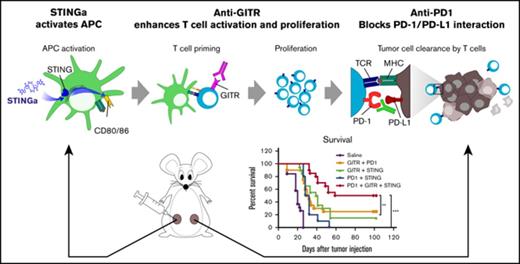
Key Points
The immunotherapeutic property of STING agonists is more potent to clear lymphoma than its cytotoxic property.
In situ vaccination with STING agonist can be enhanced by agents that improve APC or T-cell function such as anti-GITR and anti-PD-1.
Abstract
Direct activation of tumor infiltrating antigen-presenting cells (APCs) by intratumoral injection of STING agonists (STINGa) leads to regression of the treated lymphoma tumor. Because STING activation induces apoptosis in lymphoma cells in vitro, we distinguished between the direct therapeutic vs the indirect immunotherapeutic properties of STINGa in vivo. Employing wild-type or STING knockout hosts bearing either wild-type or STING knockout tumor cells, we demonstrated that local tumor regression is totally dependent on STING expression by the host and is therefore immune mediated. However, distant untreated tumors are weakly affected after injection of STINGa to a single tumor site. Therefore, using the STINGa currently being tested in clinical trials, we screened for immunomodulatory agents that could synergize with the STING pathway to induce a systemic antitumor immune response and regression of distant tumors. We combined the STINGa with agents that improve APC or T-cell function. We found that modulation of both APCs and T cells can enhance control of distant lymphoma tumors by STINGa. In particular, adding an anti-GITR antibody induced lymphocyte expansion in the lymph node draining the treated site followed by increased T-cell infiltration in the distant tumor. Furthermore, more of these CD8 T cells at the distant site expressed PD-1. Therefore, blockade of PD-1 further enhanced tumor control at the distant site, leading to cure in 50% of the mice. These preclinical data provide the rationale for testing local injection of STINGa followed by agonistic anti-GITR and anti-PD-1 antibodies as immunotherapy for human lymphoma.
Introduction
Our laboratory and others have previously explored in situ vaccination for cancer using CpG oligodeoxynucleotides (CpG), a TLR9 agonist. We have shown that intratumoral injection of CpG in combination with immunomodulatory agents induces a potent antitumor T-cell response that can affect distant untreated tumors.1-3
Cyclic dinucleotides (CDNs) are another class of immune stimulator. They activate the immune system by engaging the stimulator of interferon gene (STING). STING is therefore a receptor that recognizes CDN produced by the microorganism or endogenously produced on cytosolic DNA detection by cGAS.4 The cGAS-STING pathway is also involved in the spontaneous immune recognition of tumors.5 When injected intratumorally, CDNs are able to induce a tumor-specific T-cell response.6 They activate antigen-presenting cells (APCs), inducing them to produce cytokines and chemokines including type 1 interferon (IFN).6 Tumor endothelial cells were also shown to produce type 1 IFN on CDN stimulation.7 Injected tumors exhibit a dramatic regression that is type 1 IFN- and T-cell dependent.6,7 Intratumoral injection of CDNs has been studied in various tumor models including melanoma (B16),6,7 colorectal cancer (CT266 and MC387 ), pancreatic cancer (PancO28 ), and breast cancer (4T1).6 However, it has not been reported yet in lymphoma preclinical models. Nevertheless, 2 phase 1 clinical trials are evaluating the safety and efficacy of intratumoral injection of CDNs in patients with advanced/metastatic solid tumors or lymphomas as a single agent (NCT02675439) or in combination with anti-PD1 antibodies (NCT03172936).
Stimulation of APCs by STING agonists (STINGa) injected directly into the tumor can trigger an antitumor immune response that induces regression of the treated site. However, distant, noninjected tumor sites are less affected. Many factors can prevent the immune system from recognizing cancer cells. Regulation of the antitumor response can impair efficient antigen presentation or downregulate T-cell activity. Recent work has shown that the local effect of STINGa could be improved by adding agents that activate APCs such as CpG9 by adding antibodies that block PD-1 and CTLA-47 or that stimulate OX4010 and 4-1BB.11 Two of these studies monitored a second tumor site to evaluate the efficacy of the induced immune response to overcome immune suppression of the tumor microenvironment. They showed that treatment delayed growth of the distant tumor.
We screened for immunomodulatory agents that could synergize with a STINGa as a therapeutic in situ vaccination for lymphoma. We tested for tumor control of a distant noninjected tumor as a sign of enhancement of a systemic antitumor immune response. The STINGa used in this study is the synthetic dithio-modified cyclic diadenosine ADU-S100 that is currently being tested in clinical trials (NCT02675439 and NCT03172936). Among the candidate agents, we found an antibody reacting with the glucocorticoid-induced TNFR-related protein (GITR) to be effective. Engaging this receptor is reported to stimulate effector T-cell activity and to downregulate Tregs.12 After the STINGa and anti-GITR treatment, a higher number of CD8 T cells expressed PD-1. Therefore, blockade of PD-1 was able to further enhance tumor control at the distant site, and this final combination could cure 50% of the mice.
Methods
Reagents
STINGa, the cyclic dithio-modified diadenosine, was provided by Aduro Biotech. CpG was provided by Dynavax. Anti-OX40 (CD134) monoclonal antibody (clone OX86; European Collection of Cell Cultures) was produced from ascites in severe combined immunodeficiency mice. Anti-GITR (clone DTA-1), anti-TIGIT (clone 1G9), anti-PD-1 (clone RMP1-14), and anti-CD47 (clone: MIAP301) antibodies were purchased from Bioxcell. Resiquimod and the indoleamine 2,3-dioxygenase (IDO) inhibitor 1-methyl-d-tryptophan were purchased from Sigma-Aldrich.
