Key Points
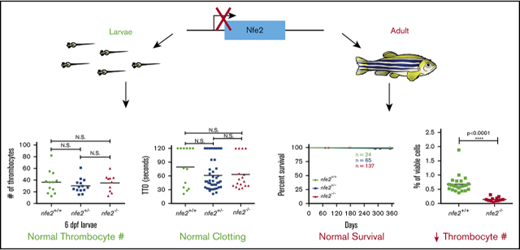
Deficiency of Nfe2 in zebrafish results in reduced thrombocyte number in adults, although survival is unaffected.
Larvae do not display functional defects, suggestive of a late block in adult thrombopoiesis consistent with mammalian phenotypes.
Abstract
The NFE2 transcription factor is expressed in multiple hematopoietic lineages with a well-defined role in regulating megakaryocyte biogenesis and platelet production in mammals. Mice deficient in NFE2 develop severe thrombocytopenia with lethality resulting from neonatal hemorrhage. Recent data in mammals reveal potential differences in embryonic and adult thrombopoiesis. Multiple studies in zebrafish have revealed mechanistic insights into hematopoiesis, although thrombopoiesis has been less studied. Rather than platelets, zebrafish possess thrombocytes, which are nucleated cells with similar functional properties. Using transcription activator-like effector nucleases to generate mutations in nfe2, we show that unlike mammals, zebrafish survive to adulthood in the absence of Nfe2. Despite developing severe thrombocytopenia, homozygous mutants do not display overt hemorrhage or reduced survival. Surprisingly, quantification of circulating thrombocytes in mutant 6-day-old larvae revealed no significant differences from wild-type siblings. Both wild-type and nfe2 null larvae formed thrombocyte-rich clots in response to endothelial injury. In addition, ex vivo thrombocytic colony formation was intact in nfe2 mutants, and adult kidney marrow displayed expansion of hematopoietic progenitors. These data suggest that loss of Nfe2 results in a late block in adult thrombopoiesis, with secondary expansion of precursors: features consistent with mammals. Overall, our data suggest parallels with erythropoiesis, including distinct primitive and definitive pathways of development and potential for a previously unknown Nfe2-independent pathway of embryonic thrombopoiesis. Long-term homozygous mutant survival will facilitate in-depth study of Nfe2 deficiency in vivo, and further investigation could lead to alternative methodologies for the enhancement of platelet production.
Introduction
In vertebrates, blood is composed of the derivatives of various cell lineages, with platelets being the second most abundant.1 They are involved in a number of processes, including maintaining hemostasis of the circulatory system, as well as infection and wound healing.2 On injury to the vascular endothelium, they initiate blood clotting to repair the injured vessel wall. Loss of platelet number or function results in the potential for pathologic hemorrhage and underlies a number of bleeding disorders.3,4 In contrast, thrombocytosis or platelet hyperactivity may initiate arterial thrombosis, resulting in heart attack or stroke.5 Platelets are produced daily from their precursor cells, megakaryocytes, and although much has been uncovered about this process, there is still a considerable amount to learn. Inherited platelet defects account for a large proportion of patients with bleeding disorders.3 These are often caused by mutations in transcription factors regulating platelet function, such as RUNX1 and ETV6.6,7 Treating platelet disorders often requires transfusions from donors. Unlike other blood components, which can be stored for long periods of time, platelets are only stable for 5 days, limiting their availability for transfusion and causing platelet shortages.8
The zebrafish has emerged as a powerful model for the study of hematopoiesis9 and hemostasis.10-12 Zebrafish are highly fecund, optically transparent during embryonic and larval stages, and display rapid development; all qualities expediting hematologic and cardiovascular analyses. In addition, they have been well established as a model to study hematopoiesis, sharing conservation of many genes and cell types involved in hematopoietic processes with mammals.13-15 In contrast to the bone marrow in mammals, hematopoiesis occurs in the kidney marrow of zebrafish. This gives rise to myeloid, erythroid, and lymphoid cell lineages.16-18 Fish do not possess megakaryocytes; rather, thrombocytes appear to develop from mononuclear precursors and do not undergo further differentiation to form platelets.19 Thrombocytes also respond to platelet agonists,20,21 and it is therefore believed that they are the functional equivalent to mammalian platelets.19,22
Nuclear factor erythroid 2 (Nfe2) is a member of the basic zipper (bZIP) and cap’n’collar (CNC) superfamily that regulates gene transcription,23-25 and is conserved in vertebrates from fish to mammals.25,26 It binds to DNA as a heterodimer with the small Maf family of transcription factors.27 It has been widely studied in mammals with a well-defined role in regulating production of several hematopoietic lineages. In addition to hematopoietic progenitor cells, Nfe2 is expressed in myeloid, erythroid, and megakaryocyte lineages.1,24 Knockout mice completely lack circulating platelets, and most die early because of hemorrhage, but show an increased number of megakaryocytes in the bone marrow,28 suggesting that Nfe2 acts as a regulator of proplatelet formation and promotes the final stages of megakaryocyte maturation.28-30 In the zebrafish, nfe2 is a single-copy gene expressed in the intermediate cell mass during erythroid differentiation, and is continued to be expressed throughout development in the circulating blood.25 The highest nfe2 expression levels are apparent during the oocyte stage as a result of maternal contribution, and again at 48 hours after fertilization during hatching.26 nfe2 morphant zebrafish display abnormal swim bladder development, but are otherwise morphologically normal.26
Here, we use transcription activator-like effector nuclease (TALEN) genome editing to engineer complete loss of Nfe2 in zebrafish to study its role in hemostasis and thrombopoiesis. Adult nfe2 homozygous mutants display a severe reduction in the number of circulating thrombocytes and a reactive increase in progenitor cells, phenotypes that are conserved with the mammalian knockout. In contrast, no hemorrhage was observed, and there was no effect on viability. Surprisingly, Nfe2 appears to be dispensable for early thrombocyte development and function. Embryonic fish display normal numbers of functional circulating thrombocytes, suggesting the potential for a distinct Nfe2-independent pathway regulating thrombopoiesis.
