Key Points
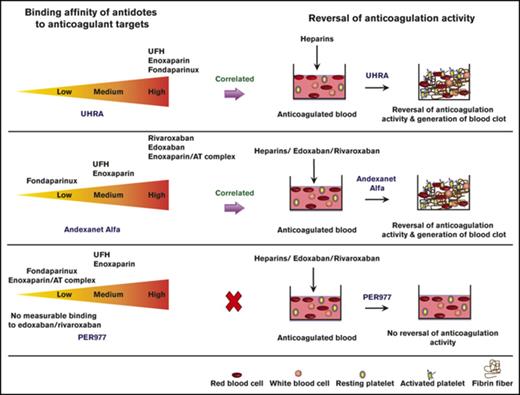
Anticoagulant therapy–associated bleeding is a concern; a specific antidote is needed for emergency reversal of anticoagulant activity.
Thermodynamic, electron microscopic, and clotting studies reveal distinct mechanisms of action for 3 different antidotes in development.
Abstract
Anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparins (LMWHs), fondaparinux, and direct oral anticoagulants (DOACs) targeting thrombin (IIa) or factor Xa (FXa) are widely used in prevention and treatment of thromboembolic disorders. However, anticoagulant-associated bleeding is a concern that demands monitoring and neutralization. Protamine, the UFH antidote, has limitations, while there is no antidote available for certain direct FXa inhibitors. Improved antidotes in development include UHRA (Universal Heparin Reversal Agent) for all heparin anticoagulants; andexanet alfa (andexanet), a recombinant antidote for both direct FXa inhibitors and LMWHs; and ciraparantag (PER977), a small-molecule antidote for UFH, LMWHs, and certain DOACs. The binding affinities of these antidotes for their presumed anticoagulant targets have not been compared. Here, isothermal titration calorimetry (ITC) was used to determine the affinity of each antidote for its putative targets. Clotting and chromogenic FXa assays were used to characterize neutralization activity, and electron microscopy was used to visualize the effect of each antidote on clot morphology in the absence or presence of anticoagulant. ITC confirmed binding of UHRA to all heparins, and binding of andexanet to edoxaban and rivaroxaban, and to the antithrombin–enoxaparin complex. PER977 was found to bind heparins weakly, but not the direct FXa inhibitors studied. For UHRA and andexanet, an affinity at or below the micromolar level was found to correlate with neutralization activity, while no reversal activity was observed for the PER977/anticoagulant systems. Standard metrics of clot structure were found to correlate weakly with PER977’s activity. This is the first study comparing 3 antidotes in development, with each exerting activity through a distinct mechanism.
Introduction
Anticoagulants are widely used to treat and prevent thromboembolism.1,2 These anticoagulants include antithrombin (AT)–dependent heparins, such as unfractionated heparin (UFH), low-molecular-weight heparins (LMWHs), the synthetic pentasaccharide fondaparinux, vitamin K antagonists (eg, warfarin), and direct oral anticoagulants (DOACs), such as direct factor Xa (FXa) inhibitors (apixaban, betrixaban, edoxaban, and rivaroxaban) or a thrombin (FIIa) inhibitor (eg, dabigatran).1,2 UFH and LMWHs remain the primary anticoagulants used to prevent and treat acute thrombotic events,3 including those arising in procedures requiring extracorporeal circulation such as hemodialysis and cardiopulmonary bypass surgery.3 Because of their superior pharmacokinetic and safety profiles compared with warfarin, DOACs are increasingly used to prevent strokes due to atrial fibrillation, treat pulmonary embolism and deep-vein thrombosis, and prevent venous thrombosis following surgery.4 However, data from real-world clinical settings show that bleeding associated with anticoagulation therapy remains a major concern.5-7 Therefore, safe and effective antidotes are needed in case of bleeding complications or emergent surgery for patients under anticoagulation.5-7
Warfarin anticoagulation activity can be reversed by administering vitamin K or prothrombin complex concentrates.8,9 Protamine is the only approved antidote for reversing the anticoagulation activity of UFH.10,11 Protamine only partially reverses the activity of LMWHs,12 with no neutralization activity against fondaparinux, and it is known to exhibit an unpredictable dose response and severe side effects.13,14 Recently, idarucizumab has been approved as a specific antidote for dabigatran.15 In contrast, effective neutralization of the anticoagulant activities of LMWHs, fondaparinux, edoxaban, and betrixaban remains lacking, thereby motivating the development of new antidotes.
