Key Points
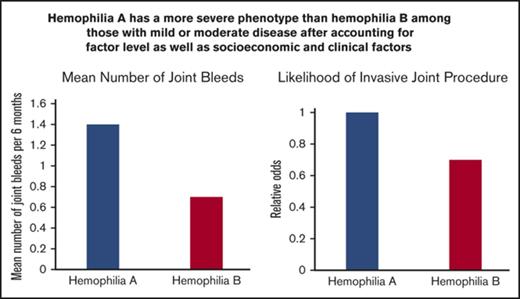
Joint hemorrhages and orthopedic procedures are more frequent in patients with hemophilia A than in those with hemophilia B.
FA levels of 20% may be required to prevent all joint hemorrhages.
Abstract
Data are needed on minimal factor activity (FA) levels required to prevent bleeding in hemophilia. We aimed to evaluate associations between hemophilia type and FA level and joint bleeding and orthopedic procedures using longitudinal data. Data were collected over an 11-year period on males with nonsevere hemophilia A or B without inhibitors who were receiving on-demand factor replacement therapy. Data on the number of joint bleeds in the previous 6 months and data on procedures from clinical records were analyzed using regression models. Data were collected on 4771 patients (hemophilia A, 3315; hemophilia B, 1456) from 19 979 clinic visits. Ages ranged from 2 to 91 years and baseline FA level ranged from 1% to 49% with a mean of 9.4%. Joint bleeding rates were heterogeneous across the FA range and were highest among men age 25 to 44 years. Adjusted for FA level, the mean number of joint bleeds per 6 months was 1.4 and 0.7 for patients with hemophilia A and B, respectively (P < .001). Regression models predicted 1.4 and 0.6 bleeds per year for hemophilia A and B patients, respectively, at an FA level of 15%. Patients with hemophilia B were 30% less likely than those with hemophilia A to have undergone an orthopedic procedure. We conclude that joint bleed rates for any given FA level were higher among hemophilia A than hemophilia B patients, and target FA levels of 15% are unlikely to prevent all joint bleeding in US males with hemophilia.
Introduction
Hemophilia A and B are clinically indistinguishable congenital bleeding disorders caused by the X-linked deficiency of coagulation factor VIII (FVIII, hemophilia A) or factor IX (FIX, hemophilia B). The bleeding phenotype (the frequency and severity of bleeding into tissues, organs, and joints) is generally related to the severity of the deficiency. Therefore, definitions of hemophilia severity have been assigned by coagulation factor activity (FA), with severe being <1%, moderate being 1% to 5%, and mild being >5% FA.1 However, heterogeneity in bleeding patterns between individuals with similar factor levels has long been recognized. In addition, recent data suggest that hemophilia B is associated with a less severe bleeding phenotype than hemophilia A, although the data are inconsistent between studies.2-6
In a study by den Uijl et al,7 rates of joint bleeding were found to decrease by 18% for each 1% increase in baseline FA level among 433 Dutch patients with moderate or mild hemophilia A who were receiving on-demand therapy. Limitations of the study were the small number of patients studied, the single data capture point for each patient, and the lack of data from patients with hemophilia B. Nonetheless, the study findings along with advances in therapeutic agents support a shift in the aspirational goal for hemophilia therapy to achieve hemostatic replacement that may result in an absence of joint bleeds for all.8,9
Since 1998, the Centers for Disease Control and Prevention (CDC) has been monitoring the health of people with bleeding disorders who receive care in federally supported hemophilia treatment centers (HTCs) through a public health surveillance system initially called the Universal Data Collection (UDC) system.10 Treatment and outcome data collected repeatedly from surveillance participants at annual comprehensive care visits provided a unique opportunity to compare rates of bleeding and orthopedic procedures among men and boys with hemophilia A and B and to make adjustments for severity on the basis of baseline FA, thus overcoming limitations of previous studies. The purpose of the study was to investigate the effects of hemophilia type and FA level on rates of joint bleeding and orthopedic procedures at the same time, accounting for other potential contributors to the severity phenotype.
Materials and methods
Details of the UDC surveillance system have been previously published.10 Briefly, people with bleeding disorders who received care in a network of nearly 130 federally funded US HTCs could participate in UDC at annual visits that took place between May 1998 and September 2011. Data on treatment and outcomes were collected by HTC staff using standardized tools, and a blood specimen was collected for infectious disease testing at CDC. All patients or parents of minor children gave informed consent for participation, and the surveillance was monitored by the institutional review boards of CDC and of every participating institution. Information on how to access UDC data can be found on the CDC Web site (https://www.cdc.gov/ncbddd/blooddisorders/udc/aboutus.html).
Study population
Males age 2 years or older with hemophilia A or B and FA between 1% and 49% were eligible for inclusion. The study population included all eligible patients who were exclusively treated with episodic (on-demand) therapy and had 2 or more UDC visits during the study period (1 January 2000 to 31 December 2010). Data collected on the results of local testing or treatment of an inhibitor during the study period were used to exclude all patients with any evidence of an inhibitor.
Data collection
At enrollment into the surveillance program, data were collected on baseline FA, self-reported race/ethnicity, and date of birth from clinical records. For the analyses, race/ethnicity was categorized as white non-Hispanic, African American non-Hispanic, Hispanic (white or African American race), and other.
During annual comprehensive clinic visits, treatment information as well as outcomes data were collected by HTC staff. Information was collected on the source of health insurance and categorized as commercial, Medicaid, or Medicare (government sponsored on the basis of income, disability, or age), other types of insurance (eg, military, state-sponsored), or uninsured as a measure of socioeconomic status.
Height and weight data from the clinic visit were used to calculate body mass index (BMI) which was then categorized as underweight, normal, overweight, or obese according to established guidelines.11 The results of testing for HIV1, hepatitis C, and hepatitis B performed at CDC on blood specimens collected on all participants as part of the surveillance were used to determine status regarding infectious disease. Hepatitis B positivity was based on a positive test for hepatitis B surface antigen.