Methods
Zebrafish strains and maintenance
nfe2 mutants were generated on a hybrid background of wild-type strains AB and TL (ABxTL). All animal experiments were in accordance with guidelines approved by the University of Michigan Animal Care and Use Committee.
TALEN-mediated genome editing
nfe2 mutants were created using TALEN genome editing technology. Two TAL effector nucleases were designed to target the third coding exon. Target sequences (5′-TCACCCACCTCTTATGAG-3′ and 5′-CATGACTACACGTGGTCA-3′) were selected using Zifit (http://zifit.partners.org/ZiFiT/) and assembled using the Restriction Enzyme and Ligation (REAL) method.31 Individual plasmids were obtained from Addgene (http://www.addgene.org/talengineering/talenkit/) and assembled into complete TALENs, followed by transcription to produce mRNA (Ambion). Single-cell embryos from a hybrid genetic background (ABxTL) were injected and raised to adulthood, followed by mating to confirm germline transmission.
Genotyping of mutant offspring
Fin clip biopsies were obtained from adult fish after anesthesia in tricaine (0.16 mg/mL; Western Chemical Inc.), and larval fish were humanely euthanized in high-dose tricaine (1.6 mg/mL), followed by lysis in buffer (10 mM Tris at pH 8.0, 2 mM EDTA at pH 8.0, 0.2% Triton x-100, 0.1 mg/mL proteinase K). Deletion mutants were detected by polymerase chain reaction, using primers 5′-GGTTCTTCCAGAATGCTTGC-3′ and 5′-CTTGTTTGATTTGTCCTGTAGAGTG-3′, followed by electrophoresis.
Laser-mediated endothelial injury
Laser injury was performed using a pulsed nitrogen dye laser system (Andor Technology), as previously described.32-35 Six-day postfertilization (dpf) larvae were anesthetized in tricaine, embedded in 0.8% low-melt agarose on a glass coverslip, and analyzed on an inverted microscope (Olympus IX71), using a 40× objective. The endothelium of the dorsal aorta was targeted and ablated at the fifth somite posterior to the anal pore at a power level of 19, using 30 pulses. The time to occlusion was recorded up to 2 minutes. Larvae that failed to occlude in 2 minutes were assigned a score of 120 seconds. After laser injury, larvae were recovered from agarose, lysed, and genotyped, as described earlier.
RNA isolation and cDNA generation
Six-dpf larvae were anesthetized in tricaine. A portion of tail was removed and placed in lysis buffer for genotyping, as described.36 Larvae were then frozen at −80°C until genotypic analysis was completed. RNA was extracted from pools of 15 embryos of each genotype, using an RNAeasy kit (Qiagen), and cDNA reverse transcribed using a Superscript III kit (Invitrogen).
Flow cytometry
Adult Tg(cd41:egfp) fish were genotyped for the nfe2 mutation by fin clip biopsy. Fish were then immobilized in tricaine and euthanized on ice. Peripheral blood was collected by cardiac puncture, using a pipette tip precoated in heparin. One to 2 µL peripheral blood was collected from each fish and immediately mixed with Fish buffer (Dulbecco's phosphate buffered saline with Ca2+ and Mg2+ and 10% fetal bovine serum) on ice. Kidneys were then dissected, placed in Fish buffer on ice, and homogenized by pipetting through a P1000 pipette tip. All samples were strained through 35-µm filters into fluorescence-activated cell sorting tubes, followed by the addition of SYTOX Blue. Samples were then analyzed using a Becton Dickinson LSR2 analyzer. At least 10 000 events were acquired per sample.