Recently, the US Food and Drug Administration approved andexanet alfa (andexanet) as an antidote for reversing anticoagulation activity of rivaroxaban and apixaban.16 Other antidotes currently in development and included in this study are UHRA (Universal Heparin Reversal Agent)17,18 (UHRA-7) and ciraparantag (PER977).19 UHRA is a synthetic multivalent dendrimeric polymer designed to reverse the activity of all clinically available heparins, and it is currently undergoing preclinical studies.17,18 Andexanet is a recombinant variant of FXa designed to reverse the activity of both direct and indirect FXa inhibitors.20-22 Ciraparantag (PER977) is a synthetic, low-molecular-weight antidote currently in phase 2 clinical trials in healthy subjects.19,23 PER977 is reported to reverse direct FXa inhibitors, UFH, and LMWHs, as well as some thrombin inhibitors.24
To date, there is no direct comparison of the binding affinities of these antidotes in development for their presumed targets. Isothermal titration calorimetry (ITC) was therefore used to identify unique and common binding partners among representative DOACs and heparins, as well as binding to relevant blood coagulation proteins as assessed by the measured equilibrium dissociation constant Kd using ITC. In order to further delineate the molecular mechanism(s) of action for each antidote, additional characterizations were performed, including (1) neutralization of anticoagulant activity measured by plasma and whole-blood clotting and chromogenic assays and (2) structures of fibrin and blood clots before and after neutralization determined by scanning electron microscopy (SEM). The 3 antidotes were found to exert their reversal activities, when observed, through distinct mechanisms of action.
Methods
Synthesis of the ciraparantag (PER977) molecule used for this study, the source of all other reagents, as well as experimental procedures are described in supplemental Methods. The synthesized PER977 molecule was characterized by proton nuclear magnetic resonance and mass spectrometry to confirm the structure identity (supplemental Figure 1); purity was determined by high-performance liquid chromatography. The synthesis, purification, and characterization of the UHRA molecule used in this study have been described previously.17,18
Blood collection and the protocols used in the current studies were approved by the Clinical Research Ethics Board (certificate number H10-01896) of the University of British Columbia, and written consent from donors was obtained in accordance with the Declaration of Helsinki.
ITC
The interaction of UHRA, andexanet, and PER977 with their putative binding partners, as well as with relevant blood coagulation proteins, was studied by ITC by following procedures described previously by Kormos et al25 using the specific conditions described in supplemental Methods.
SEM of fibrin and whole-blood clots
The morphology of clots formed in the presence of andexanet or PER977 was analyzed by SEM according to the method of Kalathottukaren et al.18 Multiple images from different areas of each clot were captured for analysis. The average fiber diameters of clots were determined with ImageJ26 as described previously.18
Anticoagulant neutralization assays
Activated partial thromboplastin time (aPTT), microplate-based whole-blood clotting assay,27 chromogenic FXa assay, and thromboelastography (TEG) were used to evaluate the anticoagulant neutralization activity of each antidote under various specified conditions. All assays except the chromogenic FXa assay were performed using plasma or whole blood collected in the absence of citrate, as PER977 is reported to interact with citrate.23 Detailed procedures are given in supplemental Methods.
Data analysis
Data are presented as mean ± standard error (SE) values from n ≥ 3 independent experiments or medians with interquartile ranges, unless otherwise specified. ITC data analysis and plots were done using Origin software from Microcal (Northampton, MA). All other results were plotted and analyzed using GraphPad Prism 7.0 (La Jolla, CA). Statistical significance was determined using a Student t test, Mann-Whitney (unpaired, 2-tailed) U tests, or the Kruskal-Wallis test with a Dunn post-test for multiple group comparisons. P < .05 was considered statistically significant.
