Key Points
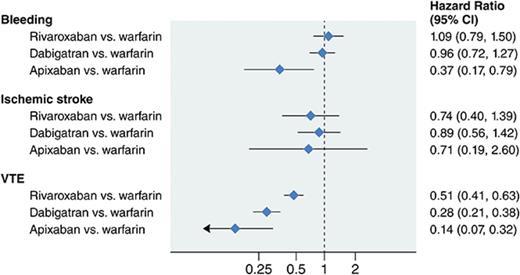
In AF and cancer patients, rate of bleeding is lower with apixaban, similar in dabigatran and rivaroxaban users, compared to warfarin users.
Ischemic stroke rates did not differ among anticoagulant users. Incident VTE risk was lower in all DOAC compared with warfarin users.
Visual Abstract
Professional illustration by Patrick Lane, ScEYEnce Studios
Abstract
Randomized clinical trials comparing direct oral anticoagulants (DOACs) to warfarin in cancer patients have not been performed. We evaluated the effectiveness and associated risk of DOACs vs warfarin, as well as comparisons of DOACs, in a large population of cancer patients with nonvalvular atrial fibrillation (AF). Using the MarketScan databases, we identified 16 096 AF patients (mean age, 74 years) initiating oral anticoagulant and being actively treated for cancer between 2010 and 2014. Anticoagulant users were matched by age, sex, enrollment date, and drug initiation date. Study end points were identified with diagnostic codes and included ischemic stroke, severe bleeding, other bleeding, and venous thromboembolism (VTE). Cox regression was used to estimate associations of anticoagulants with study end points. Compared with warfarin, rates of bleeding (hazard ratio [95% confidence interval]) were similar in rivaroxaban (1.09 [0.79, 1.39]) and dabigatran (0.96 [0.72, 1.27]) users, whereas apixaban users experienced lower rates (0.37 [0.17, 0.79]). Rates of ischemic stroke did not differ among anticoagulant users. Compared with warfarin, rate of VTE (hazard ratio [95% confidence interval]) was lower among rivaroxaban (0.51 [0.41, 0.63]), dabigatran (0.28 [0.21, 0.38]), and apixaban (0.14 [0.07, 0.32]) users. In head-to-head comparisons among DOACs, dabigatran users had lower rates of VTE than rivaroxaban users; apixaban users had lower rates of VTE and severe bleeding than rivaroxaban users. In this population of patients with AF and cancer, DOAC users experienced lower or similar rates of bleeding and stroke compared with warfarin users, and a lower rate of incident VTE.
Introduction
Cancer patients are at high risk for morbidity and mortality due to thrombosis; existing data clearly show that cancer is associated with a hypercoagulable state and poses a fourfold to sevenfold increased risk of venous thromboembolism (VTE).1 On the other hand, these patients are also at twofold higher risk of bleeding.2 As cancer is more common among older individuals, these patients are likely to have other aging-related comorbid conditions, such as atrial fibrillation (AF) and prior VTE, for which chronic anticoagulation may be indicated. Given that cancer patients are at elevated risk of both thrombotic and bleeding complications, the decisions about whether to initiate anticoagulation therapy, and which anticoagulant to choose, are complex.
Historically, warfarin has been the most commonly prescribed anticoagulant for stroke prevention in AF patients. Among cancer patients with AF, use of warfarin is challenging because of metabolic interactions with chemotherapy and antibiotics, chemotherapy-induced thrombocytopenia, and the frequent need for surgical or invasive procedures.
Since 2010, to reduce the risk of stroke and systemic embolism in patients with nonvalvular AF, the US Food and Drug Administration (FDA) has approved 4 direct oral anticoagulants (DOACs): dabigatran (a direct thrombin inhibitor),3 rivaroxaban,4 apixaban,5 and edoxaban6 (all direct Xa inhibitors). These new anticoagulants do not present some of the limitations associated with the use of warfarin, such as frequent international normalized ratio monitoring and drug interactions. However, evidence supporting the use of DOACs in cancer patients for any indication is extremely limited, and concerns have been raised about the safety and efficacy of DOACs in cancer patients.7,8 Due to the dearth of data, the American Society of Clinical Oncology does not recommend use of DOACs for VTE patients with cancer.9
Among cancer patients, additional research is necessary to determine the comparative safety and effectiveness of the DOACs in management of AF. In the absence of large randomized clinical trials comparing DOACs to warfarin in cancer patients with AF, comparative effectiveness research using existing observational data offers the opportunity to address these questions in a timely fashion with a large number of patients. Our aim was to determine the effectiveness and associated risks of DOACs vs warfarin in a large population of cancer patients with AF. We also explored the outcomes in specific cancer types, and the outcomes for head-to-head comparisons of DOACs.
Methods
Study population
We used the Truven Health MarketScan Commercial Claims and Encounters Database and the Medicare supplemental and Coordination of Benefits Database (Truven Health Analytics, Inc, Ann Arbor, MI).10 The databases include health insurance claims from all levels of care, as well as enrollment data from large employers and health plans across the United States, providing private health care coverage for employees, their spouses and dependents, and from individuals and their dependents with employer-sponsored Medicare supplemental plans. The databases contain health insurance claims and enrollment data for inpatient and outpatient services as well as outpatient pharmacy claims.