Thrombocyte counting
Relative thrombocyte counts were obtained as previously described. Briefly, Tg(cd41:egfp) larval zebrafish were anesthetized in tricaine at 6 or 18 dpf and submersed in 0.7% low-melt agarose. A manual pipette pump was used to draw larvae into a 10-mm-long, 1.5- to 1.8-mm outer diameter Pyrex glass capillary tube (Fisher). Capillaries were then submersed in system water to minimize refraction and placed under a stereomicroscope (Leica). A Canon 60D digital single lens reflex camera fitted with an AmScope microscope adapter (United Scopes, LLC, Irvine, CA) was used to record green fluorescent protein (GFP)–labeled thrombocyte movement. Movies were captured for a duration of 60 seconds and then processed.35,37
Thrombocyte spreading
Glass coverslips were coated with fibrinogen (350 µg/mL) in Tyrode’s buffer (10 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 12 mmol/L NaHCO3, 127 mmol/L NaCl, 5 mmol/L KCl, 0.5 mmol/L NaH2PO4, 1 mmol/L MgCl2, and 5 mmol/L glucose) and incubated at ambient temperature for 1 hour. Slides were washed with phosphate-buffered saline and then incubated with 2% bovine serum albumin in phosphate-buffered saline for 30 minutes, followed by additional washes in phosphate-buffered saline. Tyrode’s buffer was left on the slide until the time of imaging. Zebrafish whole blood was collected in sodium citrate-coated capillary tubes and added to Tyrode’s buffer containing 30% sodium citrate. One microliter of this mixture was then applied to Tyrode’s buffer on the slide and analyzed. An initial field of view was selected for movie recording, followed by analysis of thrombocytes in an average of 10 additional fields after completion of spreading at 30 minutes postinitiation. Fields with GFP-expressing thrombocytes were randomly selected for analysis. Thrombocyte spreading of the initial field was recorded in real time, using a 100× objective on a Zeiss Axiovert inverted fluorescent microscope and processed using Slidebook 6.0. Images were scored according to visual assessment of spreading, using a scale of 1 to 3, where 1 represents no spreading, 2 represents incomplete spreading, and 3 represents complete spreading. Thrombocyte area was calculated using ImageJ software. Images were converted to binary scale and a threshold was applied to capture only fluorescent pixels. A total of 4 wild-type and 4 homozygous fish were used for experiments. All experimentation and analysis was performed by 2 separate observers blinded to genotype.
In vitro clonal colony-forming unit assays
Unfractionated whole kidney marrow was isolated from adult Tg(cd41:egfp) fish and plated in 35-mm petri dishes with methylcellulose at 2.5 × 104 cells/mL, as described,38 in complete media39 containing 0.1% carp serum, 10% bovine serum albumin in Iscove's Modified Dulbecco’s Media (Stemcell Technologies), 0.1 μg/mL zebrafish erythropoietin (Epo), 0.1 μg/mL zebrafish granulocyte colony-stimulating factor (Gcsf), and 0.03 μg/mL zebrafish thrombopoietin (Tpo). Cultures were grown in humidified incubators at 32°C, 5% CO2. Colonies were enumerated and photographed after 7 days, on an inverted fluorescence microscope (Olympus IX53). Images were processed as described.40 Data collection was performed by an observer blinded to genotype.
Statistical analysis
Analyses of larval experiments were performed using the Mann-Whitney U or Student t test. Log-rank testing of survival curves was performed using Prism (GraphPad Software). Analyses of adult thrombocyte spreading were performed using χ2 and Student t tests.
Results
Targeted disruption of nfe2 by genome editing results in a frameshift mutation and early stop codon
TALEN-mediated genome editing was used to target nfe2 early in the third coding exon. After injection of TALEN pairs into 1-cell stage zebrafish, embryos were raised to adulthood and mated to wild-type fish to confirm germline transmission of mutations. Two mutations were identified, consisting of 10 and 8 base pair (bp) deletions that resulted in a frameshift beginning at the same amino acid (Figure 1A-B). Both mutations result in an early stop codon before the conserved DNA binding domain (Figure 1A, green), and are predicted to be null alleles. To confirm loss of function, we isolated RNA from nfe2+/+ and nfe2−/− siblings and synthesized cDNA. Sequencing of cDNA showed an 8-bp deletion in mutant larvae (Figure 1B). No grossly visible phenotypes were apparent during the embryonic/larval period in offspring with either mutation. Swim bladder defects seen in nfe2 knockdown larvae were not observed in the nfe2 knockouts. All subsequent experiments were performed using both mutant lines, unless otherwise noted, and yielded similar outcomes, but only data for the 8-bp deletion line are shown. nfe2 heterozygous fish were incrossed, and survival of the resulting clutches of offspring was tracked for 1 year. No significant die off of heterozygous or homozygous mutants was observed for either line within the first year of life (Figure 1C).
Genome editing of nfe2 results in a frameshift mutation and early stop codon. (A) Alignment of human and zebrafish Nfe2 protein sequences. Sequences are highly conserved in the CNC (blue), basic DNA binding (green), and leucine zipper domains (pink). The site of the deletion mutation and beginning of the frameshift is marked in yellow. (B) Sequencing of homozygous mutant offspring cDNA demonstrates the 8-bp deletion compared with wild-type siblings. (C) Survival of nfe2 mutant fish into adulthood is not significantly different when compared with wild-type siblings (P = .3, log rank [Mantel-Cox] test).
Genome editing of nfe2 results in a frameshift mutation and early stop codon. (A) Alignment of human and zebrafish Nfe2 protein sequences. Sequences are highly conserved in the CNC (blue), basic DNA binding (green), and leucine zipper domains (pink). The site of the deletion mutation and beginning of the frameshift is marked in yellow. (B) Sequencing of homozygous mutant offspring cDNA demonstrates the 8-bp deletion compared with wild-type siblings. (C) Survival of nfe2 mutant fish into adulthood is not significantly different when compared with wild-type siblings (P = .3, log rank [Mantel-Cox] test).