Cell lines and mice
The A20 B-cell lymphoma line was obtained from the ATCC. The BL3750 B-cell lymphoma line was provided by Thomas Tedder (Duke University).13 The 4T1 cell line was provided by S. Strober (Stanford University). The 2F3 leukemia cell line was developed in our laboratory.14
Tumor cells were cultured in RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% heat-inactivated fetal calf serum (HyClone Laboratories), 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen Life Technologies), and 50 μM 2-ME (Sigma-Aldrich), as complete medium.
Female BALB/C mice (6-8 weeks old) and female C57Bl/6 mice (8-10 weeks old) were purchased from Charles River Laboratories. STING KO C57Bl/6 mice were purchased from the Jackson Laboratory and bred in-house. All mice were housed at the comparative medicine pavilion at Stanford University.
All experiments were conducted in accordance with Stanford University Animal Facility and National Institutes of Health guidelines.
Tumor transplantation and immunotherapy
A20 cells (5 × 106), WT BL3750 cells (106), STING Knockout (KO) BL3750 cells (106), or 2F3 cells (3 × 105) were implanted at 2 subcutaneous (SC) sites on the right and left sides of the abdomen. Treatment began when tumors reached 4 to 6 mm in largest diameter. STINGa, CpG and/or Resiquimod were injected intratumorally into 1 tumor site at indicated doses every other day for 3 injections (usually days 6, 8, and 10 after tumor inoculation) unless otherwise mentioned. Anti-GITR (50 μg), anti-PD-1 (100 μg), anti-OX40 (8 μg), or anti-CD47 (50 μg) were given SC or intraperitoneally (IP) on days 6, 8, and 10 after tumor implantation. IDO inhibitor (2 mg) was given twice a day by oral gavage for 2 weeks, 5 times a week, starting day 6 after tumor inoculation. Tumor growth was measured using a caliper and expressed as a volume (length by width by height). Mice were sacrificed when tumor reached 1.5 cm in the largest diameter or when tumor sites ulcerated.
Detection of tumor-specific T cells
A week after the end of treatment; spleens were made into single-cell suspensions, and the red blood cells were lysed. A total of 5 × 105 splenocytes were cocultured with 5 × 105 irradiated A20 cells or 4T1 cells plus anti-CD28 Ab (5 μg/mL; BD Biosciences) for 24 hours at 37°C and 5% CO2. Monensin (GolgiStop; BD Biosciences) was added during the last 5 hours of culture. Cells were stained with anti-CD8 FITC, anti-CD4 PerCP, and anti-CD44 APC (BD Biosciences). Intracellular IFN-γ expression was assessed using BD Cytofix/Cytoperm kit per instructions, and BD anti-IFN-γ PE. Cells were studied by flow cytometry on a BD FACSCalibur, and fetal calf serum files were stored and analyzed using Cytobank (www.cytobank.org).
CD4 and CD8 T-cell depletion
CD4 and/or CD8 T-cell depletion was achieved by IP injection of 500 μg anti-CD4 (clone GK1.5, BioXcell) and/or 200 μg anti-CD8 (clone 2.43, BioXcell) on day −2, day −1, day 1, and day 5 of treatment. Depletion was confirmed by flow cytometry of blood showing more than 95% depletion.
Flow cytometry
One day and 1 week posttreatment, the draining lymph nodes of the treated site and the distant tumor were harvested and made into single-cell suspension. Cells were stained with the LIVE/DEADFixable Green Dead Cell Stain Kit (Invitrogen). Then, cells were stained with panel 1 or panel 2 (supplemental Table 1) and analyzed by flow cytometry on a BD LSRII and Cytobank.
Results
STING immunotherapy requires STING expression by the host
STING is expressed on various types of somatic and immune cells, as well as cancer cells. In addition, STING activation in T- and B-cell lymphoma induce apoptosis.15,16 Concordantly, we found that STING was expressed in 7 of the 7 mouse cancer cell lines we tested (supplemental Figure 1A) and that both lymphoma cell lines, A20 and BL3750, were sensitive to the STINGa (supplemental Figure 1B-E).15,16
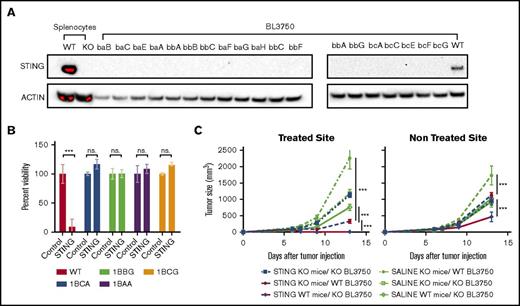
Because our work focuses on STINGa immunotherapeutic effect on lymphoma, we wanted to discriminate between the immune and the toxic effect of the STINGa in vivo. Therefore, we knocked out STING in the BL3750 B-cell lymphoma line, using CRISPR Cas9 (Figure 1A). As expected, the STING KO clones were resistant to STINGa stimulation in vitro (Figure 1B).
STING toxicity on lymphoma vs immunotherapeutic effect. (A) The Sting gene was knocked out of BL370 lymphoma cells using the CRISPR/cas9 system. Depicted is a western blot showing STING expression in BL3750 WT and STING KO clones, as well as splenocytes isolated from WT or STING KO mice. (B) Viability of WT and KO BL3750 cells after incubation with 20 µg/mL STINGa for 24 hours (n = 3). Statistical significance was calculated by a Student t test. Error bars are standard error of the mean (SEM). (C) STING knockout (KO) or wild-type (WT) BL3750 cells were implanted SC on STING KO or WT mice. Each mouse was implanted with 2 tumors, 1 on each side of the abdomen. At day 6, 8, and 10 after tumor implantation, 20 µg STINGa was injected into 1 tumor. Tumor growth of both the injected and distant tumors was monitored (5-10 mice per group). Statistical significance was calculated using 2 way analysis of variance (ANOVA). Error bars are SEM. ***P < .001. ns., not significant.