In the present analysis, we included patients enrolled in the database at some point between 1 January 2010 through 31 December 2014. We identified 532 743 AF patients, 18 to 99 years of age, with at least 1 inpatient or 2 outpatient claims for nonvalvular AF 7 to 365 days apart (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 427.3, 427.31, and 427.32 in any position), at least 1 prescription for warfarin, rivaroxaban, dabigatran, or apixaban after their first AF claim, and ≥90 days of continuous enrollment prior to their first oral anticoagulant prescription. A systematic review of studies using ICD-9-CM codes for AF identification reported a positive predicted value (PPV) of ∼90% and a sensitivity of ∼80%.11
Among eligible patients with AF, we identified 41 036 patients with at least 1 inpatient or 2 outpatient claims on different days that contained a cancer ICD-9-CM diagnosis claim in any position prior to anticoagulant initiation. Cancer was defined as ICD-9-CM codes 140.x-172.x and 174.x-209.x (173.x, nonmelanoma skin cancer, was excluded) (supplemental Table 1). Of these, a subset of 16 096 cancer patients was being actively treated at the time of anticoagulant initiation and was included in our primary analysis. Active cancer patients were defined by the use of chemotherapy, radiation therapy, or cancer surgery within 6 months prior to the start of anticoagulation. Patients were considered to have active chemotherapy treatment if they had any of the following: (a) at least 1 claim for a chemotherapy J code or chemotherapy drug code, (b) at least 1 radiation therapy code, (c) active surgical treatment, (d) inpatient Medicare Severity Diagnosis Related Group with a cancer ICD-9-CM code in the primary position of the claim, or (e) outpatient cancer surgery coded with a cancer procedure code and coded with a cancer ICD-9-CM code in the primary diagnosis of the claim (Cancer Research Network [U24 CA171524]) (supplemental Table 2).
All patient information is Health Insurance Portability and Accountability Act compliant, deidentified, commercially available secondary data, and therefore the institutional review board at the University of Minnesota deemed this analysis exempt from review.
Anticoagulant use
Patients were categorized according to the first anticoagulant prescribed after AF diagnosis from 2010 to 2014 as new warfarin user, new rivaroxaban user, new dabigatran user, or new apixaban user. Edoxaban was not FDA approved during the study period; hence, it was not considered. Validity of warfarin claims in administrative databases is excellent (PPV > 99%)12 and is likely to be similar for DOACs. We included all DOAC prescriptions independently of the dosage strength. Patients were not excluded if they switched between different anticoagulants.
Matching
Each DOAC user was matched with warfarin users by age (±3 years), sex, enrollment date (±90 days), and anticoagulant initiation date (±90 days) using a computerized “greedy” matching algorithm where the goal is to produce matched samples with balanced covariates across the treatment group and control group. It can generate matched pairs so that once a person has been matched, he or she cannot be used in another match.13 Each dabigatran and rivaroxaban user was matched with up to 4 warfarin users, and each apixaban user was matched with up to 3 warfarin users. In head-to-head comparisons of DOACs, apixaban and dabigatran were matched with up to 2 rivaroxaban users. Apixaban could not be compared with dabigatran due to small sample size after matching.
Definition of outcomes
The primary outcome of the study was severe bleeding events, with secondary outcomes of other bleeding events, ischemic stroke, and VTE. These outcomes were identified from inpatient claims (VTE events were also identified from 2 outpatient claims on different days) using validated algorithms described in the following paragraphs. Corresponding ICD-9-CM codes are listed in supplemental Table 3. In addition, a priori, we decided to use asthma as a control outcome, meaning that we expected no association between anticoagulant type and asthma risk. A similar rate of asthma by anticoagulant type would provide indirect evidence of no confounding.
Severe bleeding was defined as an intracranial hemorrhage or gastrointestinal bleeding. Intracranial hemorrhage was defined based on the presence of ICD-9-CM codes 430 (subarachnoid hemorrhage) and 431 (intracerebral hemorrhage) as the primary discharge diagnosis in an inpatient claim after the index date. The PPV has been reported as >90% in many different validation studies.14 Gastrointestinal bleeding was defined following the algorithm developed by Cunningham et al.15 This algorithm considers presence of bleeding-related ICD-9-CM codes in inpatient claims as primary and secondary diagnoses, presence of transfusion codes (hospital revenue code indicating transfusion/cross-matching for transfusion), and presence/absence of trauma codes to exclude trauma-related bleeding. The PPV of this algorithm is 86%, which is comparable to other peer-reviewed algorithms.16 Other bleeding was defined as genitourinary bleeding, hemopericardium, hemoperitoneum, hemarthrosis, epistaxis, hemoptysis, hemorrhage from throat, and unspecified hemorrhage.15
Ischemic stroke was defined based on the presence of ICD-9-CM codes 434.xx (occlusion of cerebral arteries) and 436.xx (acute but ill-defined cerebrovascular disease) as the primary discharge diagnosis in any inpatient claim following the index date. A PPV of >80% has been reported in several validation studies that used this definition.14
VTE was defined as at least 1 inpatient, 1 emergency room, or 2 outpatient claims for VTE based on ICD-9-CM codes 415.1x, 451.1x, 453.2, 453.4x, 453.5x, 453.8, or 453.9 in any position. Prior studies have reported a PPV of 64.6% to 85% for hospital/emergency department diagnosis of VTE and 30.9% for outpatient diagnosis.17-20 In the primary analysis, we not only used the combined inpatient and outpatient end point, but also conducted sensitivity analyses using inpatient claims only, given the poor PPV using outpatient diagnosis for VTE.
