Key Points
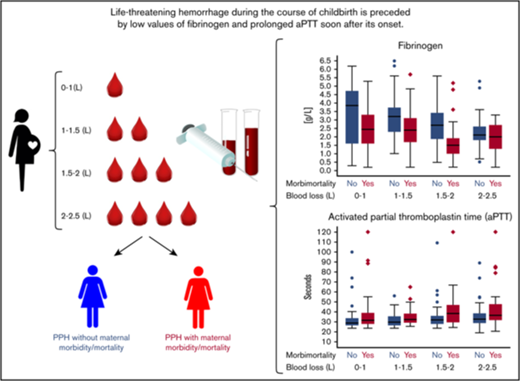
Life-threatening hemorrhage during the course of childbirth is preceded by low fibrinogen and prolonged aPTT soon after its onset.
Assessment of fibrinogen and aPTT during early postpartum hemorrhage may help to prevent progression to uncontrollable blood loss.
Abstract
We describe the pattern of change in coagulation parameters during the course of severe postpartum hemorrhage in a retrospective cohort study among 1312 women experiencing severe postpartum hemorrhage necessitating blood transfusion. Levels of hemoglobin, hematocrit, platelet count, fibrinogen, activated partial thromboplastin time (aPTT) and prothrombin time (PT) per categorized volume of blood loss during severe postpartum hemorrhage were described and compared between women with and without the composite adverse outcome. Need for surgical intervention, severe acute maternal morbidity, and maternal mortality were jointly considered the composite adverse outcome. Of the 1312 women, 463 (35%) developed the composite adverse outcome. The incidence of a fibrinogen level <2 g/L was 26% (342 per 1312). Low fibrinogen and prolonged aPTT during the first 2 L of hemorrhage were associated with a subsequent composite adverse outcome; median fibrinogen and aPTT among women with and without the composite end point after 1.5 to 2 L of hemorrhage were 1.5 g/L (interquartile range [IQR], 1.0-1.9) vs 2.7 g/L (IQR, 1.9-3.4) and 39 s (IQR, 30-47) vs 32 s (IQR, 28-36), respectively. PT and platelet count as assessed during the first 2 L of hemorrhage were not associated with morbidity or mortality. Our results suggest that detection of low levels of fibrinogen and elevated aPTT levels during early postpartum hemorrhage can contribute to the identification of women that may benefit from targeted hemostatic treatment. Essential in this identification process is the moment of reaching a level of fibrinogen of <2 g/L during the course of postpartum hemorrhage.
Introduction
Postpartum hemorrhage is a major cause of maternal morbidity and mortality with an incidence that seems to be increasing over the last decade.1-8
Efforts to prevent morbidity and mortality because of postpartum hemorrhage focus among other things on laboratory monitoring of hemostasis in order to enable timely treatment of possible coagulopathy. Hemostasis may be monitored by laboratory-based prothrombin time (PT)/activated partial thromboplastin time (aPTT), Clauss fibrinogen, platelet count, and point of care testing.9 Experts recommend that all these may be used simultaneously because there is currently no high level evidence on the best strategy.9 This advice leads to inefficiency, waste, and considerable variation in the care for patients with postpartum hemorrhage.
In order to determine the optimal strategy to monitor coagulopathy, it is crucial to know the patterns of changes in coagulation parameters in relation to the phases of postpartum hemorrhage and to identify which parameters show the fastest changes associated with the risk of severe maternal outcomes. Data on the change in coagulation parameters during the course of postpartum hemorrhage, thus per liter of ongoing hemorrhage, are limited. Earlier studies used repeated measurements at set time points or reported worst values in the course of bleeding.10-12 Some have suggested that low fibrinogen concentration might be the earliest predictor of progression toward severe postpartum hemorrhage.11,13,14 Investigators studying women with severe postpartum hemorrhage face the enormous challenge of including women in a life-threatening condition, frequently leading to failure to include the most severe cases.
Diligent observation of present-day monitoring of hemostasis and outcomes among an unselected cohort of women with ongoing postpartum hemorrhage may help to identify the hemostasis parameters that are able to recognize women with a high risk for morbidity and mortality as early as possible during postpartum hemorrhage.
The aim of this study was to describe coagulation parameters including fibrinogen during the course of severe postpartum hemorrhage per categorized volume of blood loss. Also, coagulation parameters during early postpartum hemorrhage were compared between women with and without severe acute maternal morbidity, mortality, and need for surgical intervention.
Methods
Design and study population
The Transfusion Strategies in Women during Major Obstetric Hemorrhage-1 (TeMpOH-1) study is a nationwide retrospective cohort study in 61 hospitals in The Netherlands. TeMpOH-1 included women who received at least 4 units of red cells or any transfusion of fresh frozen plasma (FFP) and/or platelets in addition to red cells because of severe obstetric hemorrhage (≥1000 mL blood loss during pregnancy, birth, or puerperium). For the present analysis, we selected women from the TeMpOH-1 cohort who met criteria for primary postpartum hemorrhage (blood loss ≥1000 mL occurring within the first 24 hours after childbirth). We excluded women for whom we did not have any coagulation parameter measured between childbirth and end of active postpartum hemorrhage. Women 18 years of age and older who met the inclusion criteria were selected. Women with a known coagulation disorder or anticoagulant were included in the study. Approval for the TeMpOH-1 study was obtained from the Medical Ethical Research Committee of the Leiden University Medical Centre (P12.273) and from the institutional review board of each participating hospital. The study was registered in the Netherlands Trial Register (NTR4079). Detailed design of the study has been reported elsewhere.15 Because of the retrospective design of the study, the need to obtain informed consent from eligible women was waived by the ethics committee. Eligible women were selected from transfusion databases and birth registries of participating hospitals with 191 772 births between 2011 and 2013. By cross-referencing electronic data from the hospitals’ blood transfusion services with local birth registers in participating hospitals, all women experiencing severe postpartum hemorrhage necessitating blood transfusion during the inclusion period of the study could be included. In most hospitals, no pregnancy-specific massive transfusion protocol is available, and in most cases, the normal (nonpregnancy) target values for hemostatic therapy are used: hemoglobin (Hb), 8 g/dL; PT and aPTT <1.5× prolonged; platelet count >50 × 109/L to 100 × 109/L; and fibrinogen >1.5 g/L.
