). Marrow from the tibias and femurs of 6- to 12-week-old Cxcl4−/− male mice was isolated and used for in vitro cell culture.13,21 Briefly, Iscove modified Dulbecco medium (Invitrogen) without modification was used to flush the marrow cavity of bones harvested from euthanized mice, and the cells were then passed through a 100-µm nylon filter (BD Biosciences). For both murine and human cells, after centrifugation at 1000g, the cell pellet was resuspended in AIM-V media (Invitrogen) at a concentration of 1 × 106 cells per mL with 50 ng/mL recombinant species-specific thrombopoietin (TPO; R&D Systems).
For inhibition studies, murine megakaryocytes were diluted to 2.2 × 106 cells per mL and exposed to hPF4 (0-50 µg/mL) for 4 to 5 days of culture. In some cultures, sodium heparin 100 IU/mL (Sigma) or ODSH 100 mg/mL were concurrently added. Cultures were supplemented with PF4 and/or heparin/ODSH every 48 hours. TPO (50 ng/mL) was also supplemented every 48 hours. Aliquots of cells (100 µL) were removed and counted using a hemocytometer in triplicate in 10-µL aliquots. In addition, at day 0 of culture, a Megacult Assay (StemCell Technologies) was performed according to the manufacturer’s instructions. At days 7 to 10, double-chamber slides were processed according to the manufacturer’s instructions, stained, and colonies were counted.
PF4 uptake studies by megakaryocytes were performed as described.13 Briefly, Cxcl4−/− murine megakaryocytes were exposed to hPF4 (50 µg/mL) after 3 to 4 days of culture. In some cultures, sodium heparin (100 IU/mL) or ODSH (100 µg/mL) were added to the media. After 24 hours, cells were washed with 1000 IU/mL sodium heparin and resuspended at 1 × 106 cells per mL in PBS (Invitrogen). To prepare cell lysates for ELISA of PF4 levels, cells were pelleted after heparin wash by centrifugation at 1000g for 5 minutes, resuspended in PBS at a concentration of 1 × 106 cells per mL, and then subjected to 3 cycles of freeze-thaw to lyse the cells. Cellular debris was then pelleted prior to analysis of the supernatant for PF4. Measurement of PF4 levels was done using a PF4 ELISA kit for hPF4 (Asserachrom PF4; Stago Diagnostica) and mouse PF4 (mPF4; R&D Systems) according to the manufacturer’s instructions.
Murine models of CIT and RIT
A 5-fluorouracil (5-FU)-induced chemotherapy-induced thrombocytopenia (CIT) model was done using intraperitoneal injection of 180 mg/kg of the drug on day 0 in 8- to 12-week-old female-only (to decrease sex variability in responses) hPF4+/Cxcl4−/− or Cxcl4−/− mice as described.10 ODSH, or vehicle control, was administered subcutaneously (SC; 25 mg/kg per dose) every 12 hours for 3 doses starting at 24 hours after 5-FU injection. Every 3 days, complete blood counts were measured as in the RIT studies. Animals were euthanized at day 30.
The RIT model involved 8- to 12-week-old female hPF4+/Cxcl4−/−, Cxcl4−/−, or WT mice receiving 660 cGy irradiation using an X-RAD 320 biological irradiator (Precision X-ray) through a fixed beam at 320 kV and 48 cGy per minute. Animals were placed in a multichamber holder held at a fixed distance (56 cm) from the irradiation source. In-run dosimetry using a Gafchromic radiographic paper (Ashland) was used to confirm dosing. Animals were treated SC with 3 doses of 25 mg/kg per dose ODSH, or vehicle control (sterile water), given at 12-hour intervals starting 24 hours after irradiation. Every 1 to 3 days, retro-orbital bleeds from anesthetized animals were done, and platelet counts were measured in an automatic cell counter (Hemavet; Drew Scientific) set for mouse parameters.8 All animals were maintained in a clean animal facility and were treated with acidified water containing antibiotics (sulfamethoxazole-trimethoprim).