Finally, asthma was defined according to an algorithm developed by the Centers for Medicare and Medicaid Chronic Condition Data Warehouse.21
Assessment of covariates
Predetermined covariates were defined based on inpatient, outpatient, and pharmacy claims that took place prior to the anticoagulation initiation date using validated published algorithms.22 Demographic characteristics, comorbidities, procedures, and pharmacy prescription fills were ascertained, and are listed in supplemental Table 4. Comorbidities of interest were defined using published algorithms from inpatient and outpatient claims, and include prior stroke/transient ischemic attack, hemorrhagic stroke, heart failure, myocardial infarction, hypertension, diabetes, peripheral arterial disease, liver disease, kidney disease, chronic pulmonary disease, metastatic cancer, history of bleeding, hematological disorders (anemia, coagulation defects), dementia, depression, and alcohol abuse. Cardiac, vascular, gastrointestinal, and neurologic procedures also were identified from inpatient and outpatient claims. Presence of prescription fills for the following medication groups were ascertained: digoxin, clopidogrel, other antiplatelets, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, calcium channel blockers, antiarrhythmics, statins, antidiabetic medications, chemotherapy, and hormonal therapy. The congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, vascular disease, age 65 to 74 years, sex (CHA2DS2-VASc) score23 was calculated at the date of anticoagulation initiation to determine stroke risk in these AF patients.
Statistical analysis
High-dimensional propensity scores (HDPSs) were calculated using methodology proposed by Schneeweiss et al,24 and included the following predefined variables: age, calendar year, sex, CHA2DS2-VASc score, any prevalent outcome before the start of the anticoagulation date, and covariates listed in the preceding section. HDPSs were calculated with SAS macros developed by Rassen et al and included the predefined covariates and the most prioritized empirical covariates from inpatient and outpatient diagnostic codes, procedure codes, and outpatient pharmacy claims.25 Variables were prioritized by their potential to control confounding not conditional on exposure and other covariates, as detailed elsewhere.25 Separate HDPSs were calculated for each of the anticoagulant comparison-outcome pairs (5 outcomes × 5 comparison groups = 25 total HDPSs).
Cox proportional hazards models were used to estimate the association between anticoagulant type and the time to each outcome (severe bleeding, ischemic stroke, other bleeding, VTE, and the control outcome asthma). Follow-up began at the date of initiation of oral anticoagulation. Time to event was considered as the time to each outcome event, health plan disenrollment, the end of study follow-up, or 31 December 2014, whichever occurred first. For each outcome, Cox proportional hazards models were adjusted for age (continuous), sex, CHA2DS2-VASc score (categorical), HDPSs (continuous; see next paragraph), and prior history of the outcome at the index date (except for VTE, in which prevalent cases were excluded).
Propensity scores can be used to control for confounding via matching or adjustment. Given our matched sample size and rare outcomes, we chose to adjust for propensity scores in the regression models, to account for patient characteristics that may differ among the comparison groups at the time of anticoagulation initiation. The additional inclusion of age and sex (which were initial matching variables) in the propensity score calculation is to be able to ensure adequate adjustment for these important covariates when creating the HDPSs.
Effect modification by sex, age (<75, ≥75 years), CHA2DS2-VASc score (<2, ≥2), and early vs late outcomes (<90 days, ≥90 days) was explored using stratified analysis. All statistical analyses were performed with SAS version 9.4 (SAS Inc, Cary, NC) and STATA version 14 (StataCorp LP, College Station, TX).
Results
Study sample
In this analysis, we included 16 096 patients with active cancer who initiated oral anticoagulation therapy for nonvalvular AF. Of these, 6075 cancer patients received DOACs and 10 021 were matched warfarin users. Patients on warfarin were slightly older (mean age, 75.4 years) and had a higher CHA2DS2-VASc score (4.6) compared with DOAC users (mean age of 74.0 years, and mean CHA2DS2-VASc score of 4.2). Approximately 40% of the patients were female, and breast cancer was the most common individual cancer. Use of chemotherapy, hormonal therapy, or other cancer therapy was similar across groups (Table 1). Mean follow-up after initiation of anticoagulation was 12 months.