The number of bleeding episodes involving joints experienced by the patient during the 6-month period before the clinic visit were collected from patient bleed logs, if available, or based on patient or parent recall. Data were collected from clinic or medical records on the occurrence of any invasive orthopedic procedures performed during the previous year.
Data analysis
We used the information collected on bleeding episodes from all visits because data from multiple visits were likely to more accurately reflect the true bleeding phenotype for the patient. Thus, for each participant, we calculated the 6-month average bleeding rate based on number of self-reported bleeds for the past 6 months from all annual comprehensive visit forms during the study period. Similarly, the average age for each patient over the course of all UDC visits was calculated and was used to assign age group in the analyses. Age groups based on standard 5-year intervals were collapsed into 4 age groups that generally correspond with the life stages of children, youth, adults, and older adults.
Bivariate associations between the demographic and clinical characteristics (including baseline FA) and the average number of joint bleeds across all visits were assessed using analysis of variance. Similarly, associations between these characteristics and the proportion of participants who had any invasive procedure during the study period were assessed for statistical significance using χ2 tests.
The independent association between the number of joint bleeds, FA, and hemophilia type was assessed using several regression models, some using repeated measures and others using the average number of joint bleeds from all visits for each patient along with various distribution assumptions. On the basis of the results of these modeling experiments and the distribution of the joint bleeds data, the model that best fit the data was a multiple linear regression analysis using the mean joint bleeds from all visits for each patient and assuming a negative binomial distribution. In a separate analysis, we used a logistic regression model to evaluate the odds of having had an invasive joint procedure at any time during the study period. The regression model covariates are listed in Tables 2 and 4. All statistical analyses were conducted using SAS statistical software (SAS Institute, Cary, NC), and P ≤ .05 was considered statistically significant.
Results
A total of 7941 males who were age 2 years or older with mild or moderate hemophilia had at least 1 UDC visit during the study period. Of these, 5495 (69%) had multiple UDC visits, and 4771 (87%) were exclusively receiving on-demand therapy and formed the study cohort. The study cohort had 19 979 clinic visits with an average of 4.2 visits per patient during the 12-year study period.
The clinical and sociodemographic characteristics of the study patients are provided in Table 1. The distribution of sociodemographic characteristics of the study participants was similar for those with hemophilia A or hemophilia B (data not shown). Nearly 60% of both cohorts were younger than age 24 years and had commercial health insurance. About 75% of participants were white, and nearly half were either overweight or obese.
Associations between demographic and clinical characteristics and joint bleeds in 4771 males with mild or moderate hemophilia
| Characteristic . | No. (%) . | Mean no. of joint bleeds per 6 months . | Invasive procedure, % . |
|---|---|---|---|
| Age group, y | |||
| 2-9 | 1019 (21.4) | 0.38* | 0.3* |
| 10-24 | 1744 (36.6) | 1.20 | 2.9 |
| 25-44 | 911 (19.1) | 2.00 | 11.1 |
| 45+ | 900 (18.9) | 1.09 | 13.9 |
| Race/ethnicity | |||
| White | 3589 (75.2) | 1.10* | 6.6* |
| African American | 362 (7.6) | 2.12 | 8.3 |
| Hispanic | 568 (11.9) | 0.82 | 2.8 |
| Other | 252 (5.3) | 1.39 | 2.0 |
| Insurance | |||
| Commercial | 2853 (59.8) | 1.01* | 5.9 |
| Medicaid/Medicare | 1225 (25.7) | 1.54 | 7.4 |
| Other | 334 (7.0) | 1.00 | 4.2 |
| Uninsured | 334 (7.0) | 1.34 | 5.1 |
| BMI | |||
| Underweight | 21 (0.4) | 1.75 | 14.3* |
| Normal | 2385 (50.0) | 1.13 | 3.4 |
| Overweight | 1145 (24.0) | 1.22 | 9.3 |
| Obese | 1037 (21.7) | 1.06 | 7.3 |
| Hemophilia type | |||
| FVIII deficiency | 3315 (69.5) | 1.28* | 6.5† |
| FIX deficiency | 1456 (30.5) | 0.89 | 5.0 |
| Hemophilia severity | |||
| Moderate | 2049 (43.0) | 2.05* | 8.0* |
| Mild | 2722 (57.0) | 0.49 | 4.6 |
| Baseline FA level, % | |||
| 1-2 | 830 (17.4) | 2.97* | 9.3* |
| 3-5 | 1268 (26.6) | 1.41 | 7.1 |
| 6-9 | 952 (20.0) | 0.68 | 5.2 |