STING toxicity on lymphoma vs immunotherapeutic effect. (A) The Sting gene was knocked out of BL370 lymphoma cells using the CRISPR/cas9 system. Depicted is a western blot showing STING expression in BL3750 WT and STING KO clones, as well as splenocytes isolated from WT or STING KO mice. (B) Viability of WT and KO BL3750 cells after incubation with 20 µg/mL STINGa for 24 hours (n = 3). Statistical significance was calculated by a Student t test. Error bars are standard error of the mean (SEM). (C) STING knockout (KO) or wild-type (WT) BL3750 cells were implanted SC on STING KO or WT mice. Each mouse was implanted with 2 tumors, 1 on each side of the abdomen. At day 6, 8, and 10 after tumor implantation, 20 µg STINGa was injected into 1 tumor. Tumor growth of both the injected and distant tumors was monitored (5-10 mice per group). Statistical significance was calculated using 2 way analysis of variance (ANOVA). Error bars are SEM. ***P < .001. ns., not significant.
To evaluate the direct toxicity effect of the STINGa as an in situ vaccine, we implanted WT or STING KO lymphoma cells into either WT or STING KO mice. Two tumors were implanted in each mouse, 1 on each side of the abdomen. We injected STINGa or vehicle intratumorally (IT) into 1 tumor and monitored both sites to assess for local and distant effect. STINGa treatment had no effect on the STING KO mice bearing the STING KO tumors. In STING KO mice bearing the WT tumors, we observed temporary impaired growth of the injected tumor and delayed tumor growth at the distant site as compared with the vehicle-treated mice. In WT mice bearing the STING KO tumors, the treatment cleared the injected tumor, causing a necrotic black scar. In addition, the therapy delayed growth of the distant tumor (Figure 1C). These data show that clearance of the injected tumor and the systemic antitumor effect in WT mice are independent of STING expression on the tumor but require STING expression in the host.
Anti-OX40, CpG, Resiquimod, and anti-GITR improve STING in situ vaccination
IT injection of STINGa has been previously shown to have a dramatic effect on the injected tumor in a variety of transplanted tumor models.6,7
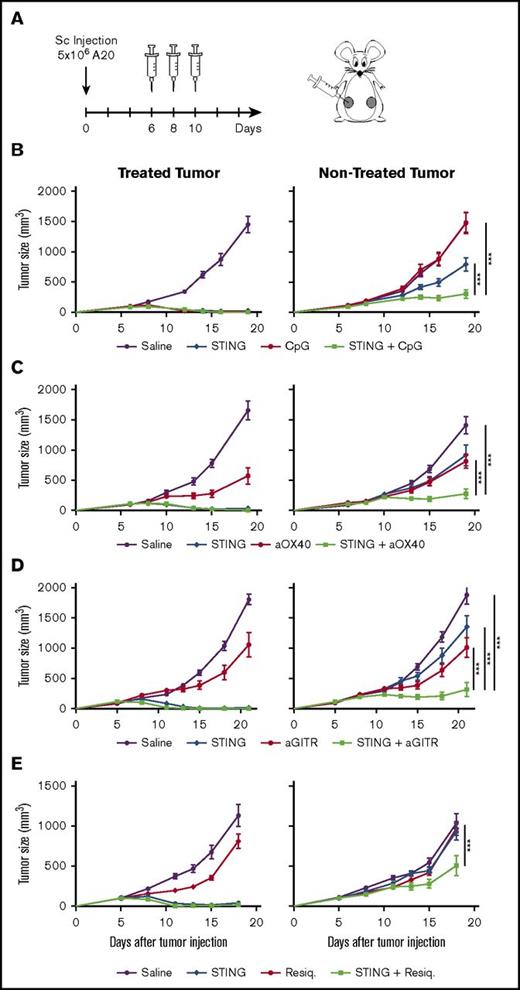
Here we sought to evaluate the induction of systemic antitumor immune responses. We employed a multiple-tumor model in which the same tumor is implanted at 2 different locations in the animal, with only 1 of the sites being treated and the others used as indicators of systemic immunity. In the current experiments, immunocompetent BALB/C mice were inoculated with the A20 B-cell lymphoma on each side of the abdomen. When tumors were palpable, the right tumor was used for IT injections of STINGa (Figure 2A).
STING in situ vaccination is improved when combined with CpG, anti-OX40, or anti-GITR. (A) Six- to 8-week-old BALB/C mice were implanted with 5 × 106 A20 cells on both flanks. One tumor was used as injection site and the other was monitored for systemic effect. Mice were treated on days 6, 8, and 10 after tumor implantation with IT injection of 5 μg STINGa and/or IT injection of 5 μg CpG (B); SC injection of 8 μg anti-OX40 antibodies (C); SC injection of 50 μg anti-GITR antibodies (D); IT injection of 5 μg Resiquimod (E). These experiments were reproduced at least twice. Error bars are SEM. Shown data are 1 representative experiment with 10 mice per group. Statistical significance of tumor growth was calculated by 2-way ANOVA. ***P < .001.
STING in situ vaccination is improved when combined with CpG, anti-OX40, or anti-GITR. (A) Six- to 8-week-old BALB/C mice were implanted with 5 × 106 A20 cells on both flanks. One tumor was used as injection site and the other was monitored for systemic effect. Mice were treated on days 6, 8, and 10 after tumor implantation with IT injection of 5 μg STINGa and/or IT injection of 5 μg CpG (B); SC injection of 8 μg anti-OX40 antibodies (C); SC injection of 50 μg anti-GITR antibodies (D); IT injection of 5 μg Resiquimod (E). These experiments were reproduced at least twice. Error bars are SEM. Shown data are 1 representative experiment with 10 mice per group. Statistical significance of tumor growth was calculated by 2-way ANOVA. ***P < .001.