Characteristics of nonvalvular AF active cancer patients by anticoagulant use, MarketScan, 2010-2014
| . | Rivaroxaban . | Dabigatran . | Apixaban . | Warfarin . |
|---|---|---|---|---|
| N | 2808 | 2189 | 1078 | 10 021 |
| Age, y | 73.8 ± 10.2 | 74.0 ± 10.3 | 74.9 ± 10.3 | 75.4 ± 10.1 |
| Female, % | 40.7 | 38.3 | 42.5 | 39.4 |
| CHA2DS2-VASc score | 4.0 ± 2.0 | 4.3 ± 2.0 | 4.2 ± 1.9 | 4.6 ± 2.0 |
| Comorbidities, % | ||||
| Hypertension | 82.3 | 83.4 | 85.6 | 85.4 |
| Prior ischemic stroke | 27.4 | 33.9 | 28.8 | 35.6 |
| Metastatic brain lesion | 2 | 1.5 | 1.2 | 2.4 |
| Prior gastrointestinal bleed | 12.1 | 14.4 | 11.6 | 16.6 |
| Prior other bleed | 8 | 10.2 | 7.2 | 13 |
| Liver disease | 9.6 | 10.3 | 9.8 | 11.5 |
| Renal disease | 13.8 | 13.3 | 17.5 | 24.4 |
| Heart failure | 32.4 | 36.3 | 34.6 | 42.5 |
| Medications (pharmacy), % | ||||
| Antiplatelet agents | 2.6 | 3.5 | 4.1 | 3.1 |
| Nonsteroidal anti-inflammatory drugs | 27.3 | 32.6 | 28 | 28.8 |
| Chemotherapy | 21.7 | 18.1 | 16.3 | 23.4 |
| Hormonal therapy | 13.4 | 11.5 | 11.7 | 12.8 |
| Radiation therapy | 12.3 | 10.3 | 8.1 | 11.7 |
| Other cancer therapy/procedures | 65.7 | 69.7 | 71.4 | 64.7 |
| Type of cancer, % | ||||
| Breast | 21.4 | 20.8 | 23.4 | 17.8 |
| Lung | 12.5 | 10.4 | 8.6 | 13.1 |
| Gastrointestinal | 10.3 | 10.7 | 11.6 | 13.9 |
| Genitourinary | 29.0 | 31.3 | 29.8 | 28.7 |
| Gyneco-oncological | 2.6 | 2.2 | 2.0 | 2.5 |
| Hematological | 9.6 | 9.2 | 8.8 | 10.1 |
| Other | 14.6 | 15.4 | 15.8 | 13.9 |
| . | Rivaroxaban . | Dabigatran . | Apixaban . | Warfarin . |
|---|---|---|---|---|
| N | 2808 | 2189 | 1078 | 10 021 |
| Age, y | 73.8 ± 10.2 | 74.0 ± 10.3 | 74.9 ± 10.3 | 75.4 ± 10.1 |
| Female, % | 40.7 | 38.3 | 42.5 | 39.4 |
| CHA2DS2-VASc score | 4.0 ± 2.0 | 4.3 ± 2.0 | 4.2 ± 1.9 | 4.6 ± 2.0 |
| Comorbidities, % | ||||
| Hypertension | 82.3 | 83.4 | 85.6 | 85.4 |
| Prior ischemic stroke | 27.4 | 33.9 | 28.8 | 35.6 |
| Metastatic brain lesion | 2 | 1.5 | 1.2 | 2.4 |
| Prior gastrointestinal bleed | 12.1 | 14.4 | 11.6 | 16.6 |
| Prior other bleed | 8 | 10.2 | 7.2 | 13 |
| Liver disease | 9.6 | 10.3 | 9.8 | 11.5 |
| Renal disease | 13.8 | 13.3 | 17.5 | 24.4 |
| Heart failure | 32.4 | 36.3 | 34.6 | 42.5 |
| Medications (pharmacy), % | ||||
| Antiplatelet agents | 2.6 | 3.5 | 4.1 | 3.1 |
| Nonsteroidal anti-inflammatory drugs | 27.3 | 32.6 | 28 | 28.8 |
| Chemotherapy | 21.7 | 18.1 | 16.3 | 23.4 |
| Hormonal therapy | 13.4 | 11.5 | 11.7 | 12.8 |
| Radiation therapy | 12.3 | 10.3 | 8.1 | 11.7 |
| Other cancer therapy/procedures | 65.7 | 69.7 | 71.4 | 64.7 |
| Type of cancer, % | ||||
| Breast | 21.4 | 20.8 | 23.4 | 17.8 |
| Lung | 12.5 | 10.4 | 8.6 | 13.1 |
| Gastrointestinal | 10.3 | 10.7 | 11.6 | 13.9 |
| Genitourinary | 29.0 | 31.3 | 29.8 | 28.7 |
| Gyneco-oncological | 2.6 | 2.2 | 2.0 | 2.5 |
| Hematological | 9.6 | 9.2 | 8.8 | 10.1 |
| Other | 14.6 | 15.4 | 15.8 | 13.9 |
Values are mean ± standard deviation or percentage. Gastrointestinal malignancies (stomach, esophagus, colon cancer, rectal cancer, pancreatic cancer, hepatocellular carcinoma, small intestine cancer), genitourinary malignancies (prostate cancer, testicular cancer, bladder cancer, kidney cancer, and penile cancer), gyneco-oncological malignancies (cancer of cervix, ovary, and uterus), hematological malignancies (leukemia and lymphomas), and “Other” for any remaining nonmelanoma rare cancers.
Rivaroxaban vs warfarin users
The hazard ratios (HRs) and 95% confidence intervals (CIs) of each outcome for rivaroxaban users compared with warfarin users are reported in Table 2. During a mean follow-up of 11 months, in models adjusted for age, sex, CHA2DS2-VASc score, prior history of the outcome (stroke or bleeding), and HDPS, rivaroxaban users had similar rates of ischemic stroke and severe bleeding compared with warfarin users: HR (95% CI) = 0.74 (0.40-1.39) and 1.09 (0.79-1.50), respectively. The rate of incident VTE was half in rivaroxaban users as compared with warfarin users: HR (95% CI) = 0.51 (0.41-0.63). There was no association between the anticoagulant type and other bleeding or the control outcome asthma (Table 2).