| 10-14 | 733 (15.4) | 0.44 | 4.0 |
| 15-24 | 661 (13.8) | 0.33 | 4.2 |
| 25-40 | 299 (6.3) | 0.33 | 5.0 |
| 41-49 | 28 (0.6) | 0.29 | 3.6 |
| HIV status | |||
| Negative | 4495 (94.2) | 1.03* | 5.4* |
| Positive | 247 (5.2) | 3.63 | 19.0 |
| Hepatitis C status | |||
| Negative | 3309 (69.4) | 0.71* | 3.0* |
| Positive | 1437 (30.1) | 2.22 | 13.2 |
| Hepatitis B status‡ | |||
| Negative | 4707 (98.7) | 1.12* | 5.9* |
| Positive | 64 (1.3) | 4.00 | 20.3 |
| Characteristic . | No. (%) . | Mean no. of joint bleeds per 6 months . | Invasive procedure, % . |
|---|---|---|---|
| Age group, y | |||
| 2-9 | 1019 (21.4) | 0.38* | 0.3* |
| 10-24 | 1744 (36.6) | 1.20 | 2.9 |
| 25-44 | 911 (19.1) | 2.00 | 11.1 |
| 45+ | 900 (18.9) | 1.09 | 13.9 |
| Race/ethnicity | |||
| White | 3589 (75.2) | 1.10* | 6.6* |
| African American | 362 (7.6) | 2.12 | 8.3 |
| Hispanic | 568 (11.9) | 0.82 | 2.8 |
| Other | 252 (5.3) | 1.39 | 2.0 |
| Insurance | |||
| Commercial | 2853 (59.8) | 1.01* | 5.9 |
| Medicaid/Medicare | 1225 (25.7) | 1.54 | 7.4 |
| Other | 334 (7.0) | 1.00 | 4.2 |
| Uninsured | 334 (7.0) | 1.34 | 5.1 |
| BMI | |||
| Underweight | 21 (0.4) | 1.75 | 14.3* |
| Normal | 2385 (50.0) | 1.13 | 3.4 |
| Overweight | 1145 (24.0) | 1.22 | 9.3 |
| Obese | 1037 (21.7) | 1.06 | 7.3 |
| Hemophilia type | |||
| FVIII deficiency | 3315 (69.5) | 1.28* | 6.5† |
| FIX deficiency | 1456 (30.5) | 0.89 | 5.0 |
| Hemophilia severity | |||
| Moderate | 2049 (43.0) | 2.05* | 8.0* |
| Mild | 2722 (57.0) | 0.49 | 4.6 |
| Baseline FA level, % | |||
| 1-2 | 830 (17.4) | 2.97* | 9.3* |
| 3-5 | 1268 (26.6) | 1.41 | 7.1 |
| 6-9 | 952 (20.0) | 0.68 | 5.2 |
| 10-14 | 733 (15.4) | 0.44 | 4.0 |
| 15-24 | 661 (13.8) | 0.33 | 4.2 |
| 25-40 | 299 (6.3) | 0.33 | 5.0 |
| 41-49 | 28 (0.6) | 0.29 | 3.6 |
| HIV status | |||
| Negative | 4495 (94.2) | 1.03* | 5.4* |
| Positive | 247 (5.2) | 3.63 | 19.0 |
| Hepatitis C status | |||
| Negative | 3309 (69.4) | 0.71* | 3.0* |
| Positive | 1437 (30.1) | 2.22 | 13.2 |
| Hepatitis B status‡ | |||
| Negative | 4707 (98.7) | 1.12* | 5.9* |
| Positive | 64 (1.3) | 4.00 | 20.3 |
All proportions do not sum to 100% because of missing data.
P ≤ .001
P ≤ .05
Hepatitis B positivity was based on a positive test for hepatitis B surface antigen.
As expected, the overall average number of joint bleeds declined as the baseline FA increased (Table 1). The proportion of patients who had undergone an invasive orthopedic procedure was highest among those with moderate hemophilia. To a varying degree, all of the other risk factors studied were identified as potential confounders of the relationship between FA and joint bleeding and procedure rates and were therefore included as covariates in the regression analyses.
The results of the multiple linear regression analysis examining the independent relations between the patient characteristics and average joint bleeds are provided in Table 2. The value of the χ2 statistic (provided as a measure of the strength of the association) can be seen to be highest for FA. The estimated value for this risk factor was interpreted as the change in the average number of bleeds for each increment in FA level (eg, on average, there are 0.09 fewer [because the estimate is a negative number] bleeds per 6 months with every 1% increase in FA). The next strongest association with joint bleeding was seen for the 10- to 24-year-old age group. Patients in this age group have 1 more bleed per 6 months on average than those in the 2- to 9-year-old age group (Table 2). Tests for statistical interaction between variables revealed no effect modification among the risk factors.
Multivariable analysis of associations between demographic and clinical characteristics and mean joint bleeds per 6-month period in 4771 males with mild or moderate hemophilia
| Characteristic . | Estimate . | Standard error . | χ2 . | P . |
|---|---|---|---|---|
| Age group, y | ||||
| 2-9 | Ref | |||
| 10-24 | 1.02 | 0.08 | 146.0 | <.001 |
| 25-44 | 0.82 | 0.11 | 52.5 | <.001 |
| 45+ | 0.27 | 0.12 | 2.2 | .03 |
| Race/ethnicity | ||||
| White | Ref | |||
| African American | 0.33 | 0.10 | 11.3 | <.001 |
| Hispanic | −0.28 | 0.09 | 9.0 | <.01 |
| Other | 0.25 | 0.12 | 4.1 | .04 |
| Insurance | ||||
| Commercial | Ref | |||
| Medicaid/Medicare | 0.41 | 0.07 | 38.9 | <.001 |
| Other | −0.004 | 0.12 | 0.0 | .9 |
| Uninsured | 0.18 | 0.11 | 2.7 | .1 |
| BMI | ||||
| Normal | Ref | |||
| Underweight | −0.26 | 0.37 | 0.5 | .5 |
| Overweight | −0.05 | 0.07 | 0.6 | .5 |
| Obese | −0.08 | 0.07 | 1.2 | .3 |