First, we tested escalating doses ranging from 5 to 200 μg. Although STINGa induced complete regression of the injected (right) tumor, it only slightly affected the growth of the noninjected tumor even at the highest tested dose (supplemental Figure 2A-B). The tissue in and around the treated tumor turned into a necrotic black scar within 3 days, and this effect was dose-dependent (supplemental Figure 2C). We presume that this adverse effect was caused by the collapse of the tumor vasculature and subsequent tissue necrosis. We selected 5 μg as an optimal dose that allowed for the therapeutic effect on the nontreated tumor and that minimized the necrosis adverse effect at the injected site.
We next assessed the STING in situ vaccination in combination with T-cell or APC modulating agents in the 2-tumor model. Seven different agents (anti-OX40, anti-GITR, anti-CD47, anti-TIGIT, IDO inhibitors, CpG, and Resiquimod) were separately tested in combination with IT STINGa. The addition of anti-OX40, anti-GITR, Resiquimod, or CpG improved the systemic antitumor effect of STINGa. These combinations reduced tumor growth at the distant site as compared with each agent alone (Figure 2B-E). Mice that were cured by these treatments were protected from tumor rechallenge, indicating a memory immune response (data not shown). In contrast, the addition of the IDO inhibitors, anti-TIGIT or anti-CD47monoclonal antibodies, had no beneficial effect on the STING vaccination. Interestingly IDO inhibitors even had an adverse effect on tumor clearance at the treated site (data not shown).
STINGa has to be injected locally: either intratumorally or peritumorally
Because both the STINGa and anti-GITR are being tested individually in clinical trials, we reasoned that the combination of these 2 agents would be logical eventually to be tested in the clinic. Therefore, we decided to study this particular combination further.
To optimize the treatment, we tested different routes of injections. We also evaluated the treatment in other tumor models.
First, we tested local (IT) vs systemic (distant SC) injections of the anti-GITR antibody. No significant difference was found between these 2 routes of administration. Systemic injection of anti-GITR was selected for further investigation because it is already being studied this way in the clinic (supplemental Figure 3A-B).
To confirm that the systemic antitumor effect is a result of in situ vaccination, we compared the therapeutic potential of local and distant injections of the STINGa. We injected the STINGa IT, peritumorally (PT) or at a distant SC site. Results showed that the STINGa has to be injected locally but not necessarily into the tumor to induce a systemic effect. The systemic effect induced by IT and PT injection of STINGa were similar, whereas the effect was mainly lost when the STINGa was administered at a distant site. However, the local effect of IT and PT injection was different; PT injection dramatically reduced the growth of the local tumor but did not clear it, whereas IT injection turned the tumor into a scar and cleared it (supplemental Figure 3C-D). Also, no scar was observed when the STINGa was injected PT or at a distant SC site. The fact that PT injection worked as well suggests that the STINGa targets APCs in the regional draining lymph node to induce a systemic antitumor effect.
We evaluated the treatment against 2 other aggressive tumor cell lines: the BL3750 B-cell line and the 2F3 pro-B leukemia cell line. In both models, the treatment delayed tumor growth at the distant site (supplemental Figure 3E-F).
The antitumor effect of STINGa and anti-GITR is T-cell mediated
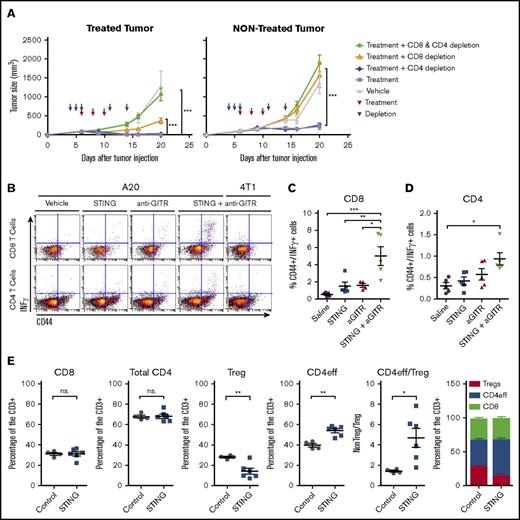
As single therapies, both anti-GITR and IT STINGa treatments were reported to induce tumor-specific T-cell responses.6,17 To assess the role of T cells and their subsets in the combination, we depleted CD4+, CD8+, or both subsets from the host, using appropriate monoclonal antibodies. Tumor growth at the injected site and the distant site were compared across the different groups. The local and systemic antitumor response required CD8+ T cells because depletion of CD8+ T cells impaired the therapeutic effect of the combination at the treated and distant site (Figure 3A). However, depletion of CD4+ T cells did not affect the local and systemic antitumor effect.
The systemic antitumor response is T-cell mediated. BALB/C mice were implanted with 5 × 106 A20 cells on both flanks. (A) Local (treated site) and systemic effect of the treatment in the context of CD4 and/or CD8 T-cell depletion. Mice were treated with IT injection of STINGa and SC injection of anti-GITR. Treatment was given on day 6, 8, and 10 after tumor implantation. Depleting antibodies for CD4 and/or CD8 T-cell depletion were given on day 4, 5, 6, 11, and 14. One experiment with 10 mice per group. Statistical significance of tumor growth was calculated using 2-way ANOVA. (B-D) Tumor-specific CD4 and CD8 T cells in the spleen. On day 6, 8, and 10, mice were treated with IT injection of STINGa and/or SC injection of anti-GITR. On day 17, spleens were harvested. INF-γ–producing cells were monitored after restimulation with tumor cells (A20) or unrelated tumor cells (4T1). (B) Raw data of representative samples. (C) Percentage of CD44+INF-γ+ cell among the CD8 T cells. Statistical significance was calculated using 1-way ANOVA. (D) Percentage of CD44+INF-γ+ cell among the CD4 T cells. Statistical significance was calculated using 1-way ANOVA. (E) Treated tumor were harvested at 2 and 4 hours after IT injection of STINGa or vehicle control. Graphs illustrate percentage of CD8 T cell, CD4 T cell, Tregs (CD4+FoxP3+), and CD4eff (CD4+FoxP3−). Statistical significance was calculated using 1-way ANOVA. Error bars are SEM. *P < .05; **P < .01; ***P < .001.