Data collection
Detailed information on maternal, pregnancy, and birth characteristics was collected from medical files. Chart reviews were conducted by trained medical students and research nurses. Data were recorded from files available at the maternity ward, operating theater, and intensive care unit for the following parameters: maternal age at the time of birth, parity, maternal body weight during early pregnancy, maternal height, ethnicity, gestational age, obstetric history, mode of birth, cause of major obstetric hemorrhage, abnormal placentation, shock, timing and volume of fluids and blood products administered, timing of surgical and hemostatic interventions, and consecutive measurements of blood loss until cessation of bleeding. Blood loss was measured by weighing gauzes and other soaked materials and by use of a collector bag and suction system in the operating theater.
Laboratory parameters
Of the included women, we documented available laboratory parameters and data on type and volume as well as timing of clear fluids and blood products administered during the course of postpartum hemorrhage, Hb level (g/dL), hematocrit (Ht; fraction), platelet count (×109/L), aPTT (seconds), PT (seconds), and fibrinogen (g/L). Laboratory parameters from the first measurement of blood loss onward were considered, including parameters drawn from women before they had reached 1000 mL of blood loss. Unlikely values were verified in the medical records. There was no preset protocol for obtaining specimens: blood samples during postpartum hemorrhage had been obtained on request of the caregiver leading to different numbers and panels of results of laboratory parameters.
Composite adverse maternal outcome
Emergency peripartum hysterectomy, ligation of the uterine arteries, B-lynch suture (in The Netherlands only used as emergency procedure), arterial embolization, or admission into an intensive care unit were jointly considered the combined end point of severe acute maternal morbidity. Women were compared with regard to whether they had developed a composite adverse maternal outcome consisting of severe acute maternal morbidity, maternal mortality, or need for surgical intervention.
Statistical analyses
Coagulation parameters are presented as median and interquartile ranges (IQRs) because of non-Gaussian distribution. The phases of ongoing postpartum hemorrhage were categorized according to increasing volumes of blood loss: 0 to 1 L, 1 to 1.5 L, 1.5 to 2 L, 2 to 2.5 L, 2.5 to 3 L, 3 to 3.5 L, 3.5 to 4 L, and >4 L. Each laboratory parameter result was assigned to the category of blood loss at which the respective blood sample had been taken. In case of multiple measurements per woman within 1 category of volume of blood loss, the mean of those values was used. In order to assign a volume of blood loss for each of the laboratory parameters, we imputed volumes of blood loss using linear interpolation of 2 consecutive blood volume measurements. In case total blood loss was the only available data point, or the blood sample was drawn before the first measurement of blood loss volume, the birth time of the baby was used as the starting point for the interpolation. Levels of coagulation parameters between groups were compared with Mann-Whitney U tests. Reference ranges of aPTT varied somewhat for the 61 participating hospitals as a result of use of different types of reagents. To examine the robustness of aPTT results, we repeated the analyses using aPTT ratios, which were calculated by dividing the observed aPTT levels by the mean of the hospital specific reference range.
Results
Patient characteristics
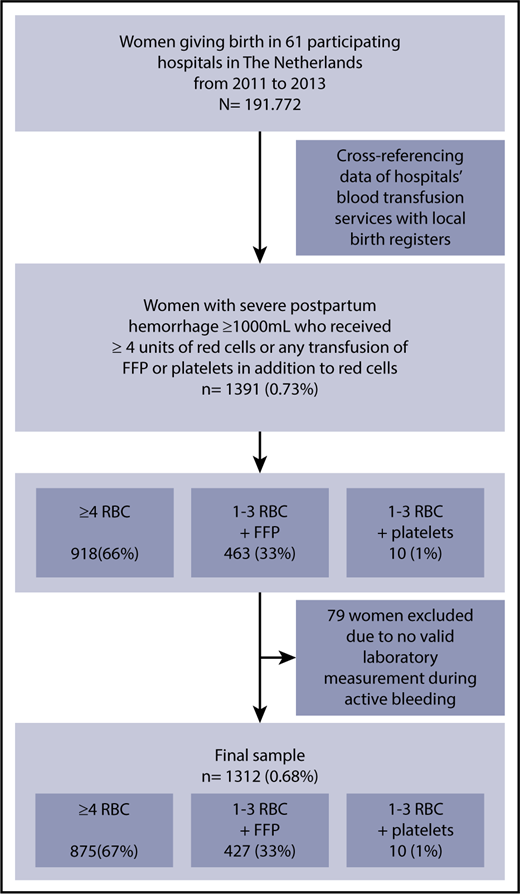
Over the 2-year inclusion period of the TeMpOH-1 study, 1391 women had received at least 4 units of red cells or FFP or platelets in addition to red cells for postpartum hemorrhage. A total of 1312 women with primary postpartum hemorrhage had at least 1 valid measurement of coagulation parameters sampled during active bleeding (Figure 1). The median volume of blood loss among these 1312 women was 3 L (IQR, 2.5-4.0). Characteristics of the study population and of women with and without the composite adverse outcome are reported in Table 1.
Inclusion flowchart for “Coagulation Parameters during the Course of Severe Postpartum Hemorrhage: A Nationwide Retrospective Cohort Study.”
Inclusion flowchart for “Coagulation Parameters during the Course of Severe Postpartum Hemorrhage: A Nationwide Retrospective Cohort Study.”