| Hemophilia type | ||||
| FVIII | Ref | |||
| FIX | −0.59 | 0.06 | 87.4 | <.001 |
| HIV infection | ||||
| Negative | Ref | |||
| Positive | 0.33 | 0.11 | 8.5 | <.01 |
| Hepatitis C infection | ||||
| Negative | Ref | |||
| Positive | 0.94 | 0.08 | 133.6 | <.001 |
| Hepatitis B infection* | ||||
| Negative | Ref | |||
| Positive | 0.28 | 0.21 | 1.8 | .2 |
| Baseline FA level | −0.09 | 0.004 | 431.8 | <.001 |
| Characteristic . | Estimate . | Standard error . | χ2 . | P . |
|---|---|---|---|---|
| Age group, y | ||||
| 2-9 | Ref | |||
| 10-24 | 1.02 | 0.08 | 146.0 | <.001 |
| 25-44 | 0.82 | 0.11 | 52.5 | <.001 |
| 45+ | 0.27 | 0.12 | 2.2 | .03 |
| Race/ethnicity | ||||
| White | Ref | |||
| African American | 0.33 | 0.10 | 11.3 | <.001 |
| Hispanic | −0.28 | 0.09 | 9.0 | <.01 |
| Other | 0.25 | 0.12 | 4.1 | .04 |
| Insurance | ||||
| Commercial | Ref | |||
| Medicaid/Medicare | 0.41 | 0.07 | 38.9 | <.001 |
| Other | −0.004 | 0.12 | 0.0 | .9 |
| Uninsured | 0.18 | 0.11 | 2.7 | .1 |
| BMI | ||||
| Normal | Ref | |||
| Underweight | −0.26 | 0.37 | 0.5 | .5 |
| Overweight | −0.05 | 0.07 | 0.6 | .5 |
| Obese | −0.08 | 0.07 | 1.2 | .3 |
| Hemophilia type | ||||
| FVIII | Ref | |||
| FIX | −0.59 | 0.06 | 87.4 | <.001 |
| HIV infection | ||||
| Negative | Ref | |||
| Positive | 0.33 | 0.11 | 8.5 | <.01 |
| Hepatitis C infection | ||||
| Negative | Ref | |||
| Positive | 0.94 | 0.08 | 133.6 | <.001 |
| Hepatitis B infection* | ||||
| Negative | Ref | |||
| Positive | 0.28 | 0.21 | 1.8 | .2 |
| Baseline FA level | −0.09 | 0.004 | 431.8 | <.001 |
The regression model included at a minimum the covariates of age group, race/ethnicity, insurance type, BMI category, hemophilia type, hepatitis B and C, HIV status, and baseline FA level.
Ref, reference.
Hepatitis B infection is based on a positive hepatitis B surface antigen test result.
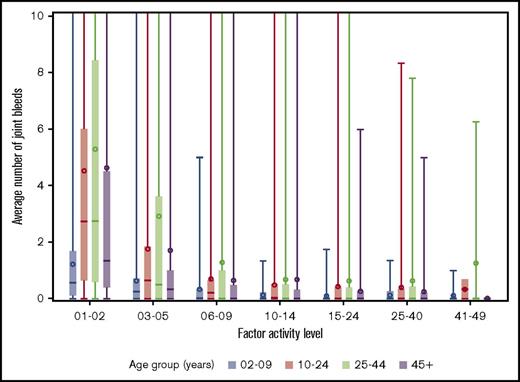
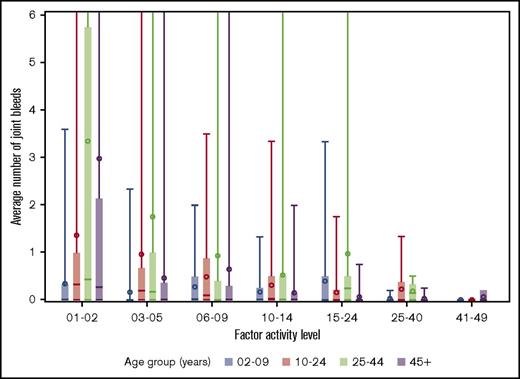
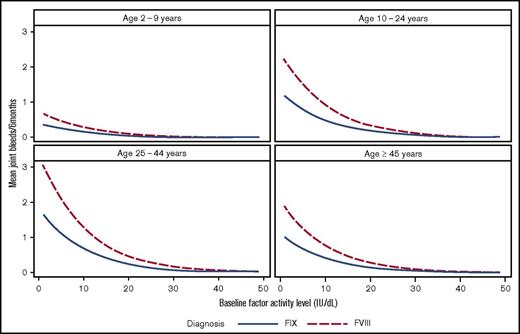
The distributions of joint bleeding by FA across the age groups are shown for the hemophilia cohorts in Figures 1 and 2. Of note is that joint bleeding rates were generally lower for hemophilia B than for hemophilia A as indicated by the different y-axis scales, although there was a great deal of heterogeneity in joint bleeding rates across the full range of FA for both hemophilia types. A linear regression model was used to account for differences in bleeding rates that were a result of demographic and other factors. The predicted frequencies of bleeds by age group across the FA range per 6-month period for a hypothetical patient with either hemophilia A or hemophilia B are shown in Figure 3. The predicted number of joint bleeds for the lowest FA was lowest in the 2- to 9-year-old age group and highest in the 25- to 44-year-old age group. The predicted number of bleeds reached zero for all age groups at an FA of 30%, and in every age group, the predicted number of joint bleeds for patients with FA below 30% was greater for patients with hemophilia A compared with that for patients with hemophilia B (Figure 3). The predicted mean bleed rates derived from the linear regression model as presented in Table 3 further highlight comparative effects of age and hemophilia type over a representative range of FA from 5% to 20%.
Distribution of average number of joint bleeds across FA level by age groups for patients with hemophilia A. In the boxplots, the central rectangle spans the first quartile to the third quartile, a horizontal line inside the rectangle shows the median, and a circle shows the mean. Vertical lines that extend to the top of the graph indicate the presence of outlier values above 10 joint bleeds per 6-month period.
Distribution of average number of joint bleeds across FA level by age groups for patients with hemophilia A. In the boxplots, the central rectangle spans the first quartile to the third quartile, a horizontal line inside the rectangle shows the median, and a circle shows the mean. Vertical lines that extend to the top of the graph indicate the presence of outlier values above 10 joint bleeds per 6-month period.
Distribution of average number of joint bleeds across factor activity level by age groups for patients with hemophilia B. In the boxplots, the central rectangle spans the first quartile to the third quartile, a horizontal line inside the rectangle shows the median, and a circle shows the mean. Vertical lines that extend to the top of the graph indicate the presence of outlier values above 6 joint bleeds per 6-month period.