The systemic antitumor response is T-cell mediated. BALB/C mice were implanted with 5 × 106 A20 cells on both flanks. (A) Local (treated site) and systemic effect of the treatment in the context of CD4 and/or CD8 T-cell depletion. Mice were treated with IT injection of STINGa and SC injection of anti-GITR. Treatment was given on day 6, 8, and 10 after tumor implantation. Depleting antibodies for CD4 and/or CD8 T-cell depletion were given on day 4, 5, 6, 11, and 14. One experiment with 10 mice per group. Statistical significance of tumor growth was calculated using 2-way ANOVA. (B-D) Tumor-specific CD4 and CD8 T cells in the spleen. On day 6, 8, and 10, mice were treated with IT injection of STINGa and/or SC injection of anti-GITR. On day 17, spleens were harvested. INF-γ–producing cells were monitored after restimulation with tumor cells (A20) or unrelated tumor cells (4T1). (B) Raw data of representative samples. (C) Percentage of CD44+INF-γ+ cell among the CD8 T cells. Statistical significance was calculated using 1-way ANOVA. (D) Percentage of CD44+INF-γ+ cell among the CD4 T cells. Statistical significance was calculated using 1-way ANOVA. (E) Treated tumor were harvested at 2 and 4 hours after IT injection of STINGa or vehicle control. Graphs illustrate percentage of CD8 T cell, CD4 T cell, Tregs (CD4+FoxP3+), and CD4eff (CD4+FoxP3−). Statistical significance was calculated using 1-way ANOVA. Error bars are SEM. *P < .05; **P < .01; ***P < .001.
In addition, we investigated the specificity of the T-cell response. CD8+ T cells isolated from mice treated with the combination responded to reexposition to A20 cells as assessed by IFN-γ measurement. In a lesser extent, CD4+ T cells from mice treated with the combination also responded to restimulation with A20 cells (Figure 3B-D).
Furthermore, we studied the phenotypes of the tumor-infiltrating T cells in the treated tumor after injection of the STINGa (Figure 3E). We found that the overall percentage of CD8+ and CD4+ T cells did not change as compared with the vehicle-treated tumors. However, we did note a lower percentage of CD4+FoxP3+ Tregs cells in the STINGa-treated tumors, suggesting that tumor-infiltrating Tregs might lose FoxP3 expression after STINGa treatment.
T-cell response starts in the draining lymph node and spreads to the distant tumor
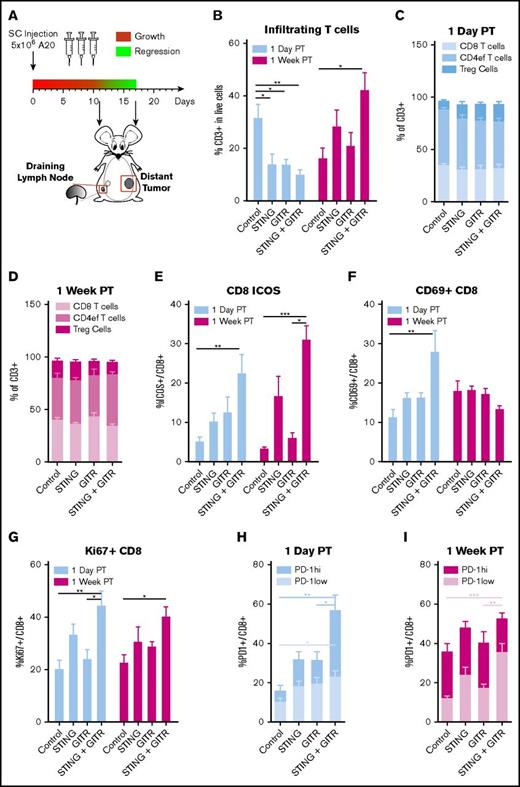
In an attempt to understand the immune mechanisms underlying the therapeutic synergy between STINGa and anti-GITR, we analyzed the draining lymph node of the treated tumor and the tumor-infiltrating cells at the distant site by flow cytometry. As illustrated by Figure 4A, we focused our analysis on 2 points; 1 day after the last injection, just before the distant tumor begins to respond, and 1 week after the last injection, when the distant tumor has already regressed.
Dissection of the immune response in the nontreated tumor. Mice bearing 2 A20 tumors were treated as in Figure 1C with IT STINGa and/or SC anti-GITR. One day posttreatment and 1 week posttreatment, mice were sacrificed and the draining lymph node of the treated site and the nontreated tumor were excised for cell population analysis. (A) Schedule of injection and organs harvest. (B-I) Cell populations in the nontreated tumor. (B) Infiltrating T cells. T-cell subsets among the T cells 1 day posttreatment (C) and 1 week posttreatment (D). Percentage of ICOS+ (E), CD69+ (F), Ki67+ (G) among CD8 T cells. Percentage of PD-1hi and PD1low among CD8 T cells 1 day posttreatment (H) and 1 week posttreatment (I). Represented are data pulled from 2 experiments with 3 mice per group. Error bars are SEM. Statistical significance was calculated using 1-way ANOVA. *P < .05; **P < .01; ***P < .001.