Reduction of circulating thrombocytes in adult, but not larval nfe2 mutants
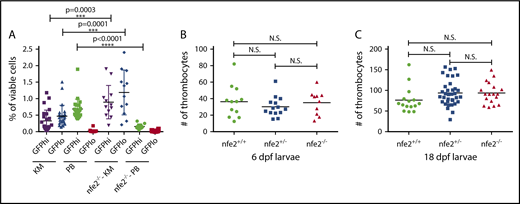
Several studies have demonstrated a complete lack of circulating platelets in NFE2-deficient mice, resulting in early lethality due to neonatal hemorrhage.28-30 However, nfe2 homozygous mutant zebrafish survive to adulthood in normal numbers (Figure 1C), suggesting that fish may be able to compensate for a lack of Nfe2. To determine whether Nfe2 is required for thrombopoiesis in fish, we quantitated circulating thrombocytes in adults. Peripheral blood was collected from wild-type and nfe2 homozygous mutant fish bred into the Tg(cd41:egfp) transgenic background at 2.5 months postfertilization. Flow cytometry on peripheral blood in this transgenic line can separate 2 distinct circulating populations, thrombocytes (GFPhigh) and progenitors (GFPlow).41 The GFPhigh population of cells was significantly reduced in nfe2−/− fish compared with wild-type siblings, whereas the GFPlow population was unaffected (Figure 2A). In contrast, kidney marrow revealed a significant increase in numbers for both the high and low compartments (P = .0003 and P = .0001, respectively), suggesting an expansion of hematopoietic progenitors in the absence of Nfe2 (Figure 2A).
Reduction of circulating thrombocytes in adult but not larval nfe2−/−fish. (A) Peripheral blood (PB) samples from 2.5-month postfertilization adult Tg(cd41:egfp) fish show a significant decrease in GFPhigh thrombocytes in nfe2−/− fish as compared with nfe2+/+ siblings (green data points; P < .0001). The numbers of GFPhigh and GFPlow cells in kidney marrow (KM) samples (purple and blue, respectively) are significantly increased between nfe2−/− and nfe2+/+ fish (P = .0003 and P = .0001, respectively). (B) Circulating thrombocyte numbers in 6-dpf larval fish are unchanged between homozygous mutants and either wild-type or heterozygous siblings (P = .86 and P = .33, respectively), or between heterozygous and wild-type siblings (P = .38). (C) Circulating thrombocyte numbers in 18-dpf larval fish were also unchanged between homozygous mutants and either wild-type or heterozygous siblings (P = .12 and P = .39, respectively), or between heterozygous and wild-type siblings (P = .42). Bars indicate the mean and SD (A) or median (B-C). N.S., nonsignificant.
Reduction of circulating thrombocytes in adult but not larval nfe2−/−fish. (A) Peripheral blood (PB) samples from 2.5-month postfertilization adult Tg(cd41:egfp) fish show a significant decrease in GFPhigh thrombocytes in nfe2−/− fish as compared with nfe2+/+ siblings (green data points; P < .0001). The numbers of GFPhigh and GFPlow cells in kidney marrow (KM) samples (purple and blue, respectively) are significantly increased between nfe2−/− and nfe2+/+ fish (P = .0003 and P = .0001, respectively). (B) Circulating thrombocyte numbers in 6-dpf larval fish are unchanged between homozygous mutants and either wild-type or heterozygous siblings (P = .86 and P = .33, respectively), or between heterozygous and wild-type siblings (P = .38). (C) Circulating thrombocyte numbers in 18-dpf larval fish were also unchanged between homozygous mutants and either wild-type or heterozygous siblings (P = .12 and P = .39, respectively), or between heterozygous and wild-type siblings (P = .42). Bars indicate the mean and SD (A) or median (B-C). N.S., nonsignificant.
To determine whether Nfe2 is also necessary for thrombocyte production in larval and juvenile fish, we quantitated GFP+ circulating thrombocytes at 6 and 18 dpf, using Tg(cd41:egfp);nfe2 heterozygous incrosses. We found no significant difference in the number of circulating thrombocytes in nfe2+/+, nfe2+/−, and nfe2−/− siblings at either age (Figure 2B-C).
Zebrafish larvae demonstrate normal thrombocyte function in the absence of Nfe2
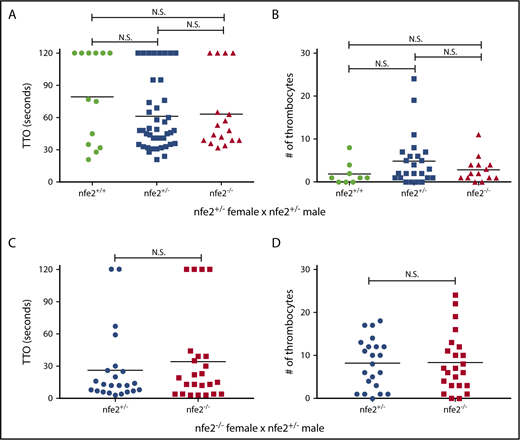
In mammals, platelets are important components in initiating blood clotting after endothelial injury, particularly in the high flow and shear of the arterial circulation. We and others have shown that induced endothelial injury in zebrafish larvae results in thrombocyte-rich clots in the arterial circulation.32,35 To assess whether thrombocytes function normally in the absence of Nfe2, we used laser-mediated induced endothelial injury in the arterial circulation. Clutches of larval zebrafish from nfe2 heterozygous incrosses were studied at 6 dpf for the ability to aggregate at the site of injury and occlude the dorsal aorta. No significant difference was found in the time to occlusion among nfe2 wild-type and mutant siblings (Figure 3A). Zebrafish oocytes have maternally generated mRNA and protein, which remains over the first 10 cell cycles, or approximately 3 hours after fertilization,42 and this contribution could result in some transient Nfe2 activity in homozygous mutants generated from nfe2+/− mothers. Therefore, we bred homozygous females with heterozygous males to produce a clutch of offspring in which the nfe2−/− larvae have no possible Nfe2 activity. Endothelial injury in these fish also showed no apparent defects in time to occlusion (Figure 3C).