Results
ITC
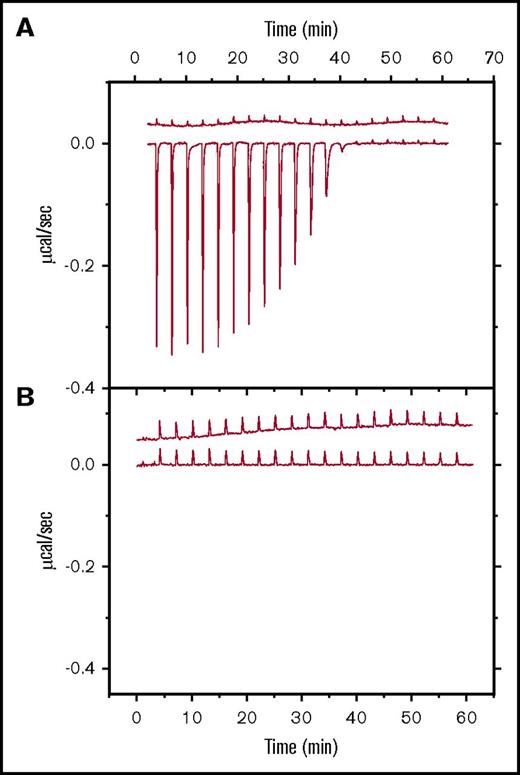
Each of the 3 antidotes studied here is thought to bind directly or indirectly to specific anticoagulants to provide reversal activity. To better delineate those targets, ITC was used to determine binding affinities to each putative target as well as to relevant components of the coagulation pathway. Table 1 reports the mean Kd (n = 3) measured by ITC for each binding partner for which a measureable (millimolar or tighter) binding interaction was observed. Figure 1 shows the representative ITC titration traces for andexanet (Figure 1A) and PER977 (Figure 1B) binding to edoxaban, respectively. Additional ITC traces are provided in supplemental Figures 2-4.
Equilibrium dissociation constants determined by ITC for each reversal agent titrated into various potential binding partners
| Putative binding partner . | Kd (M) . | ||
|---|---|---|---|
| UHRA . | Andexanet alfa . | Ciraparantag . | |
| UFH | 8.9 (± 0.3) × 10−7 | 3.4 (± 0.4) × 10−5 | 2.8 (± 0.3) × 10−5 |
| Enoxaparin | 6.0 (± 0.4) × 10−6 | 4.0 (± 0.3) × 10−5 | 1.7 (± 0.3) × 10−5 |
| Enoxaparin–AT complex | — | 7.1 (± 0.5) × 10−7 | 6.9 (± 2.8) × 10−4 |
| Fondaparinux | 2.4 (± 0.6) × 10−6 | 1.1 (± 2.0) × 10−4 | 9.6 (± 3.9) × 10−3 |
| Edoxaban | ND | 3.4 (± 0.6) × 10−9 | NB |
| Rivaroxaban | ND | 0.9 (± 0.2) × 10−9 | NB |
| h-FXa | NB | NB | NB |
| h-FIX | — | — | NB |
| h-FIXa | — | — | 3.5 (± 0.4) × 10−5 |
| h-thrombin | NB | — | NB |
| h-antithrombin | NB | — | NB |
| h-fibrinogen | NB | NB | NB |
| Putative binding partner . | Kd (M) . | ||
|---|---|---|---|
| UHRA . | Andexanet alfa . | Ciraparantag . | |
| UFH | 8.9 (± 0.3) × 10−7 | 3.4 (± 0.4) × 10−5 | 2.8 (± 0.3) × 10−5 |
| Enoxaparin | 6.0 (± 0.4) × 10−6 | 4.0 (± 0.3) × 10−5 | 1.7 (± 0.3) × 10−5 |
| Enoxaparin–AT complex | — | 7.1 (± 0.5) × 10−7 | 6.9 (± 2.8) × 10−4 |
| Fondaparinux | 2.4 (± 0.6) × 10−6 | 1.1 (± 2.0) × 10−4 | 9.6 (± 3.9) × 10−3 |
| Edoxaban | ND | 3.4 (± 0.6) × 10−9 | NB |
| Rivaroxaban | ND | 0.9 (± 0.2) × 10−9 | NB |
| h-FXa | NB | NB | NB |
| h-FIX | — | — | NB |
| h-FIXa | — | — | 3.5 (± 0.4) × 10−5 |
| h-thrombin | NB | — | NB |
| h-antithrombin | NB | — | NB |
| h-fibrinogen | NB | NB | NB |
Data are presented as mean ± SE.
h, human; NB, no binding detected; ND, not determined.