Adjusted HRs (95% CIs) comparing the safety and effectiveness of oral anticoagulant users to matched warfarin users for the treatment of nonvalvular AF in active cancer patients, MarketScan, 2010-2014
| . | No. of events . | Person-years . | No. of events . | Person-years . | HR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Rivaroxaban user (n = 2808) | Matched warfarin user (n = 5673) | |||||
| Ischemic stroke | 16 | 2277 | 59 | 5 279 | 0.74 (0.40, 1.39) | .35 |
| Severe bleeding | 68 | 2245 | 181 | 5 207 | 1.09 (0.79, 1.50) | .59 |
| Other bleeding | 50 | 2213 | 177 | 5 031 | 0.79 (0.55, 1.13) | .2 |
| VTE* | 124 | 2046 | 472 | 3 903 | 0.51 (0.41, 0.63) | <.0001 |
| Asthma (control outcome) | 41 | 2259 | 91 | 5 253 | 0.99 (0.64, 1.51) | .94 |
| Dabigatran user (n = 2189) | Matched warfarin user (n = 8339) | |||||
| Ischemic stroke | 26 | 3310 | 127 | 10 878 | 0.89 (0.56, 1.42) | .63 |
| Severe bleeding | 70 | 3273 | 329 | 10 706 | 0.96 (0.72, 1.27) | .75 |
| Other bleeding | 40 | 3236 | 306 | 10 376 | 0.58 (0.41, 0.84) | .003 |
| VTE* | 49 | 3199 | 743 | 8 206 | 0.28 (0.21, 0.38) | <.0001 |
| Asthma (control outcome) | 38 | 3302 | 183 | 10 825 | 0.75 (0.51, 1.10) | .14 |
| Apixaban user (n = 1078) | Matched warfarin user (n = 2775) | |||||
| Ischemic stroke | 4 | 550 | 18 | 1 773 | 0.71 (0.19, 2.60) | .6 |
| Severe bleeding | 10 | 551 | 84 | 1 744 | 0.37 (0.17, 0.79) | .01 |
| Other bleeding | 9 | 538 | 72 | 1 699 | 0.58 (0.25, 1.31) | .19 |
| VTE* | 7 | 540 | 218 | 1 325 | 0.14 (0.07, 0.32) | <.0001 |
| Asthma (control outcome) | 13 | 549 | 40 | 1 760 | 0.99 (0.53, 2.22) | .98 |
| . | No. of events . | Person-years . | No. of events . | Person-years . | HR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Rivaroxaban user (n = 2808) | Matched warfarin user (n = 5673) | |||||
| Ischemic stroke | 16 | 2277 | 59 | 5 279 | 0.74 (0.40, 1.39) | .35 |
| Severe bleeding | 68 | 2245 | 181 | 5 207 | 1.09 (0.79, 1.50) | .59 |
| Other bleeding | 50 | 2213 | 177 | 5 031 | 0.79 (0.55, 1.13) | .2 |
| VTE* | 124 | 2046 | 472 | 3 903 | 0.51 (0.41, 0.63) | <.0001 |
| Asthma (control outcome) | 41 | 2259 | 91 | 5 253 | 0.99 (0.64, 1.51) | .94 |
| Dabigatran user (n = 2189) | Matched warfarin user (n = 8339) | |||||
| Ischemic stroke | 26 | 3310 | 127 | 10 878 | 0.89 (0.56, 1.42) | .63 |
| Severe bleeding | 70 | 3273 | 329 | 10 706 | 0.96 (0.72, 1.27) | .75 |
| Other bleeding | 40 | 3236 | 306 | 10 376 | 0.58 (0.41, 0.84) | .003 |
| VTE* | 49 | 3199 | 743 | 8 206 | 0.28 (0.21, 0.38) | <.0001 |
| Asthma (control outcome) | 38 | 3302 | 183 | 10 825 | 0.75 (0.51, 1.10) | .14 |
| Apixaban user (n = 1078) | Matched warfarin user (n = 2775) | |||||
| Ischemic stroke | 4 | 550 | 18 | 1 773 | 0.71 (0.19, 2.60) | .6 |
| Severe bleeding | 10 | 551 | 84 | 1 744 | 0.37 (0.17, 0.79) | .01 |
| Other bleeding | 9 | 538 | 72 | 1 699 | 0.58 (0.25, 1.31) | .19 |
| VTE* | 7 | 540 | 218 | 1 325 | 0.14 (0.07, 0.32) | <.0001 |
| Asthma (control outcome) | 13 | 549 | 40 | 1 760 | 0.99 (0.53, 2.22) | .98 |
Adjusted for age, sex, CHA2DS2-VASc score, prior history of the outcome, and HDPS.
Patients with prevalent VTE excluded from the analysis.
Dabigatran vs warfarin users
During a mean follow-up of 16 months, in models adjusted for age, sex, CHA2DS2-VASc score, prior history of the outcome, and HDPS, dabigatran users had similar rates of ischemic stroke and severe bleeds compared with warfarin users: HR (95% CI) = 0.89 (0.56-1.42) and 0.96 (0.72-1.27), respectively (Table 2). The rate of other bleeding and incident VTE was significantly lower in dabigatran users as compared with warfarin users: HR (95% CI) = 0.58 (0.41, 0.84) and 0.28 (0.21-0.38), respectively. There was no association with the anticoagulant type and the control outcome asthma (Table 2).
Apixaban vs warfarin users
The FDA only approved apixaban in December 2012 and our study follow-up ended in 2014. Accordingly, apixaban users form the smallest warfarin-matched group, and follow-up is shorter for this comparison than for other DOAC vs warfarin comparisons. During a mean follow-up of 6 months, compared with warfarin users, new apixaban users had significantly lower rates of severe bleeding and incident VTE: HR (95% CI) = 0.37 (0.17-0.79) and 0.14 (0.07-0.32), respectively (Table 2). Apixaban users had similar rates of ischemic stroke, other bleeding, and asthma compared with warfarin users (Table 2).
Dabigatran vs rivaroxaban users
When comparing matched dabigatran users with rivaroxaban users, dabigatran use was associated with a higher rate of ischemic stroke: HR (95% CI) = 7.61 (1.52, 38.1), though these results were based on 12 events only (3 among rivaroxaban users and 9 in dabigatran users). Rates of VTE were lower in the dabigatran users: HR (95% CI) = 0.47 (0.21, 1.04) (Table 3).