Evaluation of ODSH effect on in vivo PF4 release by megakaryocytes
To examine the effect of ODSH on in vivo PF4 release by megakaryocytes, femurs were examined in 8-week-old female Cxcl4−/− and hPF4+/Cxcl4−/− littermates and WT mice with/without ODSH injection and with/without prior irradiation. Irradiated mice received 660 cGy 48 hours prior to being euthanized. Femurs were removed from euthanized mice and were fixed in formalin, decalcified, imbedded in paraffin, and sectioned and stained for mPF4 using a rabbit anti-mPF4 antibody (clone 140910; R&D Systems) at a 1/200 dilution or for hPF4 using a polyclonal rabbit anti-hPF4 antibody (clone 500-P05; Peprotech) at a 1/2000 dilution as reported.22 For the mPF4 antibody, a peptide-blocking step (DAKO X0909) was used, whereas for the hPF4 antibody, a rodent-blocking step (Biocare Medical) was used for 30 minutes according to the manufacturer’s instructions. Antigen retrieval was performed with E1 (Leica Microsystems) retrieval solution. Stained slides were digitally scanned at ×20 magnification (Aperio OS; Leica Bioimaging). Three sections were prepared from each animal, separated by 10 μm and 2 animals were evaluated per condition. Color deconvolution with mean measurement was used to quantitate staining using NIH ImageJ. Cxcl4−/− mice–derived marrow slides served as negative controls.
Evaluation of the effect ODSH on platelet half-life
Blood was isolated from WT-anesthetized mice, blood from the portal vein, and 600 µL of blood was placed in acid-citrate-dextrose buffer and centrifuged at 200g for 10 minutes at room temperature to prepare platelet-rich plasma.23 The platelets were then pelleted by centrifugation at 800g for 20 minutes at room temperature. This pellet was resuspended and washed once in a Tyrode buffer (134 mmol/L NaCl, 3 mmol/L KCl, 0.3 mmol/L NaH2PO4, 2 mmol/L MgCl2, 5 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 5 mmol/L glucose, 1 mg/mL albumin, 5 U/mL apyrase, and 1 mmol/L EGTA). Platelets were then labeled with the Vybrant CFDA SE Cell Tracer kit (Molecular Probes) using a 10-µM concentration of carboxy fluorescein diacetate succinimidyl ester and incubated at 37°C for 15 minutes. Platelets were then washed with Tyrode buffer and upon resuspension were immediately injected IV through the tail vein into recipient animals. Platelets isolated from 4 animals were pooled and transfused into 4 recipient animals, respectively, such that each animal received a dose of ∼1 × 108 platelets. Platelet counts were measured on the HEMAVET in blood obtained by retro-orbital puncture of recipient animals at ∼4- to 12-hour intervals. Cellular fluorescence was used as a marker for the transfused platelets, and the percentage of platelets expressing this marker was measured using flow cytometry.
Statistical analysis
Statistical analysis of the nonimaging data was performed using GraphPad Prism 6 software. Data were analyzed using 1-way analysis of variance or the Student t test with Bonferroni correction (for multiple comparisons); P < .05 was considered significant.
Results
ODSH binds to PF4 and inhibits PF4/LRP1 interactions and PF4 uptake in vitro
We previously demonstrated that PF4 negatively regulates megakaryopoiesis by binding to LRP1 on developing megakaryocytes. We now examined the effect of addition of ODSH on ability of PF4 to bind to LRP1. Addition of ODSH to PF4 significantly decreased the binding PF4 to LRP1-coated plates (Figure 1B) in a dose-dependent fashion suggestive of first-order kinetics. We then examined the effect of ODSH on megakaryocyte uptake of PF4. The addition of hPF4 (25 µg/mL) to culture media resulted in significant uptake of PF4 into murine megakaryocytes. Addition of ODSH (100 µg/mL) to these cultures significantly decreased the amount of PF4 taken up by WT murine megakaryocytes after a 48-hour exposure (Figure 1C). The same results were seen when Cxcl4−/− murine bone marrow was studied (Figure 1C).
ODSH restores colony formation and megakaryocyte growth in cultures
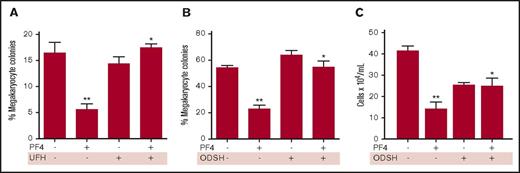
We then asked whether addition of UFH or ODSH to megakaryocyte cultures, either in semisolid or in liquid culture, would be able to restore megakaryocyte proliferation in the presence of PF4. Addition of 25 mg/mL PF4 reduced megakaryocyte colony formation by approximately one-third (Figure 2A). A supratherapeutic level of 100 IU/mL UFH was added to megakaryocyte colony assays and was able to completely restore megakaryocyte colony formation (Figure 2A). Addition of ODSH in the presence of PF4 showed similar results (Figure 2B) with reduction from 54% to 23% colonies in the presence of PF4 alone, and restoration of colony formation in the presence of both ODSH and PF4. In liquid cell cultures of human CD34+ cells, addition of PF4 reduced the numbers of megakaryocytes after 4 days, and addition of PF4+ODSH restored cell numbers (Figure 2C).