Patient and treatment characteristics of the total study population and according to the development of the composite adverse outcome
| Patient and treatment characteristics . | Total . | Severe acute maternal morbidity, mortality, and need for surgical intervention . | |
|---|---|---|---|
| No . | Yes . | ||
| Patients, n (%) | 1312 | 849 (65) | 463 (35) |
| Maternal characteristics | |||
| Age, y | 31.3 (28-35) | 31.0 (28-35) | 32.0 (29-35) |
| Body mass index, kg/m2 | 23.3 (21-26.4) | 23.1 (20.9-26.3) | 23.5 (21-27) |
| Ethnicity, white, % | 71 | 75 | 65 |
| Nulliparity, % | 52 | 54 | 47 |
| Gestational age, wk | 39.6 (38-40.7) | 39.7 (38.3-40.9) | 39.4 (37.4-40.6) |
| Mode of birth, % | |||
| Caesarean section | 25 | 19 | 36 |
| Vaginal | 75 | 81 | 63 |
| Comorbidity, % | |||
| Preeclampsia/HELLP | 11 | 9 | 14 |
| Anticoagulant use | 0.5 | 0.5 | 0.7 |
| Transfer to hospital, % | |||
| Transfer to hospital during labor | 14 | 15 | 12 |
| Postpartum transfer (birth at home) | 12 | 15 | 8 |
| Primary cause of bleeding, % | |||
| Uterine atony | 65 | 66 | 63 |
| Retained placenta | 17 | 21 | 10 |
| Pathological ingrowth of placenta | 8 | 6 | 12 |
| Placenta previa | 1 | 1 | 2 |
| Surgical bleeding | 7 | 5 | 10 |
| Placental abruption | 2 | 2 | 2 |
| Coagulopathy | 1 | 0 | 1 |
| Fibrinogen administered, % | 10 | 4 | 21 |
| Tranexamic acid administered, % | 44 | 36 | 59 |
| Recombinant FVIIa-administered, % | 3 | 0.1 | 8 |
| Bleeding rate, mL/min* | 2.4 (1.2-4.6) | 2.3 (1.2-4.2) | 2.4 (1.3-5.3) |
| Shock (systolic blood pressure <90 or heart rate >120), % | 85 | 84 | 86 |
| Total volume of clear fluids, L | 2.5 (1.7-4.0) | 2.5 (1.5-3.5) | 3.0 (2.0-4.5) |
| Total units of blood products | 6.0 (4.0-8.0) | 5.0 (4.0-6.0) | 10.0 (6.0-16.0) |
| Four or more red cells units, n (%) | 875 (67) | 481 (57) | 394 (85) |
| One to 3 red cells and 1 or more plasma units, n (%) | 427 (33) | 360 (42) | 67 (14) |
| One to 3 red cells and 1 or more platelets units, n (%) | 10 (1) | 8 (1) | 2 (0.4) |
| Total volume of blood loss, L | 3.0 (2.5-4.0) | 2.8 (2.2-3.3) | 4.0 (3.0-5.5) |
| Patient and treatment characteristics . | Total . | Severe acute maternal morbidity, mortality, and need for surgical intervention . | |
|---|---|---|---|
| No . | Yes . | ||
| Patients, n (%) | 1312 | 849 (65) | 463 (35) |
| Maternal characteristics | |||
| Age, y | 31.3 (28-35) | 31.0 (28-35) | 32.0 (29-35) |
| Body mass index, kg/m2 | 23.3 (21-26.4) | 23.1 (20.9-26.3) | 23.5 (21-27) |
| Ethnicity, white, % | 71 | 75 | 65 |
| Nulliparity, % | 52 | 54 | 47 |
| Gestational age, wk | 39.6 (38-40.7) | 39.7 (38.3-40.9) | 39.4 (37.4-40.6) |
| Mode of birth, % | |||
| Caesarean section | 25 | 19 | 36 |
| Vaginal | 75 | 81 | 63 |
| Comorbidity, % | |||
| Preeclampsia/HELLP | 11 | 9 | 14 |
| Anticoagulant use | 0.5 | 0.5 | 0.7 |
| Transfer to hospital, % | |||
| Transfer to hospital during labor | 14 | 15 | 12 |
| Postpartum transfer (birth at home) | 12 | 15 | 8 |
| Primary cause of bleeding, % | |||
| Uterine atony | 65 | 66 | 63 |
| Retained placenta | 17 | 21 | 10 |
| Pathological ingrowth of placenta | 8 | 6 | 12 |
| Placenta previa | 1 | 1 | 2 |
| Surgical bleeding | 7 | 5 | 10 |
| Placental abruption | 2 | 2 | 2 |
| Coagulopathy | 1 | 0 | 1 |
| Fibrinogen administered, % | 10 | 4 | 21 |
| Tranexamic acid administered, % | 44 | 36 | 59 |
| Recombinant FVIIa-administered, % | 3 | 0.1 | 8 |
| Bleeding rate, mL/min* | 2.4 (1.2-4.6) | 2.3 (1.2-4.2) | 2.4 (1.3-5.3) |
| Shock (systolic blood pressure <90 or heart rate >120), % | 85 | 84 | 86 |
| Total volume of clear fluids, L | 2.5 (1.7-4.0) | 2.5 (1.5-3.5) | 3.0 (2.0-4.5) |
| Total units of blood products | 6.0 (4.0-8.0) | 5.0 (4.0-6.0) | 10.0 (6.0-16.0) |
| Four or more red cells units, n (%) | 875 (67) | 481 (57) | 394 (85) |
| One to 3 red cells and 1 or more plasma units, n (%) | 427 (33) | 360 (42) | 67 (14) |
| One to 3 red cells and 1 or more platelets units, n (%) | 10 (1) | 8 (1) | 2 (0.4) |
| Total volume of blood loss, L | 3.0 (2.5-4.0) | 2.8 (2.2-3.3) | 4.0 (3.0-5.5) |
Values are median (IQR), except as noted.
Maximum.