Thrombocyte aggregation is unaffected in nfe2−/−larvae. Offspring from an nfe2+/− incross were collected and tested for the ability to form thrombocyte-rich clots in response to laser-mediated endothelial injury of the dorsal aorta at 6 dpf. (A) No significant difference was found in time to occlusion (TTO) among nfe2+/+, nfe2+/−, or nfe2−/− siblings. (B) The number of fluorescent thrombocytes recruited to the site of injury were counted using fish in the Tg(cd41:egfp) background. There was no significant difference in the total number of thrombocytes between any of the genotypes (wild-type and homozygous, P = .27; wild-type and heterozygous, P = .11; heterozygous and homozygous, P = .39; Mann-Whitney U test). (C-D) Homozygous mutant female fish were bred to heterozygous males to test whether maternal contribution might affect the observed phenotypes. No difference in either TTO (C) or total number of thrombocytes (D) was observed between nfe2−/− and nfe2+/− siblings from this cross. Horizontal bars indicate the median TTO and number of thrombocytes in panels A and C and B and D, respectively.
Thrombocyte aggregation is unaffected in nfe2−/−larvae. Offspring from an nfe2+/− incross were collected and tested for the ability to form thrombocyte-rich clots in response to laser-mediated endothelial injury of the dorsal aorta at 6 dpf. (A) No significant difference was found in time to occlusion (TTO) among nfe2+/+, nfe2+/−, or nfe2−/− siblings. (B) The number of fluorescent thrombocytes recruited to the site of injury were counted using fish in the Tg(cd41:egfp) background. There was no significant difference in the total number of thrombocytes between any of the genotypes (wild-type and homozygous, P = .27; wild-type and heterozygous, P = .11; heterozygous and homozygous, P = .39; Mann-Whitney U test). (C-D) Homozygous mutant female fish were bred to heterozygous males to test whether maternal contribution might affect the observed phenotypes. No difference in either TTO (C) or total number of thrombocytes (D) was observed between nfe2−/− and nfe2+/− siblings from this cross. Horizontal bars indicate the median TTO and number of thrombocytes in panels A and C and B and D, respectively.
When an arterial endothelial injury occurs, a signaling cascade recruits platelets to the site of injury, followed by the release of intracellular mediators, resulting in further recruitment. To assess whether this process is affected in nfe2 mutants, we counted the total number of thrombocytes adhering to and aggregating at the site of endothelial injury. We found no significant difference in the number of thrombocytes present within thrombi of nfe2+/+, nfe2+/−, and nfe2−/− larvae from heterozygous incrosses, as well as those produced by nfe2−/− females (Figures 3B,D).
Adult zebrafish have decreased thrombocyte function in the absence of Nfe2
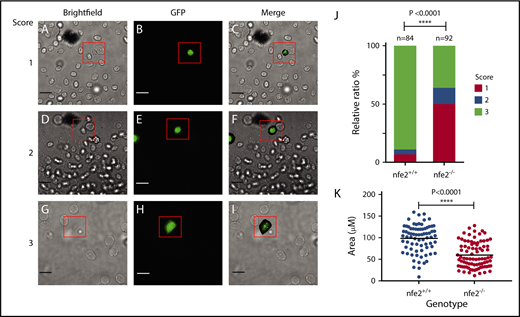
To determine whether nfe2−/− adult zebrafish, which demonstrate decreased numbers of circulating thrombocytes, also have a functional defect, we examined the ability of mutant thrombocytes to initiate spreading in response to fibrinogen. This method has been successfully used by others to demonstrate the ability of human and murine platelets to activate and spread.43,44 To examine whether zebrafish thrombocytes behave similarly to mammalian platelets, we performed a comparable assay by applying zebrafish whole blood from Tg(cd41:egfp) nfe2+/+ and nfe2−/− siblings to a glass coverslip coated with human fibrinogen. Continuous images were captured in one field 5 to 10 minutes after application of whole blood to the coverslip (supplemental Movies 1 and 2). This was followed by evaluation of a wider field at 30 minutes after application. We found that wild-type thrombocytes overwhelmingly demonstrated the ability to spread in response to fibrinogen, whereas mutant thrombocytes displayed a significant reduction in this fibrinogen-dependent spreading (Figure 4; supplemental Movies 1 and 2). We developed a quantitative scoring system (Figure 4A-J) and discovered that nfe2−/− thrombocytes spread significantly less than those from wild-type siblings (Figure 4J; P < .0001). We also quantitated the total thrombocyte area after spreading, which was significantly lower in nfe2−/− thrombocytes compared with nfe2+/+ (Figure 4K; P < .0001). These data indicate that the ability of thrombocytes to initiate activation in response to fibrinogen is diminished in adult nfe2−/− fish.