Dissection of the immune response in the nontreated tumor. Mice bearing 2 A20 tumors were treated as in Figure 1C with IT STINGa and/or SC anti-GITR. One day posttreatment and 1 week posttreatment, mice were sacrificed and the draining lymph node of the treated site and the nontreated tumor were excised for cell population analysis. (A) Schedule of injection and organs harvest. (B-I) Cell populations in the nontreated tumor. (B) Infiltrating T cells. T-cell subsets among the T cells 1 day posttreatment (C) and 1 week posttreatment (D). Percentage of ICOS+ (E), CD69+ (F), Ki67+ (G) among CD8 T cells. Percentage of PD-1hi and PD1low among CD8 T cells 1 day posttreatment (H) and 1 week posttreatment (I). Represented are data pulled from 2 experiments with 3 mice per group. Error bars are SEM. Statistical significance was calculated using 1-way ANOVA. *P < .05; **P < .01; ***P < .001.
The immune response seemed to be initiated in the draining lymph node of the treated tumor. Then, it spread to the distant tumor. In the lymph node, there were more activated cells 1 day posttreatment than 1 week posttreatment (Figure 5), whereas the opposite was observed for the distant tumor (Figure 4).
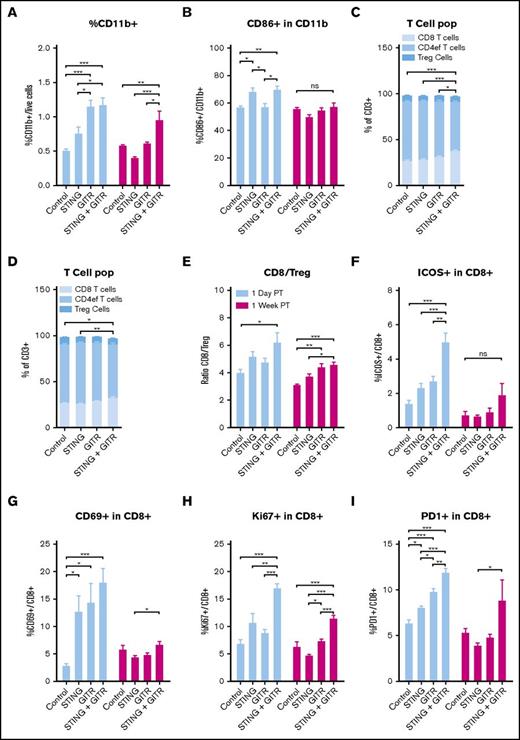
Dissection of the immune response in the draining lymph node of the treated tumor. Cell population in the draining lymph node of treated tumor harvested as described in Figure 4A. (A) Percentage of CD11b+ cells. (B) CD86+ cells among CD11b+ cells. T-cell subset 1 day posttreatment (C) and 1 week posttreatment (D). (E) CD8/Treg ratio. ICOS+ (F) CD60+ (G), Ki67+ (H), PD-1+ (I) positive cells among CD8 T cells. Represented are data pulled from 2 experiments with 3 mice per group. Error bars are SEM. Statistical significance was calculated using 1-way ANOVA. *P < .05; **P < .01; ***P < .001.
Dissection of the immune response in the draining lymph node of the treated tumor. Cell population in the draining lymph node of treated tumor harvested as described in Figure 4A. (A) Percentage of CD11b+ cells. (B) CD86+ cells among CD11b+ cells. T-cell subset 1 day posttreatment (C) and 1 week posttreatment (D). (E) CD8/Treg ratio. ICOS+ (F) CD60+ (G), Ki67+ (H), PD-1+ (I) positive cells among CD8 T cells. Represented are data pulled from 2 experiments with 3 mice per group. Error bars are SEM. Statistical significance was calculated using 1-way ANOVA. *P < .05; **P < .01; ***P < .001.
One day posttreatment, the draining lymph nodes in the treated groups were more prominent in size and contained 3 to 5 times more cells than those from the vehicle group. Anti-GITR treatment induced a greater percentage of APCs (CD11b+; Figure 5A), whereas the STINGa increased the proportion of activated APCs (CD86+; Figure 5B). The combination treatment increased T-cell activation and proliferation. The combo treated lymph nodes had an increased proportion of CD8+ T cells among the CD3+ cells (Figure 5C), which resulted in an increase in the CD8/Treg ratio (Figure 5E). Furthermore, the combination treatment was associated with increased activation of CD8+ T cells, as noted by higher percentage of cells expressing CD69, Ki67, and ICOS, as well as a low level of PD-1 (Figure 5F-I). In addition, we observed an increased percentage of activated B cells after anti-GITR treatment (supplemental Figure 4).
In the distant tumor, the combo treatment increased CD8+ T-cell activation as early as 1 day posttreatment, as indicated by higher percentages of ICOS+, Ki67+, and CD69+ cells among the CD8+ T cells (Figure 4E-G). However, T-cell infiltration increased 1 week posttreatment (Figure 4B). In addition, the percentage of cells expressing low levels of PD-1 was higher 1 week posttreatment (Figure 4I).
These data suggest that the combination treatment induced T-cell activation and expansion, which were initiated by a higher number of activated APCs in the draining lymph node of the treated tumor.
Extending in situ vaccination improves the immune antitumor effect
The flow cytometry analysis of the draining lymph node of the treated tumor suggests that the immune response was diminishing 1 week after treatment. Therefore, we investigated whether prolonging the antitumor response by extending the treatment would enhance the systemic effects.
Using the 2-tumor animal model, mice were treated by IT injection of STINGa and SC injection of anti-GITR, as described in Figure 1C. Mice were treated for 1 cycle of 3 injections every other day or for 3 such cycles separated by a 2-day gap (Figure 6A). Extending the treatment to 3 cycles did not improve the outcome at the distant site (Figure 6B).