Distribution of average number of joint bleeds across factor activity level by age groups for patients with hemophilia B. In the boxplots, the central rectangle spans the first quartile to the third quartile, a horizontal line inside the rectangle shows the median, and a circle shows the mean. Vertical lines that extend to the top of the graph indicate the presence of outlier values above 6 joint bleeds per 6-month period.
Predicted number of joint bleeds according to factor activity level and age group for patients with hemophilia A or B based on a regression model. Comparison of predicted number of joint bleeds per 6-month period according to FA level for a hypothetical white patient with commercial insurance, normal BMI, and HIV negativity with either hemophilia A (dashed line) or hemophilia B (solid line).
Predicted number of joint bleeds according to factor activity level and age group for patients with hemophilia A or B based on a regression model. Comparison of predicted number of joint bleeds per 6-month period according to FA level for a hypothetical white patient with commercial insurance, normal BMI, and HIV negativity with either hemophilia A (dashed line) or hemophilia B (solid line).
Predicted joint bleeding rate by FA level for patients with mild or moderate hemophilia A or hemophilia B
| . | Predicted no. of joint bleeds per 6 months . | |||||||
|---|---|---|---|---|---|---|---|---|
| Hemophilia A FVIII activity . | Hemophilia B FIX activity . | |||||||
| 5% . | 10% . | 15% . | 20% . | 5% . | 10% . | 15% . | 20% . | |
| Age group, y | ||||||||
| 2-9 | 0.5 | 0.3 | 0.2 | 0.1 | 0.2 | 0.1 | 0.06 | 0.04 |
| 10-24 | 1.6 | 1.0 | 0.6 | 0.4 | 0.7 | 0.4 | 0.2 | 0.1 |
| 25-44 | 2.3 | 1.4 | 0.8 | 0.5 | 1.4 | 0.8 | 0.5 | 0.3 |
| ≥45 | 1.4 | 0.8 | 0.5 | 0.3 | 0.9 | 0.5 | 0.3 | 0.2 |
| . | Predicted no. of joint bleeds per 6 months . | |||||||
|---|---|---|---|---|---|---|---|---|
| Hemophilia A FVIII activity . | Hemophilia B FIX activity . | |||||||
| 5% . | 10% . | 15% . | 20% . | 5% . | 10% . | 15% . | 20% . | |
| Age group, y | ||||||||
| 2-9 | 0.5 | 0.3 | 0.2 | 0.1 | 0.2 | 0.1 | 0.06 | 0.04 |
| 10-24 | 1.6 | 1.0 | 0.6 | 0.4 | 0.7 | 0.4 | 0.2 | 0.1 |
| 25-44 | 2.3 | 1.4 | 0.8 | 0.5 | 1.4 | 0.8 | 0.5 | 0.3 |
| ≥45 | 1.4 | 0.8 | 0.5 | 0.3 | 0.9 | 0.5 | 0.3 | 0.2 |
Prediction is for a white patient with commercial insurance and normal BMI who is HIV negative.
Five hundred orthopedic procedures were performed among 289 (6%) of the 4771 study participants. Among the 289, 75 had a total of 94 synovectomies, 126 had a total of 174 joint fusions or replacements, and 123 had a total of 143 other types of invasive orthopedic procedures. Of the 289, 185 (64%) had 1 orthopedic procedure, 55 (19%) had 2, and 49 (17%) had 3 or more during the study period. A number of factors were found to be independently associated with the likelihood of having undergone an invasive orthopedic procedure (Table 4). For every 10% increase in FA, the odds of having had a procedure were decreased by 40%. Compared with the youngest participants, those in the 10- to 24-year-old age group had twice the odds, those in the 25- to 44-year-old age group had nearly 6 times the odds, and those in the oldest age group had almost 8 times the odds of having had an invasive procedure (Table 4). Those who were overweight or obese and those who were infected with HIV or hepatitis C virus were 50% to 70% more likely to have had a procedure than patients without these conditions. Finally, participants with hemophilia B were only 70% as likely to have an invasive orthopedic procedure as those with hemophilia A after adjusting for all of the other factors (P = .04) (Table 4).