Adult nfe2−/−zebrafish demonstrate decreased ability to spread on fibrinogen. Whole blood was collected from adult zebrafish and applied to glass coverslips pretreated with fibrinogen. Thrombocytes were identified on the basis of Tg(cd41:egfp) fluorescent expression. Images were captured 30 minutes after application of whole blood and analyzed for degree of spreading. The following panels are representative of the scoring system for the spreading assay: images were scored as 1, no spreading (A-C); 2, spreading initiated, but incomplete (D-F); and 3, complete spreading (G-I) by an observer blinded to genotype. Scale bars, 10 μm. Percentages of thrombocytes displaying each score were tabulated for nfe2+/+ and nfe2−/− fish, and mutant fish displayed a significant decrease in spreading compared with wild-type siblings (J; P < .0001). Total thrombocyte area was also quantitated using ImageJ and demonstrated a statistically significant reduction in thrombocyte spreading in mutant fish as well (K; P < .0001). Each point represents the area of an individual thrombocyte. Data were collected from a total of 4 fish for each genotype.
Adult nfe2−/−zebrafish demonstrate decreased ability to spread on fibrinogen. Whole blood was collected from adult zebrafish and applied to glass coverslips pretreated with fibrinogen. Thrombocytes were identified on the basis of Tg(cd41:egfp) fluorescent expression. Images were captured 30 minutes after application of whole blood and analyzed for degree of spreading. The following panels are representative of the scoring system for the spreading assay: images were scored as 1, no spreading (A-C); 2, spreading initiated, but incomplete (D-F); and 3, complete spreading (G-I) by an observer blinded to genotype. Scale bars, 10 μm. Percentages of thrombocytes displaying each score were tabulated for nfe2+/+ and nfe2−/− fish, and mutant fish displayed a significant decrease in spreading compared with wild-type siblings (J; P < .0001). Total thrombocyte area was also quantitated using ImageJ and demonstrated a statistically significant reduction in thrombocyte spreading in mutant fish as well (K; P < .0001). Each point represents the area of an individual thrombocyte. Data were collected from a total of 4 fish for each genotype.
nfe2 mutant cells contribute to adult hematopoiesis
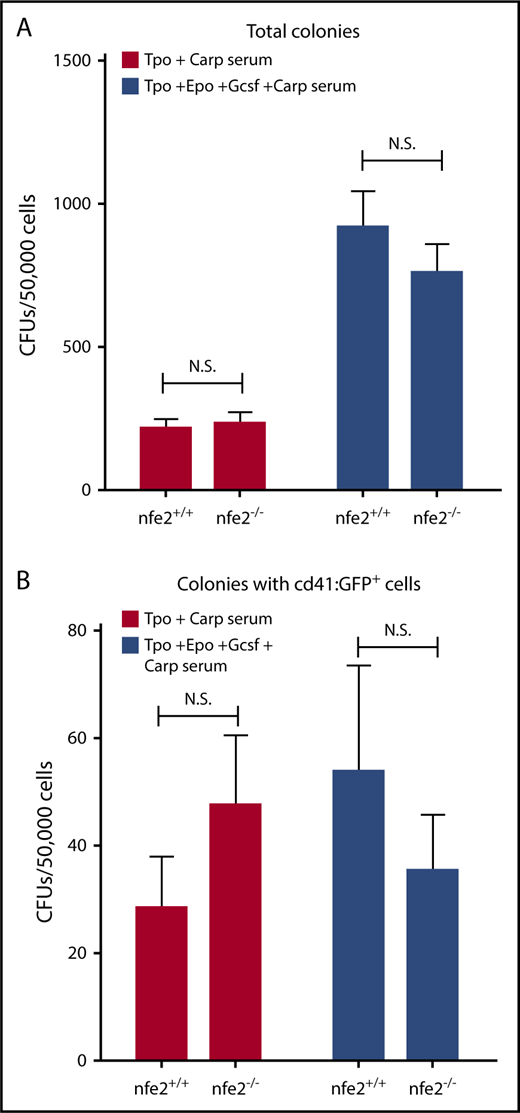
Nfe2 has also been shown to be important for the formation of several hematopoietic lineages in mammals. To evaluate the result of its loss, we performed colony-forming unit assays, using cultured extracts from kidney marrow, the site of adult hematopoiesis in fish. Whole-kidney lysates from nfe2 homozygous mutants and wild-type siblings [in the Tg(cd41:egfp) background] were cultured in the presence of Tpo + Carp serum or Tpo/Epo/Gcsf + Carp serum for 7 days. After culture, there was no significant difference in the total number of colonies produced between nfe2+/+ and nfe2−/− marrow, with or without the addition of Epo and Gcsf (Figure 5A). We also did not observe any significant difference in the number of GFP+ colonies (representative of differentiated thrombocytes) produced between nfe2+/+ and nfe2−/− marrow, with or without the addition of Epo and Gcsf (Figure 5B).