In vitro anti-PF4 activity of heparin and ODSH. (A) Megakaryocyte colony assay showing the effect of PF4 (25 µg/mL) on colony formation and the effect of heparin (100 IU/mL). N = 4 experiments performed in duplicate. *P = .01 compared with PF4. **P = .01 compared with control. (B) Same as panel A except for ODSH (100 µg/mL). N = 4 experiments performed in duplicate. **P < .001 vs control; *P < .001 vs PF4. (C) Liquid megakaryocyte culture of human CD34+ cells in the presence of PF4 (25 µg/mL) ± ODSH (100 µg/mL). N = 6 experiments performed in duplicate. *P < .01 vs PF4; **P < .001 vs control.
In vitro anti-PF4 activity of heparin and ODSH. (A) Megakaryocyte colony assay showing the effect of PF4 (25 µg/mL) on colony formation and the effect of heparin (100 IU/mL). N = 4 experiments performed in duplicate. *P = .01 compared with PF4. **P = .01 compared with control. (B) Same as panel A except for ODSH (100 µg/mL). N = 4 experiments performed in duplicate. **P < .001 vs control; *P < .001 vs PF4. (C) Liquid megakaryocyte culture of human CD34+ cells in the presence of PF4 (25 µg/mL) ± ODSH (100 µg/mL). N = 6 experiments performed in duplicate. *P < .01 vs PF4; **P < .001 vs control.
ODSH enhances platelet recovery in CIT
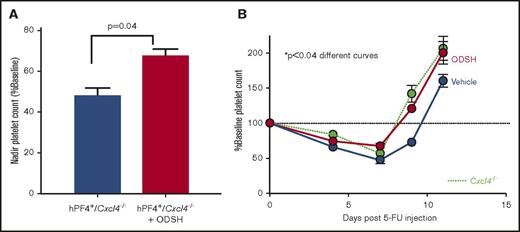
To test whether administration of ODSH by SC injection to mice could limit the fall in platelet count and/or speed platelet count recovery in CIT, mice treated with 5-FU were given ODSH (25 mg/kg) SC for 3 doses starting 24 hours after the chemotherapy dose. A higher platelet count at the nadir was observed in ODSH-treated mice than controls (Figure 3A, P = .04). The time to recovery to baseline was ∼1.5 days faster (Figure 3B). In contrast, there were no significant differences in absolute neutrophil count (ANC) or hemoglobin, whereas there was an increase in total white cell at day 14 (supplemental Figure 1). Animals that did not express PF4 (Cxcl4−/−) either did not demonstrate significant changes in platelet count recovery (supplemental Figure 1).
ODSH effect on blood counts in CIT. (A) Nadir platelet counts from mice shown in panel B demonstrating nadir platelet count was significantly higher in ODSH-treated mice vs control (P = .04) (N = 10 per arm). (B) Effect of ODSH SC injection starting 24 hours after administration of 5-FU in hPF4+/Cxcl4−/− mice (red circles, ODSH; blue circles, vehicle control) compared with Cxcl4−/− mice. Comparison of the curves showed the ODSH curve to be significantly different from the control curve; P < .04. Green circles represent WT animals examined simultaneously. Dashed line represents 100% baseline platelet count. N = 10 animals per arm.
ODSH effect on blood counts in CIT. (A) Nadir platelet counts from mice shown in panel B demonstrating nadir platelet count was significantly higher in ODSH-treated mice vs control (P = .04) (N = 10 per arm). (B) Effect of ODSH SC injection starting 24 hours after administration of 5-FU in hPF4+/Cxcl4−/− mice (red circles, ODSH; blue circles, vehicle control) compared with Cxcl4−/− mice. Comparison of the curves showed the ODSH curve to be significantly different from the control curve; P < .04. Green circles represent WT animals examined simultaneously. Dashed line represents 100% baseline platelet count. N = 10 animals per arm.