ITC data for andexanet alfa or PER977 binding to edoxaban. (A-B) Raw heat data for titrations at 25°C of (A) 100 µM edoxaban into buffer (upper data set) or 13 µM andexanet alfa (lower data set) and (B) 200 µM edoxaban into buffer (upper data set) or 20 µM Per977 (lower data set). All differential heat peaks for 2 µL injections; buffer: phosphate-buffered saline with 1% dimethyl sulfoxide.
ITC data for andexanet alfa or PER977 binding to edoxaban. (A-B) Raw heat data for titrations at 25°C of (A) 100 µM edoxaban into buffer (upper data set) or 13 µM andexanet alfa (lower data set) and (B) 200 µM edoxaban into buffer (upper data set) or 20 µM Per977 (lower data set). All differential heat peaks for 2 µL injections; buffer: phosphate-buffered saline with 1% dimethyl sulfoxide.
UHRA.
UHRA (UHRA-7) has micromolar binding affinity for each heparin-based anticoagulant studied (supplemental Figure 2), with no measureable interaction with FXa, AT, or fibrinogen (Table 1). UHRA was not designed to neutralize DOACs, so they were not included in the panel of putative UHRA-binding partners studied.
Using fluorescence and enzyme-linked immunosorbent assay studies, we have shown that the UFH–AT complex is disrupted by the addition of UHRA, resulting in loss of anticoagulant activity.17,28 The ITC results show that UHRA directly binds UFH, LMWH (enoxaparin), or fondaparinux with micromolar affinity (Table 1). The binding of affinity of UFH-UHRA is similar in magnitude to that of ATIII-UFH–binding data previously reported from ITC experiments.29 Taken together, the fluorescence, enzyme-linked immunosorbent assay and ITC results support direct binding of UHRA to heparin-based anticoagulants (at a strength that enables displacement of AT in the heparin–AT complex) as the primary mechanism by which UHRA neutralizes the activity of UFH or LMWH.
Andexanet.
Andexanet binds the largest range of anticoagulants. Direct binding to UFH and enoxaparin was observed (supplemental Figure 3) at affinities roughly an order of magnitude weaker than that observed for UHRA. However, a Kd commensurate with that reported for the UHRA–UFH complex was observed for binding of andexanet to the AT–enoxaparin complex, suggesting that andexanet preferably binds to heparin-activated AT, which is consistent with the mechanism of action for reversal of AT-dependent FXa inhibitors by andexanet. In addition, andexanet binds each FXa inhibitor studied with nanomolar affinity (Table 1; Figure 1A), with binding to rivaroxaban being exceptionally tight (Kd = 0.9 nM) (supplemental Figure 3).
As a modified human FXa, andexanet retains the high-affinity binding to both direct and AT-dependent indirect FXa inhibitors.20 The ITC data support those findings by showing that andexanet directly binds rivaroxaban and edoxaban with nanomolar affinity (and 1:1 stoichiometry), consistent with its proposed mechanism of action in reversing DOAC-mediated FXa inhibition.
Although direct binding of andexanet to UFH, enoxaparin, and fondaparinux is also observed, nevertheless, the relatively weak binding (Kd = 34-110 µM) of andexanet to any of these heparins (in the absence of AT) suggests this interaction is not the physiological mechanism of action for the reversal of AT-dependent anticoagulant by andexanet. Conversely, the high binding affinity (Kd = 0.71 µM) of andexanet to the enoxaparin–AT complex was observed (Table 1). This observation is in agreement with the binding affinity of S195A FXa (inactive form) to free AT and AT–heparin complex reported by Izaguirre et al.30 Their fluorescence studies revealed that the binding affinity of S195A FXa to free AT is weak (Kd > 30 µM) compared with AT complexed with heparin (Kd = 8.7 nM).30 These results suggests that the major mechanism of action for the reversal of AT-dependent inhibitors by andexanet is via binding to heparin-activated AT in the AT–anticoagulant complex and not through direct sequestration of the heparin anticogulant.31,32
PER977.