Cultured kidney marrow from nfe2−/−zebrafish supports normal hematopoietic development. Unfractionated kidney marrow was isolated from Tg(cd41:egfp);nfe2−/− mutant and wild-type siblings and was cultured in the presence of Tpo + Carp serum alone, or with the addition of Epo/Gcsf. After culture, the total number of colony-forming units (CFUs) (A) and GFP+ CFUs (B) were counted. There were no significant differences in either total number of cells (A) or cells expressing GFP (B) between wild-type and homozygous mutant fish, with or without the addition of Epo and Gcsf.
Cultured kidney marrow from nfe2−/−zebrafish supports normal hematopoietic development. Unfractionated kidney marrow was isolated from Tg(cd41:egfp);nfe2−/− mutant and wild-type siblings and was cultured in the presence of Tpo + Carp serum alone, or with the addition of Epo/Gcsf. After culture, the total number of colony-forming units (CFUs) (A) and GFP+ CFUs (B) were counted. There were no significant differences in either total number of cells (A) or cells expressing GFP (B) between wild-type and homozygous mutant fish, with or without the addition of Epo and Gcsf.
Discussion
Here, we report targeted mutagenesis of zebrafish nfe2, using genome editing TALEN technology. Our data show that similar to mammals, adult zebrafish display a reduction of circulating thrombocytes while the precursors in the kidney marrow are upregulated. This suggests that loss of Nfe2 prevents differentiation of fully matured thrombocytes from their precursor cells. A similar phenomenon is seen in nfe2 knockout mice, which completely lack circulating platelets but display an increased number of megakaryocytes in their bone marrow.28 In addition, both fish and mouse retain colony forming activity. However, unlike mice, which die at neonatal stages, the zebrafish mutants are able to survive well into adulthood for up to 1 year. There are several possible reasons for the lack of observed lethality in nfe2 homozygous mutant zebrafish. Physiological differences could be relevant, with the main one being that zebrafish undergo external fertilization and do not experience a traumatic birth process as mammals do. Beyond that, the laboratory environment for both species does not have stresses present in the wild. There are no significant opportunities for trauma-induced bleeding, making it less likely for fish to experience hemorrhage and allowing survival with reduced numbers of circulating thrombocytes. Through targeting of coagulation factor X in fish, we have previously shown that spontaneous hemorrhage can indeed occur in the laboratory setting at juvenile and adult stages.45 This suggests that the environment is not solely responsible for the lack of bleeding seen in the nfe2 knockout fish. In humans, the risk for spontaneous nontraumatic hemorrhage is low even at platelet counts 10% to 20% of the lower limit of normal. Thus, it is possible that the minimal quantity of residual thrombocytes may be sufficient to prevent spontaneous hemorrhage in zebrafish.
There are known differences in hematopoietic programs between the 2 model systems. In mammals, megakaryocytes undergo several differentiation steps before ultimately shedding functional platelets. Zebrafish do not possess megakaryocytes, but are believed to derive thrombocytes directly from a myeloid-erythroid progenitor cell, similar to avian species.46 Despite this, zebrafish express many genes found in megakaryocytes including runx1, gata1, fog1, and fli1, in addition to nfe2.19 Studies have shown varying degrees of involvement of these genes in thrombocyte development. Morpholino knockdown demonstrated that zebrafish deficient in fog1 did not generate mature thrombocytes, indicating that Fog1 is necessary for thrombocyte development.47 A separate study using adult zebrafish demonstrated that expression from the gata1 promoter becomes weaker and the fli1 promoter stronger in maturing thrombocytes, indicating that these genes may be required at different stages of development.48 It is possible that some of these genes are able to compensate for the loss of Nfe2 at early stages in a hypothetical simplified differentiation program, allowing larvae to produce thrombocytes. These data also suggest the presence of distinct embryonic and adult thrombopoietic differentiation programs, analogous to primitive and definitive erythropoiesis.49 The switch from primitive to definitive erythropoiesis is conserved in fish50,51 ; thus it is not inconceivable that such a thrombopoietic switch might also exist, as has been shown in mammals.52,53
Several studies in mammals have shown that NFE2 is a direct regulator of genes important for platelet function, including thromboxane A synthase1 (TBXAS1), required for the production of thromboxane,54 as well as TUBB1 and RAB27B, deficiency of which have also been linked to thrombocytopenia.1 Our finding that thrombocytes from adult nfe2 mutant fish have a diminished ability to spread on fibrinogen shows that loss of Nfe2 results in a reduction in thrombocyte activity, which could have effects on clotting. These data suggest that as in mammalian platelets, Nfe2 may be required for regulation of downstream targets important for thrombocyte activation and function. However, it remains puzzling that larval mutant thrombocytes are able to aggregate in response to endothelial injury. This lends further evidence to the possibility of differences in embryonic/larval and adult thrombopoiesis.
We were surprised to find that the number of thrombocytes is unchanged in larval fish, suggesting the potential for an unknown Nfe2-independent pathway of embryonic/larval thrombopoiesis. Consistent with this observation, Potts et al55 previously demonstrated that mouse diploid platelet-forming cells (DPFCs) are the first platelet progenitors (found in the embryonic day 8.5-10.5 mouse yolk sac), but do not require NFE2 for development. On the basis of these data, it is tempting to speculate that DPFCs and larval thrombocytes descend from a common progenitor. However, normal DPFCs were able to produce platelets, indicating they do possess the mammalian megakaryocytic program. Overall, the fish and murine data suggest the presence of alternative Nfe2-independent programs during embryonic phases of development compared with fetal and adult stages.