Laboratory parameters during postpartum hemorrhage
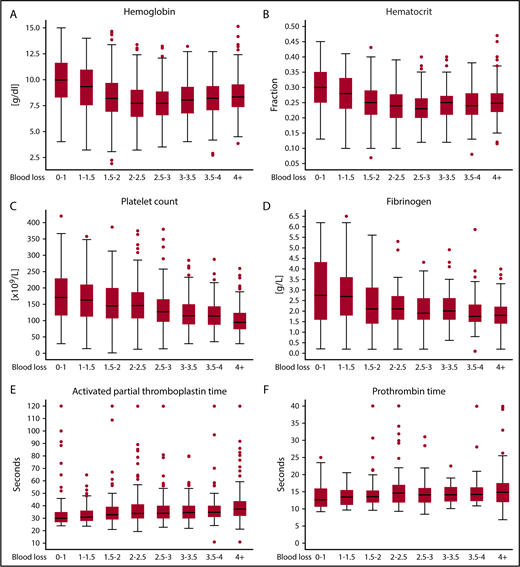
Hb concentration was measured on 2605 occasions, Ht on 2245 occasions, platelet count on 1581 occasions, fibrinogen concentration on 775 occasions, PT on 876 occasions, and aPTT on 1075 occasions. Women had a median amount of 3 (IQR, 2-5) blood loss measurements during active postpartum hemorrhage. Figure 2 shows results of the laboratory test results according to increasing volumes of hemorrhage. The accompanying patient count, mean, standard deviation, median, IQR, and lowest and highest values of laboratory parameters according to increasing volumes of blood loss are presented in supplemental Table 1. Levels of Hb tended to decrease up to 2.0 to 2.5 L of blood loss to an Hb level of 7.7 g/dL (IQR, 6.4-9.0) and an Ht of 0.24 (IQR, 0.20-0.28), after which stabilization occurred. At 2.5 L of blood loss, 203 out of 443 (46%) women had been transfused with blood products.
Coagulation parameters of women during the course of severe postpartum hemorrhage per categorized amount of blood loss. (A) Hemoglobin, (B) hematocrit, (C) platelet count, (D) fibrinogen, (E) aPTT, and (F) PT. Laboratory parameters are presented in box plots. Circles are outliers. The box represents the 25th and 75th percentiles, and the whiskers are the upper and lower adjacent values.
Coagulation parameters of women during the course of severe postpartum hemorrhage per categorized amount of blood loss. (A) Hemoglobin, (B) hematocrit, (C) platelet count, (D) fibrinogen, (E) aPTT, and (F) PT. Laboratory parameters are presented in box plots. Circles are outliers. The box represents the 25th and 75th percentiles, and the whiskers are the upper and lower adjacent values.
Platelet counts also decreased with increasing volume of blood loss. Women with 2.0 to 2.5 L of blood loss had a median platelet count of 146 × 109/L (IQR, 108-186). Four percent (10/253) of these women had received a platelet transfusion at that time. For 128 women with blood loss of 3.5 to 4.0 L the median platelet count was 115 × 109/L (IQR, 89-143); 21 of these 128 (16%) women had received platelet transfusions, and 113/128 (88%) had received a blood product.
There were 342 (0.18% of all births in the 61 hospitals and 26% of the women in our study cohort) women who developed a fibrinogen level below 2 g/L. A fibrinogen level below 1 g/L was reached by 78 women. Five percent (70/1312) of the women in our cohort reached a fibrinogen level below 2 g/L after losing <2 L of blood. Four women reached this level because of postpartum hemorrhage because of placental abruption. Median baseline fibrinogen level during early postpartum hemorrhage was 2.8 g/L (IQR, 1.6-4.3). Fibrinogen levels tended to decrease up to 2 to 2.5 L of blood loss at 2.1 g/L (IQR, 1.6-2.7). Among 152 women who had lost >4 L, median level of fibrinogen was 1.8 g/L (IQR, 1.4-2.2); 41% of these women had been treated with fibrinogen concentrates. In the subgroup of patients with postpartum hemorrhage because of uterine atony or retained placenta, we observed a similar trend (supplemental Figure 4).
Median PT values showed a slight increase with increasing volumes of blood loss. During the earliest phase of postpartum hemorrhage, median PT was 12.7 seconds (IQR, 10.7-15.9); among women who lost >4 L, the median PT was 14.9 (IQR, 12.0-17.5). Median aPTT increased with increasing volumes of blood loss from 30.0 seconds (IQR, 27.0-35.0) during early bleeding to 37.5 seconds (IQR, 32.0-43.6) in the maximum blood loss category. Sensitivity analyses on the aPTT ratio showed similar results (supplemental Figure 5).
Laboratory parameters and adverse maternal outcome
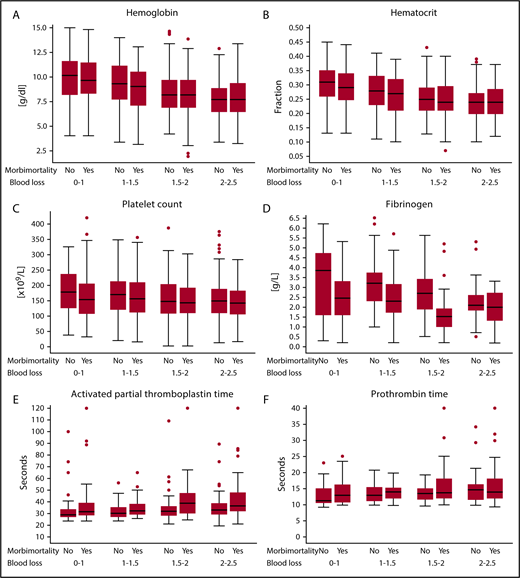
Of the 1312 women, 463 (35%) developed a combined end point of severe acute maternal morbidity, mortality, or the need for surgical intervention; 37% (172/463 women) of these women developed >1 of the items composing the combined adverse end point. To arrest bleeding, hysterectomy was necessary in 72 (5%) women, 164 (13%) were treated with arterial embolization, and in 46 (4%) women an emergency B-lynch procedure or ligation of arteries was performed. Of the women in our study cohort, 386 (29%) were admitted to the intensive care unit and 7 (0.5%) died as a result of severe hemorrhage. Figure 3 shows laboratory results during postpartum hemorrhage of women with and women without the composite adverse outcome. Women who developed the composite adverse outcome had lower fibrinogen concentrations and longer aPTTs than women who did not develop the end point, which was already apparent and most pronounced during the earliest phases of postpartum hemorrhage. There were no noteworthy differences in Hb, Ht, PT, and platelet levels during the early phases of postpartum hemorrhage (blood loss <2 L; Table 2). Women who developed the composite adverse outcome had a median fibrinogen level of <2g/L at 1.5 to 2 L of blood loss, whereas women without the composite adverse outcome had a median fibrinogen of <2 g/L after a volume of >3.5 L of blood loss. Additional results based on first sample during postpartum hemorrhage irrespective of blood loss volume and receiver-operating characteristic analyses of progression to the severe morbidity end point based on the first blood test are provided in supplemental Tables 2 and 3. Sensitivity analyses with the aPTT ratio showed similar results (supplemental Table 6). Patient characteristics of the women with fibrinogen measurements are also presented (supplemental Table 7).