Expending in situ vaccination in a second treated site improve the treatment. (A-B) Six- to 8-week-old BALB/C mice were implanted with 5 × 106 A20 cells on both flanks. One tumor was used as injection site and the other was monitored for systemic effect. Mice were treated with IT injection of 5 μg STINGa and SC injection of 50 μg anti-GITR. Mice were treated every other day 3 or 9 times. (A) Schedule of injections. (B) Growth curve of the treated and nontreated tumor. Data represent 1 experiment with 10 mice per group. (C-D) Six- to 8-week-old BALB/C mice were implanted with 5 × 106 A20 cells at 3 sites on the abdomen. Two tumors were used as injection site; the tired 1 was monitored for systemic effect. Mice were treated with IT injection of 1 μg STINGa and SC injection of 10 μg anti-GITR antibodies. One group of mice was treated every other day 3 times in the tumor 1. A second group of mice was treated 3 times every other day in tumor 1 and then received another 3 injections every other day in the second tumor. (C) Schedule of the injection. (D) Growth curve of the 3 tumors and survival of the mice. Data represent 1 experiment with 10 mice per group. Error bars are SEM. Statistical significance of tumor growth was calculated using 2-way ANOVA. Survival significance was calculated using Mantel-Cox. *P < .05; ***P < .001.
Expending in situ vaccination in a second treated site improve the treatment. (A-B) Six- to 8-week-old BALB/C mice were implanted with 5 × 106 A20 cells on both flanks. One tumor was used as injection site and the other was monitored for systemic effect. Mice were treated with IT injection of 5 μg STINGa and SC injection of 50 μg anti-GITR. Mice were treated every other day 3 or 9 times. (A) Schedule of injections. (B) Growth curve of the treated and nontreated tumor. Data represent 1 experiment with 10 mice per group. (C-D) Six- to 8-week-old BALB/C mice were implanted with 5 × 106 A20 cells at 3 sites on the abdomen. Two tumors were used as injection site; the tired 1 was monitored for systemic effect. Mice were treated with IT injection of 1 μg STINGa and SC injection of 10 μg anti-GITR antibodies. One group of mice was treated every other day 3 times in the tumor 1. A second group of mice was treated 3 times every other day in tumor 1 and then received another 3 injections every other day in the second tumor. (C) Schedule of the injection. (D) Growth curve of the 3 tumors and survival of the mice. Data represent 1 experiment with 10 mice per group. Error bars are SEM. Statistical significance of tumor growth was calculated using 2-way ANOVA. Survival significance was calculated using Mantel-Cox. *P < .05; ***P < .001.
After the first round of treatment, the injected tumor disappeared and turned into a scar. Therefore, there were no more tumor antigens available at the treated site for the second cycle of STING vaccination. As a consequence, we hypothesized that having an additional tumor site for injecting the STINGa during the second cycle would improve the results. To test this hypothesis, we implanted 3 A20 tumors on the abdomen of mice. Two tumor sites were used for IT injection, whereas the third tumor site was used to evaluate the systemic antitumor immune response (Figure 6C). In this scheme, the tumor growth at the third site showed a higher growth delay in mice that received 2 consecutive cycles of treatment compared with mice receiving only 1 cycle of treatment (Figure 6D). Also, mice treated for 2 cycles survived significantly longer than mice treated for only 1 cycle. From these data, we can conclude that the antitumor effects of the STINGa may be extended by repeated cycles (ie, IT injection of different sites).
PD-1 blockade improves anti-GITR + STING combination
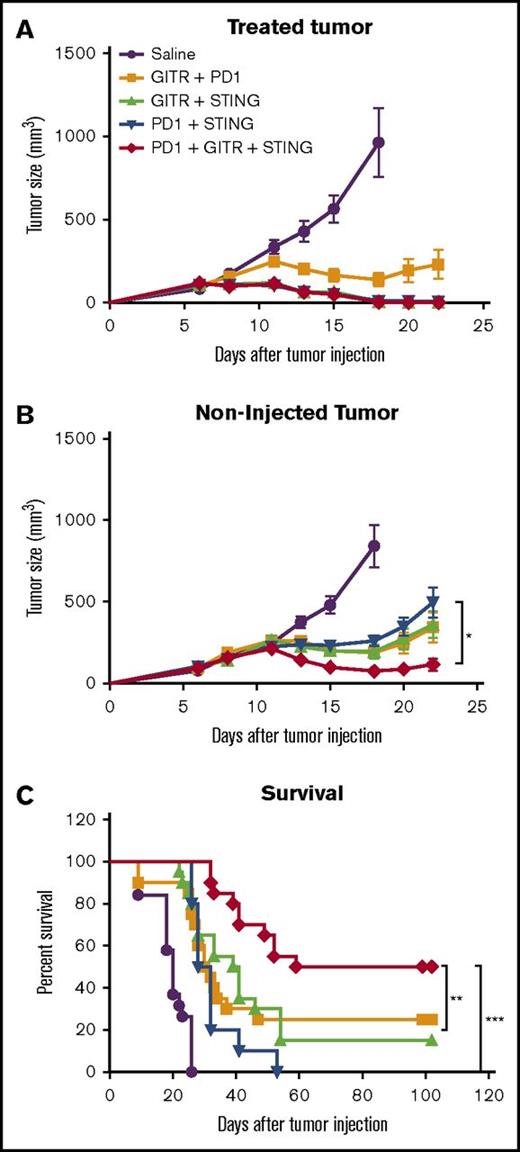
After STINGa and anti-GITR treatment, we observed an increase in PD-1-expressing cells in the local lymph node and the distant tumor. PD-1 has been described as a marker for activation and exhaustion. In animal models, T cells expressing a low level of PD-1 are mostly activated cells, whereas T cells expressing high levels of PD-1 are unresponsive and considered exhausted.18 As shown, in the lymph node, PD-1 expression on T cells was low; however, in the tumor, CD8+ T cells were subdivided into PD-1hi and PD-1low. This might suggest that activated T cells that originated from the lymph node may be inhibited in the tumor because of the interaction of PD-1 with PD-L1 expressed on tumor cells.19 Therefore, we assessed whether the addition of PD-1 blockade could enhance our therapy. Using the 2 A20 tumor model as described in Figure 1C, mice were treated with either 2 or 3 agents consisting of IT injection of STINGa, IP injection of anti-PD-1, and IP injection of anti-GITR. The triple combination showed an improved tumor control at the distant site as compared with any double combination (Figure 7B). This was also reflected by an improvement in survival for the tri-therapy as compared with any bi-therapies (Figure 7C).