In addition to the presence of DPFCs, there have been several other differences observed in adult versus neonatal platelet formation in both mice and humans. Tpo levels are higher in human neonates compared with adults, and several studies have shown that fetal and neonatal megakaryocyte progenitors proliferate faster than adult megakaryocyte progenitors.56-59 In addition, in vitro assays have demonstrated that although hematopoietic progenitor cells cultured from cord blood generate a greater number of megakaryocytes than those cultured from adult peripheral blood, these megakaryocytes are smaller in size and produce fewer platelets.56-59 Further, several additional studies indicate that although the megakaryocyte lineage is primarily derived from megakaryocyte-erythroid progenitors in neonates, these cells are derived from hematopoietic stem cells in adult tissues.60,61 Collectively, these data indicate that fetal and neonatal megakaryocytes are a biologically distinct cell type compared with adult megakaryocytes. This is consistent with our observations that zebrafish thrombocytes seem to replicate this phenomenon as well, suggesting that these distinctive pathways evolved in a common ancestor.
In summary, our studies contribute to the body of knowledge indicating that thrombocytes are orthologous to platelets and their megakaryocyte precursors. We present data demonstrating overall conservation of Nfe2 function across vertebrate species, with a clear requirement for adult thrombopoiesis and thrombocyte function. Our findings that Nfe2 is not required for embryonic thrombopoiesis supports the concept of distinct embryonic and adult thrombopoietic pathways, previously demonstrated in mammalian studies. The fact that zebrafish nfe2 mutants are able to survive well into adulthood provides a model system that will facilitate the study of Nfe2 function in thrombopoiesis and possibly other hematopoietic cell types on a mechanistic level not possible in mammals.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Benjamin Tourdot, and Reheman Adili for technical assistance. The authors also thank Martha Sola-Visner and Joe Italiano for critical reading of the manuscript.
This work was supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) R01-HL124232 and R01-HL125774; American Society of Hematology Faculty Scholar Award (J.A.S.); NIH, NHLBI T32-HL007622 (M.S.R.); American Cancer Society Postdoctoral Fellowship 122577PF1214601DDC (I.S.); NIH, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R15-DK114732-01 (D.L.S.); and NIH, NHLBI R01-HL04880, P01-HL032262, and U01-HL134812 and NIDDK U54-DK110805, R01-DK053298, and R24-DK092760 (L.I.Z.). J.A.S. is the Diane and Larry Johnson Family Scholar of Pediatrics and Communicable Diseases. L.I.Z. is an investigator of the Howard Hughes Medical Institute.
Authorship
Contribution: M.S.R. designed and performed research, analyzed data, and wrote the manuscript; I.S., Y.L., A.H.V., C.E.R., S.M.E., and D.L.S. designed and performed research and analyzed data; M.H. designed research and analyzed data; L.I.Z. analyzed data; and J.A.S. designed and supervised research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: J.A.S. has been a consultant for Bayer, Shire, CSL Behring, and Octopharma. D.L.S. is a scientific advisor for Finless Foods. M.H. is a consultant and stockholder for Veralox Therapeutics. L.I.Z. is a founder and stockholder of Fate Therapeutics, Marauder Therapeutics, and Scholar Rock. The remaining authors declare no competing financial interests.
The current affiliation for I.S. is Bluebird bio, Cambridge, MA.
The current affiliation for Y.L. is Molecular Innovations, Inc., Novi, MI.
The current affiliation for A.H.V. is Northwestern University Medical School, Chicago, IL.
Correspondence: Jordan A. Shavit, Department of Pediatrics, University of Michigan, Room 8301, Medical Science Research Building III, 1150 W Medical Center Dr, Ann Arbor, MI 48109-5646; e-mail: jshavit@umich.edu.

![Figure 1. Genome editing of nfe2 results in a frameshift mutation and early stop codon. (A) Alignment of human and zebrafish Nfe2 protein sequences. Sequences are highly conserved in the CNC (blue), basic DNA binding (green), and leucine zipper domains (pink). The site of the deletion mutation and beginning of the frameshift is marked in yellow. (B) Sequencing of homozygous mutant offspring cDNA demonstrates the 8-bp deletion compared with wild-type siblings. (C) Survival of nfe2 mutant fish into adulthood is not significantly different when compared with wild-type siblings (P = .3, log rank [Mantel-Cox] test).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/23/10.1182_bloodadvances.2018021865/8/m_advances021865f1.png?Expires=1767723082&Signature=s9dNbX7CiJoszbLVMnDc7aQOo2BWPrN637O81MTMOEXBxduV2QxfJpJkrRkR-q2j5h5mKh2C8YJIh6rtNXGjUXi~KtVOUEymNtbTF0Rj7aATjktiLB77dW5-WTW7Bj2IvfT-hhzpbQmkcrYCdCVcMg1yJpR9y2sxqJRh2SmmtJ-aoyNutUW9kpNIr6-HIBnLq6~PQdBB9HCmu996g-mVB97ukHwZm20wajPMfDAobxnFdoNzEiTiw3rPj4930UDSgtos6IOtm5ii9fAZEEBMDzuEWpf0ZYClDyC0vAIz6uua3WcHLxWK5rbPoeHMAZW-h-ga018icgCV4~rzrL86NA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)