Coagulation parameters of women with and without combined end point of severe acute maternal morbidity or mortality per categorized amount of blood loss. (A) Hemoglobin, (B) hematocrit, (C) platelet count, (D) fibrinogen, (E) aPTT, and (F) PT. Box plots of coagulation parameters per categorized amount of blood loss comparing women experiencing postpartum hemorrhage with and without the composite adverse outcome. Morbi-mortality comprises the composite adverse outcome of severe acute maternal morbidity, mortality, or need for surgical intervention. Circles are outliers. The box represents the 25th and 75th percentiles, and the whiskers are the upper and lower adjacent values.
Coagulation parameters of women with and without combined end point of severe acute maternal morbidity or mortality per categorized amount of blood loss. (A) Hemoglobin, (B) hematocrit, (C) platelet count, (D) fibrinogen, (E) aPTT, and (F) PT. Box plots of coagulation parameters per categorized amount of blood loss comparing women experiencing postpartum hemorrhage with and without the composite adverse outcome. Morbi-mortality comprises the composite adverse outcome of severe acute maternal morbidity, mortality, or need for surgical intervention. Circles are outliers. The box represents the 25th and 75th percentiles, and the whiskers are the upper and lower adjacent values.
Coagulation parameters during the course of postpartum hemorrhage of women with and without composite adverse maternal outcome
| Blood loss category, L . | Hb, g/dL . | Ht (fraction) . | Platelet count, ×109/L . | Fibrinogen, g/L . | aPTT, s . | PT, s . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composite end point . | Composite end point . | Composite end point . | Composite end point . | Composite end point . | Composite end point . | |||||||||||||
| No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | |
| 0.00 to 1.0 | 10.1 | 9.7 | 0.28 | 0.31 | 0.29 | 0.23 | 178 | 154 | 0.24 | 3.9 | 2.5 | 0.09 | 29 | 32 | 0.11 | 11 | 13 | 0.26 |
| 1.01 to 1.5 | 9.3 | 9.0 | 0.04 | 0.28 | 0.27 | 0.04 | 170 | 156 | 0.29 | 3.2 | 2.3 | 0.01 | 30 | 33 | 0.03 | 13 | 14 | 0.44 |
| 1.51 to 2.0 | 8.2 | 8.2 | 0.91 | 0.25 | 0.24 | 0.84 | 147 | 144 | 0.47 | 2.7 | 1.5 | <0.01 | 32 | 39 | <0.01 | 14 | 14 | 0.26 |
| 2.01 to 2.5 | 7.7 | 7.7 | 0.61 | 0.24 | 0.24 | 0.55 | 150 | 142 | 0.32 | 2.1 | 2.0 | 0.19 | 33 | 37 | <0.01 | 15 | 14 | 0.54 |
| 2.51 to 3.0 | 7.9 | 7.6 | 0.29 | 0.24 | 0.23 | 0.13 | 136 | 119 | 0.19 | 2.0 | 1.8 | <0.01 | 33 | 38 | <0.01 | 14 | 14 | 0.42 |
| 3.01 to 3.5 | 7.7 | 8.4 | 0.01 | 0.24 | 0.25 | 0.02 | 117 | 111 | 0.21 | 2.2 | 2.0 | 0.40 | 33 | 36 | 0.04 | 15 | 14 | 0.82 |
| 3.51 to 4.0 | 8.3 | 8.2 | 0.58 | 0.25 | 0.24 | 0.22 | 128 | 110 | 0.06 | 2.0 | 1.7 | 0.05 | 34 | 36 | 0.09 | 15 | 14 | 0.09 |
| 4.01 or more | 8.2 | 8.4 | 0.06 | 0.24 | 0.25 | 0.04 | 115 | 93 | <0.01 | 1.7 | 1.8 | 0.76 | 34 | 38 | <0.01 | 15 | 15 | 0.93 |
| Blood loss category, L . | Hb, g/dL . | Ht (fraction) . | Platelet count, ×109/L . | Fibrinogen, g/L . | aPTT, s . | PT, s . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composite end point . | Composite end point . | Composite end point . | Composite end point . | Composite end point . | Composite end point . | |||||||||||||
| No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | No . | Yes . | P* . | |
| 0.00 to 1.0 | 10.1 | 9.7 | 0.28 | 0.31 | 0.29 | 0.23 | 178 | 154 | 0.24 | 3.9 | 2.5 | 0.09 | 29 | 32 | 0.11 | 11 | 13 | 0.26 |
| 1.01 to 1.5 | 9.3 | 9.0 | 0.04 | 0.28 | 0.27 | 0.04 | 170 | 156 | 0.29 | 3.2 | 2.3 | 0.01 | 30 | 33 | 0.03 | 13 | 14 | 0.44 |
| 1.51 to 2.0 | 8.2 | 8.2 | 0.91 | 0.25 | 0.24 | 0.84 | 147 | 144 | 0.47 | 2.7 | 1.5 | <0.01 | 32 | 39 | <0.01 | 14 | 14 | 0.26 |
| 2.01 to 2.5 | 7.7 | 7.7 | 0.61 | 0.24 | 0.24 | 0.55 | 150 | 142 | 0.32 | 2.1 | 2.0 | 0.19 | 33 | 37 | <0.01 | 15 | 14 | 0.54 |
| 2.51 to 3.0 | 7.9 | 7.6 | 0.29 | 0.24 | 0.23 | 0.13 | 136 | 119 | 0.19 | 2.0 | 1.8 | <0.01 | 33 | 38 | <0.01 | 14 | 14 | 0.42 |
| 3.01 to 3.5 | 7.7 | 8.4 | 0.01 | 0.24 | 0.25 | 0.02 | 117 | 111 | 0.21 | 2.2 | 2.0 | 0.40 | 33 | 36 | 0.04 | 15 | 14 | 0.82 |
| 3.51 to 4.0 | 8.3 | 8.2 | 0.58 | 0.25 | 0.24 | 0.22 | 128 | 110 | 0.06 | 2.0 | 1.7 | 0.05 | 34 | 36 | 0.09 | 15 | 14 | 0.09 |
| 4.01 or more | 8.2 | 8.4 | 0.06 | 0.24 | 0.25 | 0.04 | 115 | 93 | <0.01 | 1.7 | 1.8 | 0.76 | 34 | 38 | <0.01 | 15 | 15 | 0.93 |
Mann-Whitney U test.