PER977 binds fondaparinux weakly and binds UFH or enoxaparin with an affinity similar to that observed for direct binding of andexanet to the same targets (Kd ∼ 25 µM) (Table 1; supplemental Figure 4). Unlike with andexanet, binding of PER977 is weakened when enoxaparin is complexed with AT. Finally, we did not observe binding of PER977 to either edoxaban or rivaroxaban using ITC, but we did observe direct binding to FIXa (Table 1; Figure 1B; supplemental Figure 4). Direct binding to other blood coagulation proteins studied was not observed with any of the 3 antidotes.
By ITC, we saw no conclusive evidence of a direct binding interaction between PER977 and either edoxaban or rivaroxaban in phosphate-buffered saline (with no citrate or other chelator present). This result is at odds with a previous study33 reporting noncovalent binding of PER977 to DOACs based on dynamic light scattering (DLS) data. Based in part on those DLS data,33 direct binding to DOACs has been proposed as the putative mechanism of action of PER977, but our findings suggest that PER977 might derive its purported DOAC-neutralization activity in a different manner.
Some insight into the alternate mechanism of action is provided by our finding that PER977 binds FIXa. Since FIXa is the key enzyme in the intrinsic Xase that activates FX to FXa, leading to thrombin generation, it has long been recognized as a potential target for modulating coagulation.34,35 The observed binding of PER977 to FIXa raises the question as to whether it might serve to alter the activity of the FIXa in activating FX to FXa, similar to polylysine.36,37
In concordance with previously reported DLS data,33 we do observe direct binding of PER977 to enoxaparin (Table 1). However, the Kd for binding of PER977 to enoxaparin is weaker than that for either UHRA binding to enoxaparin or andexanet binding to the enoxaparin–AT complex. Interestingly, that binding affinity is attenuated when enoxaparin is complexed with AT. Finally, activity against fondaparinux through a direct binding mechanism is not supported by our ITC data, as weak affinity (Kd ∼ mM) is observed for that complex.
Fibrin polymerization and clot imaging
Bleeding at the site of injury is ceased via polymerization of fibrin from thrombin-cleaved fibrinogen to form a fibrin clot.38 A number of factors modulate clot formation kinetics, structure, and stability, including thrombin, calcium, and fibrinogen concentrations, as well as pH.39,40 In general, high thrombin levels produce thinner clot fibers that are more resistant to lysis when compared with the generally thicker fibers formed at lower thrombin concentrations.39,40 Both andexanet and PER977 are reported to halt bleeding caused by DOACs and heparin anticoagulants to generate a hemostatic clot.41 To better define their mechanisms of action, we therefore asked whether these anticoagulant reversal agents have any impact on fibrin clot formation and structure. Our study focused on andexanet and PER977, as we have recently shown that UHRA does not alter fibrin polymerization, plasma clotting, or clot structure in the absence of heparin anticoagulants.18 Moreover, the structure of blood clots obtained after neutralizing UFH with UHRA was comparable to the buffer control clots.18
Fibrin polymerization in the presence of andexanet (up to 200 µg/mL) or PER977 (up to 500 µg/mL) did not significantly change the maximum optical density, which correlates with the average fiber cross-sectional area,40 in comparison with the buffer control (supplemental Figure 5A-D). However, andexanet at a supratherapeutic concentration more than threefold above the highest andexanet plasma levels (500 µg/mL, ∼12 µM) produced clots with reduced optical density, suggesting impaired clot formation and/or the formation of thinner fibrin fibers.
To gain further insights, we visualized the ultrastructures of fibrin and blood clots formed in the presence of each antidote using SEM to determine whether these molecules have any effect on fibrin fiber diameter. No anticoagulant was present in these studies, as our goal was to study any effect of these molecules alone on fibrin clot. In addition, this experimental condition could mimic an overdose scenario where the antidote molecules are present in the free form in blood. Based on available reports related to PER977, the therapeutic concentration of PER977 ranges from ∼20 to 60 µg/mL (∼39-117 µM),19,23 and the therapeutic concentration of andexanet ranges from ∼80 to 160 µg/mL (∼1.9-3.8 µM)21,22 (see supplemental Table 1 for a µg/mL to µM conversion). Hence, the concentration used in this study ranged from therapeutic to supratherapeutic for these 2 antidotes.