Adjusted HRs (95% CIs) of selected outcomes comparing DOAC use in nonvalvular AF patients with active cancer, MarketScan, 2010-2014
| . | No. of events . | Person-years . | No. of events . | Person-years . | HR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Dabigatran user (n = 859) | Matched rivaroxaban user (n = 922) | |||||
| Ischemic stroke | 9 | 980 | 3 | 1059 | 7.61 (1.52, 38.12) | .01 |
| Severe bleeding | 22 | 968 | 22 | 1045 | 1.07 (0.50, 2.32) | .86 |
| Other bleeding | 12 | 972 | 19 | 1030 | 0.81 (0.32, 2.06) | .65 |
| VTE* | 13 | 956 | 33 | 982 | 0.47 (0.21, 1.04) | .06 |
| Asthma (control outcome) | 11 | 978 | 15 | 1049 | 1.66 (0.58, 4.77) | .34 |
| Apixaban user (n = 1126) | Matched rivaroxaban user (n = 2016) | |||||
| Ischemic stroke | 3 | 568 | 13 | 1123 | 0.52 (0.13, 2.17) | .37 |
| Severe bleeding | 10 | 567 | 43 | 1112 | 0.29 (0.13, 0.65) | .002 |
| Other bleeding | 9 | 554 | 31 | 1088 | 0.64 (0.28, 1.49) | .3 |
| VTE* | 10 | 556 | 92 | 976 | 0.23 (0.12, 0.47) | <.0001 |
| Asthma (control outcome) | 14 | 565 | 25 | 1116 | 0.75 (0.34, 1.67) | .48 |
| . | No. of events . | Person-years . | No. of events . | Person-years . | HR (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Dabigatran user (n = 859) | Matched rivaroxaban user (n = 922) | |||||
| Ischemic stroke | 9 | 980 | 3 | 1059 | 7.61 (1.52, 38.12) | .01 |
| Severe bleeding | 22 | 968 | 22 | 1045 | 1.07 (0.50, 2.32) | .86 |
| Other bleeding | 12 | 972 | 19 | 1030 | 0.81 (0.32, 2.06) | .65 |
| VTE* | 13 | 956 | 33 | 982 | 0.47 (0.21, 1.04) | .06 |
| Asthma (control outcome) | 11 | 978 | 15 | 1049 | 1.66 (0.58, 4.77) | .34 |
| Apixaban user (n = 1126) | Matched rivaroxaban user (n = 2016) | |||||
| Ischemic stroke | 3 | 568 | 13 | 1123 | 0.52 (0.13, 2.17) | .37 |
| Severe bleeding | 10 | 567 | 43 | 1112 | 0.29 (0.13, 0.65) | .002 |
| Other bleeding | 9 | 554 | 31 | 1088 | 0.64 (0.28, 1.49) | .3 |
| VTE* | 10 | 556 | 92 | 976 | 0.23 (0.12, 0.47) | <.0001 |
| Asthma (control outcome) | 14 | 565 | 25 | 1116 | 0.75 (0.34, 1.67) | .48 |
Adjusted for age, sex, CHA2DS2-VASc score, prior history of the outcome, and HDPS.
Patients with prevalent VTE excluded from the analysis.
Apixaban vs rivaroxaban users
Compared with rivaroxaban, apixaban use was associated with a lower rate of severe bleeding and a lower rate of incident VTE: HR (95% CI) = 0.29 (0.13, 0.65) and 0.23 (0.12, 0.47), respectively. Rates were not significantly different for the outcomes of ischemic stroke, other bleeding, and asthma (Table 3).
Stratified analysis
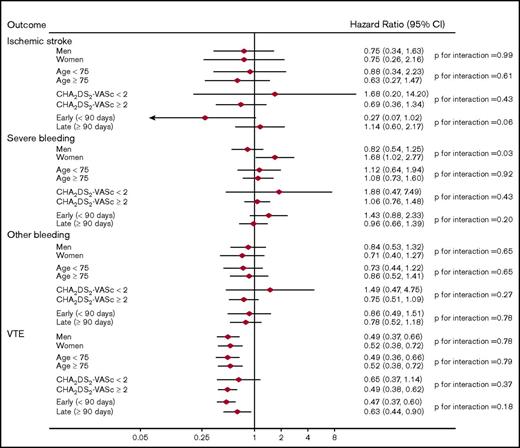
Each DOAC was independently compared with warfarin in analyses stratified by sex, age (<75, ≥75 years), CHA2DS2-VASc score (<2, ≥2), and early vs late outcomes (<90 days, ≥90 days). In stratified analysis, the same adjustment models were used as for the original analysis. Rivaroxaban (vs warfarin) was associated with a significantly increased rate of severe bleeding in women but not in men: HR (95% CI) = 1.68 (1.02, 2.77) and 0.82 (0.54, 1.25), respectively (P for interaction = .03). No other significant interactions were observed (Figure 1).
Adjusted HRs (95% CIs) of outcomes among anticoagulant users, stratified by subgroups: rivaroxaban vs warfarin users.
Adjusted HRs (95% CIs) of outcomes among anticoagulant users, stratified by subgroups: rivaroxaban vs warfarin users.