Discussion
Among women with severe postpartum hemorrhage requiring blood transfusion, the occurrence of low levels of fibrinogen and prolonged aPTTs in the earliest phases of hemorrhage was associated with progression toward severe acute maternal morbidity, mortality, or need for surgical intervention.
Strength and limitations of this study
Our study describes coagulation parameters and morbidity of women with severe postpartum hemorrhage. The results are therefore only generalizable to women suffering severe postpartum hemorrhage. The unique strength of this retrospective study is that we were able to include all women with severe postpartum hemorrhage necessitating blood transfusion that had occurred in the 61 participating hospitals during the study period, including the most severe cases, enabling reliable and generalizable estimation of percentages of women with coagulopathy during the course of postpartum hemorrhage. In addition, the large sample size allowed us to examine patterns of laboratory parameters throughout the course of ongoing postpartum hemorrhage. The retrospective study design also has limitations. We did not have control over the number and specific panels of coagulation samples. Therefore, our results are based on different selections of women in the categories of blood loss. Obviously, more blood samples were drawn from women with more severe bleeding. It is therefore possible that women with low fibrinogen or prolonged aPTT were missed in women with lower blood loss as these parameters were not measured, because there were no measurements. This may have led to an underestimation of the occurrence of abnormal laboratory parameters. Moreover, laboratory measurements were performed in local laboratories of the 61 hospitals, leading to significant variation in measurements and possible misclassification. Such variation will also influence the results toward underestimation of the strength of the association of coagulation parameter abnormalities with morbidity and mortality. To be certain of the accuracy of all low fibrinogen values in our study cohort, we returned to the 61 participating hospitals and verified all values of fibrinogen with a level <2 g/L (and all other outliers of laboratory parameters) in the medical files. During the inclusion period of our study, none of the participating hospitals used thromboelastometry in women experiencing postpartum hemorrhage.
Comparison with other studies
The occurrence of coagulopathy in postpartum hemorrhage and its association with maternal morbidity and mortality has been studied previously. For a correct interpretation of the results of this study in the context of previous studies, it should be taken into account that our study differs from previous studies with regard to its source population: as opposed to previous studies, only women with severe postpartum hemorrhage necessitating the administration of blood products were included. Fibrinogen levels of lower than 2 g/L were strongly associated with progression toward severe postpartum hemorrhage in a study among 128 women with postpartum hemorrhage.11 However, in this study measurements were done at predefined hours after enrollment, and information on the corresponding amount of blood loss at the time of the measurements was lacking. It therefore remained unclear whether the level of fibrinogen was a predictor of progression toward more severe bleeding or a result of blood loss at time of blood sampling. Another study among 456 women with postpartum hemorrhage from a large UK unit reported results of Hb, platelet count, PT, and aPTT tests that were categorized based on the worst value of the total amount of blood loss at the end of bleeding.10 Fibrinogen was found to be the parameter that best correlated with increasing volume of hemorrhage. PT and aPTT remained within the normal range in most women despite large bleeds. In a review article, Collis et al summarized results of 5 studies that tried to determine a value of fibrinogen that could serve as a biomarker for progression of postpartum hemorrhage.14 These values varied between studies: fibrinogen level 3.3/3.4/1.8/3.1/2.8 g/L.10,11,13,16,17 Their overall conclusion was that a fibrinogen level of <3 g/L and, in particular, <2 g/L was associated with progression toward more severe postpartum hemorrhage.
Another study on women in need of massive transfusion because of postpartum hemorrhage (≥8 units of red cells within 24 hours of delivery) was also based on the first and worst values measured, regardless of volume of blood loss at sampling.12 Also, in this study a difference was made between levels of coagulation parameters for different primary causes of bleeding. Because the primary cause of bleeding often remains unclear during active postpartum hemorrhage, and in some cases is only clarified when additional tests have been performed after the event, we find it of great clinical importance to study the pattern of change of coagulation parameters over time in relation to volume of blood loss, regardless of primary cause of bleeding.
In our cohort, we observe a higher occurrence of a fibrinogen concentration below 2 g/L compared with results suggested in randomized trials. This can be explained by differences in patient selection. In a Danish multicenter double-blind randomized trial, women experiencing severe postpartum hemorrhage were treated with a dose of 2 g of fibrinogen concentrate or placebo.18 Of the 244 randomized women, only 5 had a fibrinogen value <2 (mean value in both groups 4.5). A more recent trial randomized 55 women at a FIBTEM value of 15 mm (considered to be the equivalent to Clauss fibrinogen value of 3 g/L) to fibrinogen concentrate or placebo.19 No improvement in outcome was observed in women who were administered fibrinogen; only 7 women (out of a cohort of 663 women with postpartum hemorrhage) developed a fibrinogen level below 2 g/L confirming, as discussed by the authors, the challenges related to consenting women with severe bleeding and undertaking trial procedures while treating acutely ill women. This challenge was also experienced by a Finnish research group that aimed to perform a randomized controlled trial comparing prothrombin complex concentrate and fibrinogen concentrate to FFP as a treatment of women experiencing postpartum hemorrhage exceeding 2 L (#NCT01910675). Obtaining informed consent in an acute situation with severe bleeding turned out to be impossible (Jouni Ahonen, Helsiniki University, e-mail communication, 15 April 2018).
The results of the TeMpOH-1 study confirm the results of previous studies on this subject, however, with 1 very important addendum for acute clinical decision making: the dimension of time. We found 342 women with a fibrinogen level ≤2 g/L and a further 78 women with a fibrinogen level ≤1 g/L. We have elucidated that women who experienced postpartum hemorrhage without developing a composite outcome of maternal morbidity and mortality only sporadically reached a fibrinogen value of <2 g/L (blood loss >3.5 L). Women who did develop the composite adverse outcome reached this low fibrinogen level much earlier (1.5-2 L of blood loss) during postpartum hemorrhage. This difference in the moment of reaching a level of fibrinogen of <2 g/L during the course of postpartum hemorrhage is essential for the selection of the right target population for future studies into the potential benefit of administering fibrinogen concentrate.