Multivariable analysis of associations between demographic and clinical characteristics and having an invasive orthopedic procedure in 4771 males with mild or moderate hemophilia
| Characteristic . | OR . | 95% CI . | P . |
|---|---|---|---|
| Age group, y | |||
| 2-9 | Ref | ||
| 10-24 | 2.1 | 1.1-4.0 | .02 |
| 25-44 | 5.7 | 3.0-10.9 | < .001 |
| 45+ | 7.7 | 4.1-14.7 | < .001 |
| Race/ethnicity | |||
| White | Ref | ||
| African American | 1.1 | 0.7-1.8 | .7 |
| Hispanic | 0.5 | 0.3-0.9 | .03 |
| Other | 0.5 | 0.2-1.2 | .1 |
| Insurance | |||
| Commercial | Ref | ||
| Medicaid/Medicare | 1.2 | 0.9-1.6 | .3 |
| Other | 0.7 | 0.4-1.3 | .3 |
| Uninsured | 0.7 | 0.4-1.2 | .2 |
| BMI | |||
| Normal | Ref | ||
| Underweight | 1.3 | 0.4-5.0 | .7 |
| Overweight | 1.6 | 1.2-2.2 | .004 |
| Obese | 1.5 | 1.1-2.2 | .01 |
| Hemophilia type | |||
| FVIII | Ref | ||
| FIX | 0.7 | 0.5-0.9 | .04 |
| HIV infection | |||
| Negative | Ref | ||
| Positive | 1.6 | 1.1-2.4 | .03 |
| Hepatitis C infection | |||
| Negative | Ref | ||
| Positive | 1.7 | 1.2-2.3 | .004 |
| Hepatitis B infection | |||
| Negative | Ref | ||
| Positive | 1.4 | 0.7-2.8 | .4 |
| Baseline FA level | 0.96 | 0.95-0.98 | < .001 |
| Characteristic . | OR . | 95% CI . | P . |
|---|---|---|---|
| Age group, y | |||
| 2-9 | Ref | ||
| 10-24 | 2.1 | 1.1-4.0 | .02 |
| 25-44 | 5.7 | 3.0-10.9 | < .001 |
| 45+ | 7.7 | 4.1-14.7 | < .001 |
| Race/ethnicity | |||
| White | Ref | ||
| African American | 1.1 | 0.7-1.8 | .7 |
| Hispanic | 0.5 | 0.3-0.9 | .03 |
| Other | 0.5 | 0.2-1.2 | .1 |
| Insurance | |||
| Commercial | Ref | ||
| Medicaid/Medicare | 1.2 | 0.9-1.6 | .3 |
| Other | 0.7 | 0.4-1.3 | .3 |
| Uninsured | 0.7 | 0.4-1.2 | .2 |
| BMI | |||
| Normal | Ref | ||
| Underweight | 1.3 | 0.4-5.0 | .7 |
| Overweight | 1.6 | 1.2-2.2 | .004 |
| Obese | 1.5 | 1.1-2.2 | .01 |
| Hemophilia type | |||
| FVIII | Ref | ||
| FIX | 0.7 | 0.5-0.9 | .04 |
| HIV infection | |||
| Negative | Ref | ||
| Positive | 1.6 | 1.1-2.4 | .03 |
| Hepatitis C infection | |||
| Negative | Ref | ||
| Positive | 1.7 | 1.2-2.3 | .004 |
| Hepatitis B infection | |||
| Negative | Ref | ||
| Positive | 1.4 | 0.7-2.8 | .4 |
| Baseline FA level | 0.96 | 0.95-0.98 | < .001 |
The regression model included at a minimum the covariates of age group, race/ethnicity, insurance type, BMI category, hemophilia type, hepatitis B and C, HIV status, and baseline FA level. Invasive orthopedic procedures included arthrodesis; joint replacement; arthroscopic, radioisotopic, or open synovectomy; and any other invasive procedure.
Although there is a significant founder mutation affecting people of Amish heritage in the United States (moderate hemophilia B population), both the bleeding rate and the invasive procedure analyses were repeated after removing data from all individuals with mild or moderate hemophilia B living in the geographic areas having Amish communities (n = 195 or 13.4% of this study’s hemophilia B population), and the outcome results were unchanged.12,13
Discussion
Although standard definitions of hemophilia severity are based upon laboratory FA, the determinants of clinical bleeding phenotype are far more complex. The large UDC surveillance allowed us to account for potential contributors to clinical severity (including hemophilia type), as measured by both average semiannual reported joint bleeding rates for up to 11 years and orthopedic procedures. Of the demographic and clinical characteristics we accounted for, we found that age was the most important predictor of bleeding rate. In general, joint bleeding rates were highest in the 10- to 24-year-old and 25- to 44-year-old age groups compared with the oldest and youngest patients. We observed a large degree of heterogeneity in joint bleeding rates regardless of the FA, but on average, joint bleeding rates decreased as FA increased. We also found that the likelihood of having had an invasive orthopedic procedure increased with age and body weight. We assessed severity of hemophilia A compared with hemophilia B by both bleeding rate and orthopedic procedures, accounting for the variables already determined to be important contributors to clinical severity (most importantly being age). We found that hemophilia A was more severe than hemophilia B at each FA level.
In evaluating the associations between clinical factors and hemophilia severity (bleeding and joint procedures), we found that besides hemophilia type (A vs B) and FA, age was also strongly associated, and men age 25 to 44 years in particular reported high joint bleed rates (Figures 1 and 2). This challenges the notion that the need for prophylaxis decreases with age and presumably a more sedentary lifestyle. The observation of high bleeding rates in both 10- to 24-year-old and 25- to 44-year-old men also suggests a cumulative effect, in which prior bleeds and synovitis predispose to future frequent bleeding. This finding is consistent with a study by den Uijl et al14 which found that patients with moderate hemophilia who had a joint bleed before age 5 years were highly likely to require prophylaxis at a later time. Similarly, others have demonstrated that early initiation of primary prophylaxis predicts preservation of joint range of motion and avoidance of arthropathy.15-17 We conclude that caution should be taken in stopping primary prophylaxis in adulthood, a strategy that has been debated.18,19
We also observed a large degree of heterogeneity in the semiannual bleed rate that is consistent with the well-described heterogeneity of clinical phenotypes in hemophilia.20-22 Extremely high bleeding rates were reported in small minorities of UDC participants across all age groups and FA (Figures 1 and 2). Because of concern about whether the findings of the study were overly influenced by these extreme outliers, we excluded them in the analysis and found that the findings remained essentially unchanged (results not shown).
An analysis of bleeding rate data from a nationwide questionnaire of individuals with hemophilia in the Netherlands reported that joint bleeding rates decreased by 18% for every 1% increase in FA.7 Bleeding decreased by 10% for every 1% increase in FA in the UDC population, but the decrease was not linear, and the clinical translational relevance of this figure is uncertain. The Dutch authors noted that the single collection time point (representing 1 year of follow-up) potentially introduced variation in their results, and the small number of patients resulted in wide confidence intervals. Nevertheless, the data generated from their population predicted that baseline FVIII expression in the range of 15% is associated with a joint bleeding rate that approaches zero. That report includes 10-fold more individuals with moderate hemophilia A (n = 1201) and 848 individuals with moderate hemophilia B. In the US population reported here, the FA of 15% is not adequate to prevent all joint bleeding (Figure 3; Table 3). Instead, in the United States, a typical (white, commercial insurance, normal BMI, HIV negative) 25- to 44-year-old hemophilia A patient with a FVIII activity of 20% is predicted to have 1 joint hemorrhage per year. In contrast, a typical 25- to 44-year-old hemophilia B patient with an FIX activity of 20% is predicted to have 1 joint bleed every 2 years.