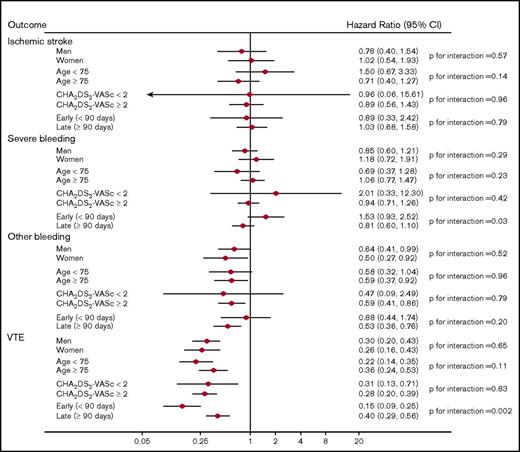
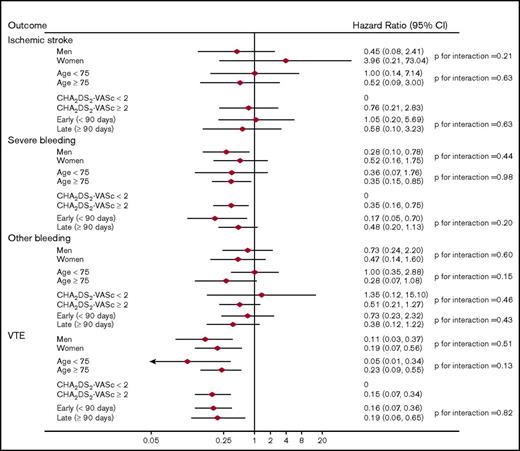
When dabigatran users were compared with the warfarin users, there was significant interaction with a lower rate of VTE occurring early (<90 days) vs late (≥90 days) after anticoagulant initiation: HR (95% CI) = 0.15 (0.09, 0.25) and 0.40 (0.29, 0.56), respectively (P for interaction = .002). There was also significant interaction associated with a higher rate of severe bleeding occurring early (<90 days) after anticoagulant initiation relative to later: HR (95% CI) = 1.53 (0.93, 2.52) and 0.81 (0.60, 1.10), respectively (P for interaction = .03). No other significant interactions were present (Figure 2). Finally, there were no significant interactions in the analysis comparing apixaban users to warfarin users (Figure 3).
Adjusted HRs (95% CIs) of outcomes among anticoagulant users, stratified by subgroups: dabigatran vs warfarin users.
Adjusted HRs (95% CIs) of outcomes among anticoagulant users, stratified by subgroups: dabigatran vs warfarin users.
Adjusted HRs (95% CIs) of outcomes among anticoagulant users, stratified by subgroups: apixaban vs warfarin users.
Adjusted HRs (95% CIs) of outcomes among anticoagulant users, stratified by subgroups: apixaban vs warfarin users.
The comparisons of rivaroxaban vs warfarin and dabigatran vs warfarin were also stratified by major cancer type (supplemental Tables 5 and 6). We performed separate analyses for the most prevalent cancers, that is, breast, lung, colon, and prostate, with the remaining nonmelanoma cancers lumped into 1 subgroup as other cancers. Despite the small number of cases in each of the subgroups, lower rates of VTE with rivaroxaban or dabigatran vs warfarin were observed across cancers. Similarly, we also found an overall trend of lower rates of “other bleeding” in dabigatran and, to a lesser extent, rivaroxaban for all examined specific cancer types except lung cancer for rivaroxaban and prostate cancer for dabigatran (supplemental Tables 5 and 6). However, we view these results as extremely preliminary given the limited number of events. The numbers of events in the apixaban subgroup was so low that no definite conclusions can be drawn for cancer-specific effects. Similarly, head-to-head comparison of DOACs was limited by the paucity of events in the comparator groups.
Sensitivity analysis for inpatient-only VTE
Sensitivity analyses were conducted with inpatient-only claims for VTE as the outcome, due to the low reported PPV for outpatient VTE claims in previous studies. Compared with warfarin, the HR (95% CI) for rivaroxaban was 0.73 (0.53, 1.01), for dabigatran was 0.38 (0.25, 0.57), and for apixaban was 0.14 (0.03, 0.59). Comparing to matched patients using rivaroxaban users as a reference group, the HR (95% CI) for dabigatran was 0.37 (0.11, 1.21) and for apixaban was 0.08 (0.02, 0.35) (Table 4).
Adjusted HRs (95% CIs) for inpatient VTE outcome comparing DOAC users to matched control users for the treatment of nonvalvular AF in active cancer patients, MarketScan, 2010-2014
| No. of events . | Person-years . | No. of events . | Person-years . | HR (95% CI) . | P . | |
|---|---|---|---|---|---|---|
| DOAC | Warfarin | |||||
| Rivaroxaban vs warfarin | 66 | 2085 | 160 | 4156 | 0.73 (0.53, 1.01) | .06 |
| Dabigatran vs warfarin | 28 | 3217 | 281 | 8734 | 0.38 (0.25, 0.57) | <.0001 |
| Apixaban vs warfarin | 2 | 541 | 67 | 1407 | 0.14 (0.03, 0.59) | .008 |
| DOAC | Rivaroxaban | |||||
| Dabigatran vs rivaroxaban | 6 | 961 | 15 | 997 | 0.37 (0.11, 1.21) | .10 |
| Apixaban vs rivaroxaban | 2 | 558 | 43 | 1004 | 0.08 (0.02, 0.35) | .0008 |
| No. of events . | Person-years . | No. of events . | Person-years . | HR (95% CI) . | P . | |
|---|---|---|---|---|---|---|
| DOAC | Warfarin | |||||
| Rivaroxaban vs warfarin | 66 | 2085 | 160 | 4156 | 0.73 (0.53, 1.01) | .06 |
| Dabigatran vs warfarin | 28 | 3217 | 281 | 8734 | 0.38 (0.25, 0.57) | <.0001 |
| Apixaban vs warfarin | 2 | 541 | 67 | 1407 | 0.14 (0.03, 0.59) | .008 |
| DOAC | Rivaroxaban | |||||
| Dabigatran vs rivaroxaban | 6 | 961 | 15 | 997 | 0.37 (0.11, 1.21) | .10 |
| Apixaban vs rivaroxaban | 2 | 558 | 43 | 1004 | 0.08 (0.02, 0.35) | .0008 |
Adjusted for age, sex, CHA2DS2-VASc score, prior history of the outcome, and HDPS. Bold values represent statistical significance.