PD-1 blockade improve STING + anti-GITR therapy. Mice bearing 2 A20 tumors were treated as described in Figure 1C. Mice were treated with IT STINGa and IP anti-PD-1, IT STING and IP anti-GITR, IP anti-GITR and IP anti-PD-1, or, the triple combination, IT STINGa, IP anti-GITR and IP anti-PD-1. Treatment efficacy was assessed measuring tumor growth at the treated site (A), tumor growth at the distant nontreated site (B), and overall survival (C). (A-B) One experiment with 10 mice per group. Experiment was repeated twice. Error bars are SEM. Statistical significance of tumor growth was calculated using 2-way ANOVA. (C) Data pulled from 2 experiment with 10 mice per group. Survival significance was calculated using Mantel-Cox. *P < .05; **P < .01; ***P < .001.
PD-1 blockade improve STING + anti-GITR therapy. Mice bearing 2 A20 tumors were treated as described in Figure 1C. Mice were treated with IT STINGa and IP anti-PD-1, IT STING and IP anti-GITR, IP anti-GITR and IP anti-PD-1, or, the triple combination, IT STINGa, IP anti-GITR and IP anti-PD-1. Treatment efficacy was assessed measuring tumor growth at the treated site (A), tumor growth at the distant nontreated site (B), and overall survival (C). (A-B) One experiment with 10 mice per group. Experiment was repeated twice. Error bars are SEM. Statistical significance of tumor growth was calculated using 2-way ANOVA. (C) Data pulled from 2 experiment with 10 mice per group. Survival significance was calculated using Mantel-Cox. *P < .05; **P < .01; ***P < .001.
Discussion
We have shown that STING in situ vaccination can be enhanced by T cell or APC modulation in syngeneic animal models. We examined the treated and distant tumors as well as the draining lymph node to better understand the mechanism of the immune response.
The underlying antitumor mechanism of the STINGa is still unclear. Previous studies in solid tumors have demonstrated the involvement of APCs and endothelial cells, as well as type 1 IFN signaling.6,7 Unlike in solid tumors, STING stimulation in lymphoma cells leads to apoptosis.15,16 We have demonstrated that clearance of the injected tumor is entirely dependent on STING activation in the host cells and that the direct toxicity of STINGa on lymphoma cells has only a minor effect. In accordance with observations by others, we have shown that tumor regression at the treated site is T-cell mediated6 and is associated with an early decrease in CD4+FoxP3+ T cell in the treated tumor.
The STING pathway was shown to contribute to cellular invasion and metastases in cancer with chromosomal instability.20 According to these findings, STINGa treatment could potentially augment tumor metastases. However, we did not observe increased dissemination of tumor cells after STINGa treatment in our in vivo models. In addition, STINGa treatment was shown to decrease metastasis burden in 4T1 and B16F10 tumor model.6,21
In our screen for compounds that could enhance STING in situ vaccination, we tested APC stimulators (anti-CD47, CpG, and Resiquimod) and T-cell modulators (any-OX40, anti-GITR, anti-TIGIT, and IDO inhibitors). Among these, CpG, Resiquimod, anti-OX40, and anti-GITR showed efficacy, indicating that modulating APCs or T cells can enhance STING in situ vaccination. Some of these combinations have been studied by others. Temizoz et al showed that combination of TLR9 and STINGa is a potent type 1 adjuvant that induces a cytotoxic CD8+ T-cell response after OVA vaccination and that IT injection of the combination reduced the size of the injected tumor.9 Foote et al showed that adding PD-L1 blockade to OX40 receptor activation and STING in situ vaccination overcomes immune tolerance and induces breast tumor regression in tolerized neu/N mice.10
Here we demonstrated that the combination of STINGa and GITR modulation stimulated a local and systemic T-cell response. Our data suggest that the STINGa activated APCs in the draining lymph node of the treated site, which, together with GITR costimulation, induced activation and proliferation of T and B cells in this lymph node. Then, activated T cells migrated from this lymph node to the distant tumor and attacked the tumor cells, resulting in tumor regression. However, at the distant tumor site, these activated T cells are soon inhibited by the interaction between PD-1 that is expressed on the T cell, and PD-L1 expressed on tumor cells. T cells with high PD-1 expression are reported to be fully exhausted and unable to eliminate cancers.18 Mice treated with the combination of STINGa and anti-GITR had a higher proportion of PD-1low CD8+ T cells. This subset of T cells was shown to be the 1 responding to PD-1 blockade.22 Blocking PD-1/PD-L1 interaction using an anti-PD-1 antibody was able to enhance the therapeutic effect of the STINGa and anti-GITR combination and cure half the mice.
STING in situ vaccination combined with GITR and PD-1 modulation is a promising therapy for lymphoma. Intratumoral injection of the STINGa induces local toxicity, but this could be avoided by peritumoral injection.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Aduro Biotech for providing the STING agonist and Dynavax for providing the CpG oligonucleotides. The authors also thank D. Czerwinski for excellent technical support with flow cytometry.
This work was supported by the National Institutes of Health, National Cancer Institute (R35CA197353). A.S. was supported by fellowships from the Belgian American Education Foundation and the Wallonie-Bruxelles International.
Authorship
Contribution: A.S. designed and performed experiments, analyzed data, and wrote the paper; S.R. performed experiments and analyzed data; A.K. performed experiments and analyzed data; and R.L. designed experiments, reviewed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.R. is Clinical Operations, Roche Genentech, South San Francisco, CA.
Correspondence: Ronald Levy, Division of Oncology, Department of Medicine, Stanford University, 269 Campus Dr, Stanford, CA 94305; e-mail: levy@stanford.edu.