Three previous retrospective, single-institution studies have attempted to determine whether the bleeding rates in hemophilia A and hemophilia B differ.5,6,23 These studies included between 20 and 35 hemophilia B patients per study and between 68 and 252 hemophilia A patients, and they had conflicting results. Others have examined surrogate outcomes to compare hemophilia A and B. National studies report higher rates of prophylaxis (Canada) and hospitalization (Scotland) and suggest that the severity of hemophilia A is greater than that of hemophilia B, but they contrast with a multinational pediatric study that found no differences in age at first bleed, first joint bleed, or first exposure to clotting factor.24-26 Our large national database demonstrates that in each age group, patients with hemophilia A had higher bleeding rates than those with hemophilia B (Figure 3). Of note, an earlier examination of UDC data revealed that limitation of joint range of motion in 4343 males age 2 to 19 years was significantly greater for those with hemophilia A than for those with hemophilia B.27 The multivariable analysis of the characteristics of the young patients undergoing orthopedic procedures in that study found that the likelihood of having had an invasive orthopedic procedure increased with age and body weight. We found that procedure rates were highest among underweight patients (Table 1). This seemingly contradictory finding is probably the result of having only a few patients in this BMI category. In addition, this small cluster of patients had an average age greater than that of the total population (37 years) and a high proportion of other risk factors, including hepatitis and HIV infections. Overall, hemophilia B patients with similar FA and risk factors, when compared with hemophilia A patients, were 30% less likely to have had an invasive orthopedic procedure.
Two earlier studies compared orthopedic procedures in severe hemophilia A and hemophilia B populations. A retrospective, multi-institutional Italian survey collected data from 366 joint replacement surgeries among 253 hemophilia A and 15 hemophilia B patients, most of whom had little or no lifelong access to factor prophylaxis.4 The odds ratio for arthroplasty was 3.4 times greater for severe hemophilia A when compared with severe hemophilia B (95% confidence interval, 2.0-5.78). In contrast, a Dutch retrospective, single-institution study of a hemophilia population with broad early access to factor prophylaxis reported that 161 arthroplasties were performed in 30% of patients with severe hemophilia B and in 31% of those with severe hemophilia A.5 The results from the US database include orthopedic procedures in addition to arthroplasty in patients using episodic factor replacement. The more frequent progression to orthopedic surgery among UDC individuals with hemophilia A (compared with hemophilia B) suggests a natural history that is consistent with the Italian experience. Although some patients with mild disease, particularly older individuals, may have joint disease and orthopedic procedures unrelated to their bleeding disorder, the relationship that we found between factor level and orthopedic procedures supports a hemophilia-related risk. In addition, any age-associated frequencies of orthopedic procedures that are unrelated to hemophilia do not confound our current multivariable analysis of likelihood of procedures in hemophilia A vs hemophilia B.
Although the strengths of this study include the large number of men and boys with mild or moderate hemophilia collected over a long study period with repeat measures, the study does have limitations. We excluded 2441 men and boys with a single UDC visit, which likely excluded those with the mildest phenotypes. However, 60% of these patients had mild disease, which is not different from the 57% of patients with mild disease in our study population (Table 1), and were otherwise similar to the study group in terms of demographic and clinical characteristics. Conversely, we excluded 724 men and boys who were treated with prophylaxis during the study period, which excludes those on secondary prophylaxis who had the more severe phenotypes. A higher proportion of these patients were younger than age 25 years, had hemophilia A, and had moderate disease compared with the group studied. However, because the format of the UDC surveillance does not allow us to effectively differentiate primary from secondary prophylaxis, we could not use secondary prophylaxis use as another measure of severity. Because the moderate hemophilia A patients were overrepresented in the excluded prophylaxis group, our multivariable models may have underestimated the magnitude of the decreased bleeding rates and decreased invasive procedures among patients with hemophilia B relative to hemophilia A.
A potential limitation is that the baseline FA entered into the UDC database is determined in the patient’s local laboratory rather than in a central laboratory. The great variability described between laboratories that perform specific FA assays may be related to differences in reagents as well as to patient-related variables; this has been observed even when the same testing platforms are used.28 Because this variability affects both FVIII and FIX activity testing,29 it is unlikely to result in consistent biases in any direction that would invalidate our findings.
An additional limitation is that upon the patient’s initial enrollment in the UDC, the FA was entered a single time. In hemostatically normal populations, mean FVIII and FIX activity increase at similar rates with age.30 A recent examination that focused on mild or moderate hemophilia A demonstrated that increasing FVIII activity with aging contributes to interindividual and intraindividual differences in baseline FA.28 This finding does not definitively establish that the bleeding phenotype of hemophilia becomes milder with advanced age. In addition, specific mutations in the F8 or F9 genes demonstrate age-responsiveness that may be more extreme than the population mean (eg, mutations in the F8 gene that differentially impair FVIII intracellular trafficking or binding with von Willebrand factor or mutations in the F9 gene that affect transcription factor binding sites 5′ or 3′ to the coding sequence).28,31 The UDC database did not contain genotype data; therefore, no genotype or phenotype correlation was possible.