ODSH in RIT
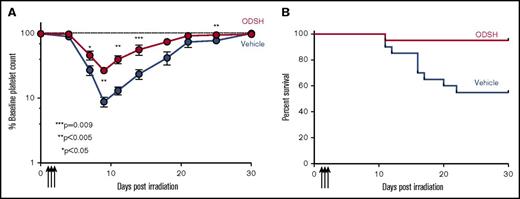
hPF4+/Cxcl4−/− mice exposed to sublethal radiation (expected to result in ∼50% lethality at day 30; supplemental Figure 2) were treated with ODSH or vehicle control starting 24 hours after irradiation. Animals treated with ODSH had significantly higher nadir platelet counts (Figure 4A) and faster recovery (Figure 4B). Hemoglobin and total white cell count were unaffected by treatment (supplemental Figure 3), whereas there was a slight increase in ANC after day 21; however, there was a significantly improved survival (Figure 4C; P < .01). In addition, in hPF4+/Cxcl4−/− ODSH-treated mice, there was significantly less weight loss than controls (Figure 4D; P < .01). In contrast, animals that did not express mPF4 or hPF4 (Cxcl4−/−) did not demonstrate significant changes in platelet count recovery or survival with ODSH treatment, suggesting that PF4 was the major mediator of the ODSH effect on altered recovery (supplemental Figure 4). Because we were interested in the relevance of ODSH protection in a WT population, we performed similar experiments in C57BL/6 mice, and found that there was both improved platelet count recovery (Figure 5A) and survival (Figure 5B). To determine whether the effect of ODSH in bone marrow recovery was due to changes in intramedullary PF4 content, we examined bone marrow histologic sections obtained 48 hours after treatment in irradiated mice given vehicle (Figure 6A: hPF4+; Figure 6B: WT) or ODSH (Figure 6C: hPF4+; Figure 6D: WT). PF4 content within bone marrow was significantly reduced in ODSH-treated mice (supplemental Figure 5). Of note, ODSH did not have a demonstrable effect on the half-life of transfused platelets (supplemental Figure 6), again supporting a role of ODSH on PF4 directly in the bone marrow.
ODSH effect on blood counts in RIT in hPF4+/Cxcl4−/−mice. (A) Nadir platelet counts of irradiated (6.5 Gy) hPF4+/Cxcl4−/− animals as percentage of baseline platelet count, which occurred at day 10 for ODSH-treated animals vs control Cxcl4−/− at day 12. P < .05. N = 20 animals per treatment arm. (B) Same as panel A but showing recovery of platelet count to baseline (dashed line demonstrates 100% baseline) after irradiation showing statistically different platelet counts starting at day 12 postirradiation (**P < .01 by Student t test after Bonferroni correction). N = 5 animals per arm. (C) Same as panel A, but showing survival of animals after irradiation, demonstrating improved survival in ODSH-treated animal (P < .04 by the Gehan-Breslow-Wilcoxon test). N = 20 animals per arm. (D) Same as panel A, but showing weight loss as percentage starting body weight for animals treated with ODSH or vehicle control. Dashed line is baseline body weight. N = 20 animals per arm. P < .001 for difference in curves.
ODSH effect on blood counts in RIT in hPF4+/Cxcl4−/−mice. (A) Nadir platelet counts of irradiated (6.5 Gy) hPF4+/Cxcl4−/− animals as percentage of baseline platelet count, which occurred at day 10 for ODSH-treated animals vs control Cxcl4−/− at day 12. P < .05. N = 20 animals per treatment arm. (B) Same as panel A but showing recovery of platelet count to baseline (dashed line demonstrates 100% baseline) after irradiation showing statistically different platelet counts starting at day 12 postirradiation (**P < .01 by Student t test after Bonferroni correction). N = 5 animals per arm. (C) Same as panel A, but showing survival of animals after irradiation, demonstrating improved survival in ODSH-treated animal (P < .04 by the Gehan-Breslow-Wilcoxon test). N = 20 animals per arm. (D) Same as panel A, but showing weight loss as percentage starting body weight for animals treated with ODSH or vehicle control. Dashed line is baseline body weight. N = 20 animals per arm. P < .001 for difference in curves.