Discussion
In this analysis of a large health care claims database, we found that, among patients with both nonvalvular AF and active cancer, the rate of severe bleeding was significantly lower among apixaban users as compared with warfarin and rivaroxaban users, whereas the rate was similar in dabigatran and rivaroxaban users compared with warfarin users. Also, incidence of ischemic stroke was similar between DOAC and warfarin users. Prevention of VTE was not the primary intent for use of anticoagulation in this population; however, we found that DOAC users had a significantly lower rate of incident VTE compared with warfarin users. VTE rate reduction was similar for all of the DOACs. In general, results were consistent across cancers at different sites.
In the aging population, the incidence and prevalence of AF is increasing,26 and cancer patients often have AF as a comorbid condition.27 DOACs offer an effective anticoagulant choice that does not require monitoring. However, leaders in the field and professional societies (including the American Society of Clinical Oncology) have expressed concerns and advised caution for the use of DOACs in cancer patients due to the dearth of data among these patients.7-9 Our data clearly show, though, that the use of DOACs is widespread among cancer patients with AF.
Our data also suggest that DOACs may be at least as safe and effective as warfarin for prevention of ischemic stroke and may even have a more beneficial bleeding profile, similar to what is found in nonselected populations of anticoagulated patients with AF. All DOACs were associated with 50% to 85% reductions in the rate of incident VTE compared with warfarin in our study population. These results were consistent across different types of cancer. In head-to-head comparisons, dabigatran and apixaban were associated with lower rates of VTE than rivaroxaban. Apixaban was also associated with lower bleeding rates compared with rivaroxaban. However, some of these comparisons are based on small numbers of events and, therefore, future analyses, as the use of DOACs becomes more widespread, will be able to provide more robust answers.
Our findings have major implications for cancer patients, as thrombotic events are the second leading cause of mortality in this patient population.28 If use of DOACs among cancer patients, as compared with warfarin, does really reduce the rate of incident VTE and/or bleeding events, more widespread use of DOACs would have a clinically significant impact on morbidity and mortality of cancer patients.
Limitations of our study include the retrospective nature of the study and the use of a claims database, with the accompanying issues of confounding, selection bias, misclassification of the exposure and outcome, unmeasured confounders such as frailty, and lastly, patient vs provider preferences. We tried to address all of these variables with the use of validated algorithms for outcome definitions and appropriate statistical methods for comparative effectiveness research. Confounding was addressed by adjusting for HDPS, which uses predefined variables and a wide range of empirically identified confounders. Our approach for selection of covariates considered the most likely variables to be generating confounding based on their association with treatment and with the outcome. This approach has shown to be an effective approach for control of confounding.24 We stratified by the cancer types as well in our models and the results were similar across the different cancers. In addition, we included a control outcome (ie, asthma) in our analysis. The lack of an association between anticoagulant type and rate of asthma provides indirect evidence of no residual uncontrolled confounding. Despite implementing all of these state-of-the-art statistical approaches, we acknowledge the limitations of statistical methods in large database retrospective studies. Moreover, even though the PPV of most algorithms for outcome ascertainment is adequate, some events were likely missed and some detected outcomes were false positives. Also, the PPV and sensitivity for codes for active cancer have not been validated in a systematic fashion in any study.
We performed a sensitivity analysis with a more specific definition of VTE; estimates from this analysis were similar to those of the analysis including the definition using both inpatient and outpatient claims to define VTE events although they were slightly attenuated in some of the comparisons. Another issue is the limited duration of follow-up data, but this is expected given the recent approval of the DOACs and the turnover in health care plan membership. In addition, claims provide limited information about staging for the majority of the cancers, which may result in uncontrolled confounding if type of oral anticoagulant (warfarin vs DOAC) varies by cancer stage. Lastly, there is no information on time in therapeutic range for the warfarin group. These patients may or may not be well controlled for international normalized ratio monitoring.
Despite the limitations, our study has several strengths that are worth highlighting. Our study, although retrospective, characterizes practice-based outcomes in a large population, which will complement randomized clinical trials once they are available. The landmark studies showing noninferiority of DOACs compared with warfarin in AF patients did not specifically look at the subgroup of patients with cancer.3-6 In the studies of DOACs vs warfarin for VTE, subset analysis of cancer patients was not preplanned, although it appears that DOACs were safe in the small group for which information is available.29-31
Until results of randomized control trials specifically designed to look at the safety and efficacy of DOACs in cancer patients are available, clinicians have to rely on robust observational data. Based on our findings, all of the DOACs appear to be safe compared with warfarin from the standpoint of rate of severe bleeding. DOACs appear to be superior to warfarin in preventing the development of VTE, which carries major implications in the morbidity and mortality of cancer patients. Our data give some reassurance to clinicians that a DOAC may be a reasonable option for cancer patients who need anticoagulation for which warfarin or low-molecular-weight heparin may not be suitable.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01-HL122200 and R01-HL131579 and American Heart Association grant 16EIA26410001 (A.A.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: S.S. designed research, analyzed data, and wrote the manuscript; F.L.N. performed research and analyzed the data; Y.H.D. and P.L.L. analyzed the data and provided feedback on the manuscript preparation; R.F.M. and L.Y.C. provided feedback on the manuscript preparation; and A.A. obtained funding, designed research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Surbhi Shah, Division of Hematology, Oncology and Transplantation, University of Minnesota Medical School, Mayo Mail Code 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: shahx090@umn.edu.