Clinical implications
By the timely detection of changes in levels of relevant coagulation parameters, targeted hemostatic therapy to restore deficiencies could be administered. However, assessment of fibrinogen levels by a standard coagulation test like the Clauss fibrinogen assay has a turnaround time of up to 60 minutes making it unsuitable for acute clinical decision making.20 Point-of-care devices like ROTEM thromboelastometry are able to detect essential changes in the coagulation system within 10 minutes after blood sampling.21 ROTEM FIBTEM could potentially be a worthwhile addition to postpartum hemorrhage management.22,23 To make progress in this field, we need to monitor women experiencing postpartum hemorrhage closely during the process of active blood loss. In our next currently ongoing study (#NCT02149472), we will further elucidate the predictive value of early changes in coagulation parameters (including thromboelastometry) for the development of severe acute maternal morbidity and mortality in women experiencing postpartum hemorrhage. The results provided by this study provide a solid knowledge base to be used when making the transition toward the evidence-based use of rapid point-of-care testing.
Conclusion
In this nationwide retrospective cohort study on the change of coagulation parameters in 1312 women experiencing severe postpartum hemorrhage requiring blood transfusion, we provide a solid knowledge base of common patterns of change in coagulation parameters during postpartum hemorrhage. Our results suggest that detection of low levels of fibrinogen and elevated aPTT levels during early postpartum hemorrhage can contribute to the identification of women that may benefit from targeted hemostatic treatment. Based on these results, we advise to assess levels of fibrinogen and aPTT in all women who experience postpartum hemorrhage with blood loss exceeding 1 L.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors would like to thank all 61 participating hospitals and the Dutch Consortium for Healthcare Evaluation and Research in Obstetrics and Gynaecology–NVOG Consortium 2.0; medical students R. M. Loeff, R. J. van Goeverden, B. Eijlers, A. Hillebrand, S. E. Spelmink, T. J. Beunder, V. Harskamp, M. Wind Koning, R. A. Cramer, A. Veenstra, S. M. Smith, and E. E. Ensing; data managers C. J. van Brussel-de Groot and O. Zouitni; and research nurses C. Kolster-Bijdevaate, M. S. Bourgonje-Verhart, C. E. Bleeker-Taborh, E. Roos-van Milligen, and A. de Graaf-Dijkstra for their contributions to the TeMpOH-1 study. The authors would also like to thank J. C. M. Meijers for critically reading and providing feedback on the manuscript.
Authorship
Contribution: A.G., T.v.d.A., D.D.C.A.H., and J.G.v.d.B. designed the research; A.G. wrote the original draft of the manuscript; A.G., C.C.-D., and D.D.C.A.H. were responsible for data curation; C.C.-D. and A.G. analyzed results and made the figures and tables; D.D.C.A.H., J.J.Z., M.P.M.d.M., J.J.M.v.R., J.E., K.W.M.B., and J.G.v.d.B. were involved in conceptualization and methodology and reviewed and edited the paper; and J.G.v.d.B. and T.v.d.A. had supervision over the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of members of the TeMpOH-1 Study Group appears in “Appendix.”
Correspondence: Johanna G. van der Bom, Center for Clinical Transfusion Research, Sanquin Research, Plesmanlaan 1a–Fifth floor, 2333 BZ Leiden, The Netherlands; e-mail: j.g.vanderbom@lumc.nl.
Appendix: study group members
The members of the TeMpOH-1 Study Group are: H. J. Adriaanse, Gelre Hospital, Medical Laboratory Head; E. S. A. van den Akker, Onze Lieve Vrouwe Hospital, Obstetrician; M. I. Baas, Hospital Rivierenland Tiel, Obstetrician; C. M. C. Bank, Admiraal de Ruyter Hospital, Medical Laboratory Head; E. van Beek, St. Antonius Hospital, Obstetrician; B. A. de Boer, Atalmedial, Medical Laboratory Head; K. de Boer, Rijnstate Hospital, Obstetrician; D. M. R. van der Borden, Regional Hospital Koningin Beatrix, Obstetrician; H. A. Bremer, Reinier de Graaf Hospital, Obstetrician; J. T. J. Brons, Medical Centre Twente, Obstetrician; J. M. Burggraaff, Scheper Hospital, Obstetrician; H. Ceelie, Vlietland Hospital, Medical Laboratory Head; H. Chon, Tergooi Hospital, Medical Laboratory Heads; J. L. M. Cikot, Van Weel-Bethesda Hospital, Obstetrician; F. M. C. Delemarre, Elkerliek Hospital, Obstetrician; J. H. C. Diris, Bernhoven Hospital, Medical Laboratory Head; M. Doesburg–van Kleffens, Maas Hospital Pantein, Medical Laboratory Head; I. M. A. van Dooren, St. Jans Hospital, Obstetrician; J. L. P. van Duijnhoven, Elkerliek Hospital, Medical Laboratory Head; F. M. van Dunné, Medical Centre Haaglanden, Obstetrician; J. J. Duvekot, Erasmus Medical Centre, Obstetrician; P. Engbers, Bethesda Hospital, Medical Laboratory Head; M. J. W. van Etten–van Hulst, Fransiscus Hospital, Obstetrician; H. Feitsma, Haga Hospital, Obstetrician; M. A. Fouraux, Ikazia Hospital, Medical Laboratory Head; M. T. M. Franssen, University Medical Centre Groningen, Obstetrician; M. A. M. Frasa, Groene Hart Hospital, Medical Laboratory Head; A. J. van