ODSH in RIT in WT animals. (A) Platelet counts of WT C57BL/6 animals as percentage of baseline platelet count after irradiation, showing statistically different platelet counts starting at day 8 postirradiation in animals treated with ODSH (25 mg/kg) starting 24 hours after irradiation and continuing for 3 doses every 12 hours. N = 15 animals per arm. P values by the Student t test after Bonferroni correction. (B) Same as panel A, but showing survival of irradiated animals. N = 15 animals per arm. P < .004 for survival difference by the Gehan-Breslow-Wilcoxon test.
ODSH in RIT in WT animals. (A) Platelet counts of WT C57BL/6 animals as percentage of baseline platelet count after irradiation, showing statistically different platelet counts starting at day 8 postirradiation in animals treated with ODSH (25 mg/kg) starting 24 hours after irradiation and continuing for 3 doses every 12 hours. N = 15 animals per arm. P values by the Student t test after Bonferroni correction. (B) Same as panel A, but showing survival of irradiated animals. N = 15 animals per arm. P < .004 for survival difference by the Gehan-Breslow-Wilcoxon test.
Effect of ODSH on intramedullary PF4 levels. (A) hPF4+/Cxcl4−/− marrow 48 hours after irradiation stained with anti-hPF4 antibody. (B) Same as panel A, but for hPF4+/Cxcl4−/− marrow treated with a total of 75 mg/kg ODSH. (C) As in panel A, but for WT animal without irradiation. (D) As in panel B, but for WT animal treated with ODSH without irradiation. (E) Same as panel C, but 48 hours after irradiation in a WT animal (treated with PBS). (F) Same as panel D, but 48 hours after irradiation in a WT animal treated with ODSH. For all panels, scale bar = 100 µm; immunohistochemical stain and counterstained with hematoxylin.
Effect of ODSH on intramedullary PF4 levels. (A) hPF4+/Cxcl4−/− marrow 48 hours after irradiation stained with anti-hPF4 antibody. (B) Same as panel A, but for hPF4+/Cxcl4−/− marrow treated with a total of 75 mg/kg ODSH. (C) As in panel A, but for WT animal without irradiation. (D) As in panel B, but for WT animal treated with ODSH without irradiation. (E) Same as panel C, but 48 hours after irradiation in a WT animal (treated with PBS). (F) Same as panel D, but 48 hours after irradiation in a WT animal treated with ODSH. For all panels, scale bar = 100 µm; immunohistochemical stain and counterstained with hematoxylin.
Discussion
Both mPF4 and hPF4 have previously been shown to influence steady-state platelet counts, as well as recovery from CIT in mice, and this interaction can be blocked using PF4-specific antibodies.10 We have previously shown that, both in vitro and in vivo, PF4 is an important negative paracrine regulator of the effect of radiation on megakaryocyte formation.9-10 We have also previously shown that irradiation markedly increases release of PF4 from megakaryocytes and that this chemokine is the only biologically relevant negative regulator of megakaryopoiesis as evidenced by the ability of blocking anti-PF4 antibodies to restore colony formation.9 Our current studies explore therapeutic intervention with a low-anticoagulant formulation of heparin ODSH, and its ability to mitigate the PF4 effect on CIT and RIT even when administered 24 hours after injury.
Thrombocytopenia in bone marrow injured states is a significant concern post–chemotherapy or radiation exposure. Thrombocytopenia after chemotherapy results in therapy delays and increases risk of morbidity and mortality.24-26 In pediatric patients with leukemia, we have previously shown that PF4 levels inversely correlated with platelet count recovery.11 Previous studies have demonstrated that these doses of ODSH in mice minimally prolong the activated partial thromboplastin time (whereas the same dose of heparin causes significant prolongation of the activated partial thromboplastin time).27 As this dose is equivalent to doses currently being studied in human subjects20 as an adjuvant to chemotherapy for ODSH’s anti-inflammatory properties (Steven Marcus, Cantex Pharmaceuticals, written communication, 2011 and ongoing), we elected to study dosing that could be started 24 hours after bone marrow injury. We now demonstrate that use of ODSH in an animal CIT model can improve platelet count recovery, and our data suggest that this mechanism is through modulation of PF4 in bone marrow. Because animals lacking PF4 did not benefit from administration of ODSH, our data suggest that the benefit to survival in the RIT model is primarily through amelioration of thrombocytopenia, as would be suggested by other data studying the effects of Mpl ligands in RIT (see Dicarlo et al3 for review).