Self-reporting of joint hemorrhage at annual visits is an imperfect measure of joint hemorrhage. Self-reporting is subject to recall bias, and bleeding is stochastic, so rates of bleeding will vary from year to year. However, we would not expect recall bias to differentially affect those with hemophilia A vs B, and we used the average reported semiannual bleed rate in an attempt to account for the variability of joint hemorrhage over time. Because joint bleeding was not objectively measured, the rate of joint bleeding could also be falsely inflated, particularly in the older cohorts, because it is challenging to differentiate synovitis and arthritic pain from hemorrhage.32 The UDC did not collect measures of physical activity, which conceivably influence bleeding rates. However, we are unaware of systematic differences in activity between hemophilia A and B that could have accounted for the results derived from our self-reported joint hemorrhage rates. The results are strengthened by observing differences between hemophilia A and B using the more objective measure of orthopedic procedures.
Our attempt to use the mild or moderate hemophilia data to draw direct conclusions regarding target trough levels for FVIII and FIX clotting factor concentrate infusions is complicated by the limitations imposed by the pharmacokinetics of factor concentrates.7-9 The short circulating half-life of unmodified therapeutic FVIII and FIX proteins requires higher peak activity levels to maintain a greater minimum trough level. Therefore, clotting factor infusion therapy to maintain a targeted trough will result in enormously greater FA area under the curve than is maintained by the individual with mild hemophilia. The trough level associated with absent bleeding during prophylactic factor replacement in most individuals with severe hemophilia may be somewhat lower than the steady state FA associated with no bleeding in the individual with mild hemophilia who does not experience regular post-infusion peaks of FA. Nevertheless, the data reported here show very large interindividual variability in bleeding rates at every FA level, suggesting that achieving the aspiration of eliminating all joint and spontaneous bleeding may require individualized approaches to therapy.
However, the predictive value of the data collected here may allow direct conclusions about goals for steady-state FA to be targeted by gene therapy. This study emphasizes that the predictive value of the FA must take into account age, particularly at FA of <10%. It is interesting to speculate whether the propensity to more joint bleeding at any given FA in the 25- to 44-year-old age group is inherent to some feature of this age (eg, joint physiology, hemostasis) or whether this risk will diminish over decades with more individuals entering adulthood who have practiced regular prophylactic therapy throughout their development. The predicted annualized joint bleeding rate at any given FA is lower for hemophilia B than hemophilia A, which in theory could define somewhat different targets for FA for hemophilia A when compared with hemophilia B gene therapy. Nevertheless, if levels of FVIII and FIX of >20% to 25% can indeed be maintained after human gene therapy, as suggested by recently reported first-in-human trials, the ability to discriminate differences in joint outcomes between hemophilia A and B may be small.33-35
Acknowledgments
The authors acknowledge the staff of the US Hemophilia Treatment Center Network (US HTCN) for recruiting patients to the Universal Data Collection (UDC) surveillance project and collecting the data.
This work was supported by the Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention (CDC). The UDC Project was funded by a cooperative agreement (“Prevention of Bleeding Disorder Complications through Regional Hemophilia Treatment Centers”) between the CDC and the US HTCN comprising >130 clinical centers located throughout the United States.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Authorship
Contribution: J.M.S. conceptualized the study design, analyzed the data, conducted statistical analysis, wrote the manuscript, and created the tables and figures; M.A.M. analyzed the data and wrote the manuscript; B.A.K. and R.K. analyzed the data and edited the manuscript; and P.E.M. conceptualized the study design, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: B.A.K. received consulting fees from Bioverativ, CSL Behring, Genentech, and Shire; and research support from Bioverativ, Sangamo, and Shire. P.E.M. received research support through the University of North Carolina from Asklepios, Novo Nordisk, and Baxter Healthcare; he holds patents that have been licensed to Asklepios for which he receives royalties; has received payment for consultation, services, and for speaking for Asklepios, Chatham LLC, Baxter Healthcare, Pfizer, Bayer, Novo Nordisk, and Biogen; and was employed by Shire during drafting of the manuscript. The remaining authors declare no competing financial interests.
The current affiliation for P.E.M. is Spark Therapeutics, Philadelphia, PA.
A list of the members of the US Hemophilia Treatment Center Network appears in “Appendix.”
Correspondence: J. Michael Soucie, Centers for Disease Control and Prevention, Chamblee Campus, 4770 Buford Hwy, MS E64, Atlanta, GA 30341-3717; e-mail: msoucie@cdc.gov.
Appendix: study group members
The US Hemophilia Treatment Center Network (US HTCN) includes about 130 regionally organized hemophilia treatment centers. The CDC UDC Cooperative Agreement Grantees/Regional Directors of the 12 regions of the US HTCN at the time of final UDC data cleaning and research evaluations include: Doreen B. Brettler, New England Hemophilia Center, Worcester, MA; Christopher E. Walsh, Mount Sinai School of Medicine, New York, NY; Regina B. Butler, Children’s Hospital of Philadelphia, Philadelphia, PA; Paul Monahan, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ruth Brown, Hemophilia of Georgia, Inc., Atlanta, GA; Ivan C. Harner, Hemophilia Foundation of Michigan, Ypsilanti, MI; Danielle L. Baxter, Great Lakes Hemophilia Foundation, Milwaukee, WI; Deborah L. Brown, Gulf States Hemophilia and Thrombophilia Center, Houston, TX; Brian M. Wicklund, Kansas City Regional Hemophilia Center, Kansas City, MO; Marilyn J. Manco-Johnson, University of Colorado Hemophilia and Thrombosis Center, Aurora, CO; Diane J. Nugent, Children’s Hospital of Orange County, Orange, CA; Michael Recht, The Hemophilia Center at Oregon Health and Science University, Portland, OR. In addition to coordinating regional data collection, the Regional Coordinators from the 12 regions of the US HTCN validated specific data elements and categories to verify the precision of this study and include: Ann D. Forsberg, Mariam Voutsis, Danielle L. Deery, Steven Humes, Karen Droze, Suzanne Kapica, Kathryn Reese, John Drake, Becky Dudley, Judith R. Baker, Brenda K. Riske and Robi Ingram-Rich.
References
Author notes
J.M.S. and P.E.M. contributed equally to this study.