Gammeren, Amphia Hospital, Medical Laboratory Head; N. van Gemund, Sint Fransiscus Hospital, Obstetrician; F. van der Graaf, Máxima Medical Centre, Medical Laboratory Head; C. J. M. de Groot, VU Medical Centre, Obstetrician; C. M. Hackeng, St. Antonius Hospital, Medical Laboratory Head; D. P. van der Ham, Martini Hospital, Obstetrician; M. J. C. P. Hanssen, Bethesda Hospital, Obstetrician; T. H. M. Hasaart, Catharina Hospital, Obstetrician; H. A. Hendriks Sint Lucas Andreas Hospital, Medical Laboratory Head; Y. M. C. Henskens, Maastricht University Medical Centre, Medical Laboratory Head; B. B. J. Hermsen, Sint Lucas Andreas Hospital, Obstetrician; S. Hogenboom, Flevo Hospital, Medical Laboratory Head; A. Hooker, Zaans Medical Centre, Obstetrician; F. Hudig, Haga Hospital, Medical Laboratory Head; A. M. G. Huijssoon, Vlietland Hospital, Obstetrician; A. J. M. Huisjes, Gelre Hospital, Obstetrician; N. Jonker, Wilhelmina Hospital, Medical Laboratory Head; P. J. Kabel, St. Elisabeth Hospital, Medical Laboratory Head; C. van Kampen, Gelderse Vallei Hospital, Medical Laboratory Head; M. H. de Keijzer, Rivierenland Tiel Hospital, Medical Laboratory Head; D. H. van de Kerkhof, Catharina Hospital, Medical Laboratory Head; J. F. W. Keuren, Zuwe Hofpoort Hospital, Medical Laboratory Head; J. F. W. Keuren, Groene Hart Hospital, Medical Laboratory Head; G. Kleiverda, Flevo Hospital, Obstetrician; J. H. Klinkspoor, Amsterdam Medical Centre, Medical Laboratory Head; S. G. A. Koehorst, Slingeland Hospital, Medical Laboratory Head; M. Kok, Amsterdam Medical Centre, Obstetrician; R. D. Kok, Bernhoven Hospital, Obstetrician; J. B. de Kok, Deventer Hospital, Medical Laboratory Head; A. Koops, Wilhelmina Hospital, Obstetrician; W. Kortlandt, Diakonessen Hospital, Medical Laboratory Head; J. Langenveld, Atrium Medical Centre, Obstetrician; M. P. G. Leers, Atrium Medical Centre, Medical Laboratory Head; A. Leyte, Onze Lieve Vrouwe Gasthuis, Medical Laboratory Head; A. de Mare, Medlon, Medical Laboratory Head; G. D. M. Martens, Zuwe Hofpoort Hospital, Obstetrician; J. H. Meekers, University Medical Centre Groningen, Employee laboratory; C. A. van Meir, Groene Hart Hospital, Obstetrician; G. C. H Metz, Ikazia Hospital, Obstetrician; E. C. H. J. Michielse, St. Jans Hospital, Medical Laboratory Head; L. J. Mostert, Van Weel-Bethesda Hospital, Medical Laboratory Head; S. W. H. Nij Bijvank, Isala clinics, Obstetrician; E. Oostenveld, Tjongerschans Hospital, Obstetrician; N. Osmanovic, Zaans Medical Centre, Medical Laboratory Head; M. A. Oudijk, University Medical Centre Utrecht, Obstetrician; C. Pagano Mirani–Oostdijk, Fransiscus Hospital, Medical Laboratory Head; E. C. M. van Pampus, University Medical Centre St. Radboud, Medical Laboratory Head; D. N. M. Papatsonis, Amphia Hospital, Obstetrician; R. H. M. Peters, Tjongerschans Hospital, Medical Laboratory Head; G. A. E. Ponjee, Medical Centre Haaglanden, Medical Laboratory Head; M. Pontesilli, Medical Centre Alkmaar, Fertility doctor; M. M. Porath, Máxima Medical Centre, Obstetrician; M. S. Post, Medical Centre Leeuwarden, Obstetrician; J. G. J. Pouwels, Scheper Hospital, Medical Laboratory Head; L. Prinzen, Sint Fransiscus Hospital, Medical Laboratory Head; J. M. T. Roelofsen, Lange Land Hospital, Obstetrician; J. J. M. Rondeel, Isala clinics, Medical Laboratory Head; P. C. M. van der Salm, Meander Medical Centre, Obstetrician; H. C. J. Scheepers, Maastricht University Medical Centre, Obstetrician; D. H. Schippers, Canisius-Wilhelmina Hospital, Obstetrician; N. W. E. Schuitemaker, Diakonessen Hospital, Obstetrician; J. M. Sikkema, Hospital group Twente, Obstetrician; J. Slomp, Medical Spectre Twente, Medical Laboratory Head; J. W. Smit, Martini Hospital, Medical Laboratory Head; Y. S. Snuif–de Lange, Admiraal de Ruyter Hospital, Obstetrician; J. W. J. van der Stappen, Canisius-Wilhelmina Hospital, Medical Laboratory Head; P. Steures, St. Elisabeth Hospital, Obstetrician; G. H. M. Tax, Reinier de Graaf Hospital, Medical Laboratory Head; M. Treskes, Tergooi Hospital, Medical Laboratory Heads; H. J. L. M. Ulenkate, Zorgsaam Zeeuws-Vlaanderen Hospital, Medical Laboratory Head; G. A. van Unnik, Diaconessen Hospital, Obstetrician; B. S. van der Veen, Medical Centre Leeuwarden, Medical Laboratory Head; T. E. M. Verhagen, Slingeland Hospital, Obstetrician; J. Versendaal, Maasstad Hospital, Obstetrician; B. Visschers, Zorgsaam Zeeuws-Vlaanderen Hospital, Obstetrician; O. Visser, VU Medical Centre, Hematologist; H. Visser, Tergooi Hospital Obstetrician; K. M. K. de Vooght, University Medical Centre Utrecht, Medical Laboratory Head; M. J. de Vries, Rijnland Hospital, Obstetrician; H. de Waard, Rijnstate Hospital, Medical Laboratory Head; F. Weerkamp, Maasstad Hospital, Medical Laboratory Head; M. J. N. Weinans, Gelderse Vallei Hospital, Obstetrician; H. de Wet, Refaja Hospital Stadskanaal, Obstetrician; M. van Wijnen, Meander Medical Centre, Medical Laboratory Head; W. J. van Wijngaarden, Bronovo Hospital, Obstetrician; A.C. de Wit, Maas Hospital Pantein, Obstetrician; M. D. Woiski, University Medical Centre St. Radboud, Obstetrician.