Current therapeutic approaches using TPO–receptor agonist in CIT have not demonstrated the robust recovery benefits that were anticipated, although clinical trials are ongoing. Use of eltrombopag in severe aplastic anemia has demonstrated interesting effects on both platelet and stem cell populations and further studies are ongoing to better understand these findings and to define patient populations. We have previously shown that PF4 levels inversely correlate with platelet transfusion need and platelet count nadir in pediatric patients with leukemia (whereas TPO levels did not correlate),11 and baseline TPO levels in our various murine models with different PF4 expression levels also do not correlate with platelet counts or platelet count recovery. Current studies are under way to determine whether TPO stimulation would complement blocking PF4 or whether stimulating megakaryopoiesis by TPO administration might have a deleterious effect on count recovery by enhancing marrow-PF4 release.
In radiation injury, platelet count drop correlates with risk of death.6-7 Therefore, in this setting, development of an agent that is able to significantly improve nadir platelet counts and speed count recovery is of vital importance. Our studies demonstrate efficacy of ODSH in a radiation injury model without adjuvant treatment. Future studies in large animal models should be able to establish a therapeutic range for proof-of-principle studies.
Previous studies have demonstrated that the effects of PF4 on platelet recovery after irradiation are blocked by infusion of anti-PF4 F(ab′)2 fragments, but not by the intact antibody, which we posit is the ability of the smaller fragments to penetrate sufficiently into the marrow.9 In a similar fashion, heparin that binds PF4 with high avidity28 and neutralizes its effect on megakaryopoiesis in culture was ineffective to raise platelet counts in vivo (data not shown), which we assumed was due to its known poor penetrance into the marrow.13 ODSH has 10-fold lower anticoagulant activity compared with native heparin,15 allowing for higher plasma concentrations without significant anticoagulant effect. Its decreased negative charge may also enhance its entry into the protected bone marrow niche. Our observation that ODSH leads to removal of intramedullary PF4 suggests bone marrow penetration by ODSH.
We observed significantly improved survival in both WT and hPF4+/Cxcl4−/− animals after treatment with ODSH without an increase in bleeding or greater drop in hemoglobin. The improved survival is not due to changes in hemoglobin, white blood cell count, or ANC, but rather appears to be related to improved platelet count recovery. Other studies have shown that platelet count is significantly correlated with recovery after radiation injury,6-7 and these data support the hypothesis that improving platelet count recovery can significantly improve survival after radiation injury. The contribution of platelets to survival may be due to improved coagulation with lessened gastrointestinal bleeding or from the role played by platelets in resistance to sepsis.
In conclusion, we present evidence that treatment with ODSH beginning 24 hours after radiation or chemotherapy injury can markedly improve platelet count and animal recovery by removing intramedullary free PF4. Further studies need to be done to translate these findings to the clinic in the care of patients with RIT and/or CIT, and perhaps other states such as immune thrombocytopenia purpura and myelodysplastic syndrome where megakaryocyte injury and release of intramedullary PF4 may be present.29
The full-text version of this article contains a data supplement.
Acknowledgments
The authors gratefully acknowledge Steven Marcus of Cantex, Inc, for the generous donation of ODSH for these studies.
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (HHSN272201400033C).
Authorship
Contribution: M.P.L. and M.P. designed and conceptualized the experiments, analyzed and interpreted the data, and edited the manuscript; E.T., A.A., J.T., and L.X. performed the experiments, and analyzed, interpreted, and maintained the data; E.T. prepared the first draft of the manuscript; S.A. performed the dosimetry and edited the manuscript; T.P.K. and G.J. developed ODSH, provided substantial aid in interpretation of data and experiment design, and edited the manuscript; B.T.-S. and K.A. analyzed data, provided aid in experimental design and planning, and edited the manuscript; and N.V.R. designed the anti-PF4 ELISA and helped with data interpretation and manuscript editing.
Conflict-of-interest disclosure: T.P.K. is the original inventor of 2-O, 3-O desulfated heparin. He was the scientific founder and is a current stockholder of Cantex Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: Michele P. Lambert, Division of Hematology, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Abramson Research Building, Room 316G, Philadelphia, PA 19104; e-mail: lambertm@email.chop.edu.