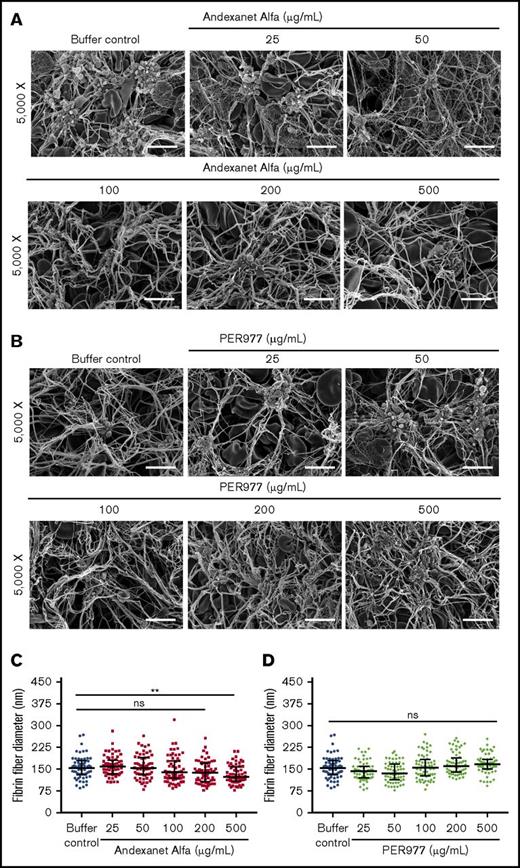
Andexanet up to 200 µg/mL showed no change in fibrin clot structure or fibrin fiber diameter relative to the buffer control (supplemental Figure 6A,C). However, consistent with the fibrin-polymerization data, andexanet at 500 µg/mL gave thinner fiber structures. Fibrin clots formed in the presence of PER977 at concentrations up to 500 µg/mL showed no change in clot structure or fibrin fiber diameter (supplemental Figure 6B-C). Likewise, anticoagulant-free whole-blood clots formed in presence of andexanet or PER977 did not show any significant morphological changes, having normal clot components and normal fibrin fibers when compared with the buffer control (Figure 2). At a supratherapeutic concentration (500 µg/mL), andexanet yields thinner fibers (Figure 2A,C).
Antidotes alone do not influence blood clot morphology. Blood clots were generated by incubating antidotes in noncitrated whole blood at 37°C. (A-B) Blood clots formed in the presence of both andexanet alfa and PER977 did not show any major morphological changes. Clot images were taken at 2 magnifications (original magnification ×2500 and ×5000, respectively. Only images from the ×5000 magnification are depicted (scale bars, 5 µm). (C-D) Blood clot fiber diameters formed in the presence of andexanet alfa or PER977. Fiber diameter was measured from scanning electron micrographs using ImageJ software. A total of 60 fibers were analyzed from 2 independent experiments. Fibers for size analysis were selected from 4 images by probing 4 different spots in each image. Data are median with interquartile ranges (n = 60). Black horizontal lines represent median, and error bars represent interquartile range. Statistical significance for fiber diameter was determined by comparing the antidote-treated group to the buffer control using a Kruskal-Wallis test followed by a Dunn post-test. Asterisks indicate significant differences in comparison with the buffer control. Fibers formed in the presence of 500 µg/mL andexanet alfa are significantly thinner than those in the control clot (**P < .005). ns, not significant.
Antidotes alone do not influence blood clot morphology. Blood clots were generated by incubating antidotes in noncitrated whole blood at 37°C. (A-B) Blood clots formed in the presence of both andexanet alfa and PER977 did not show any major morphological changes. Clot images were taken at 2 magnifications (original magnification ×2500 and ×5000, respectively. Only images from the ×5000 magnification are depicted (scale bars, 5 µm). (C-D) Blood clot fiber diameters formed in the presence of andexanet alfa or PER977. Fiber diameter was measured from scanning electron micrographs using ImageJ software. A total of 60 fibers were analyzed from 2 independent experiments. Fibers for size analysis were selected from 4 images by probing 4 different spots in each image. Data are median with interquartile ranges (n = 60). Black horizontal lines represent median, and error bars represent interquartile range. Statistical significance for fiber diameter was determined by comparing the antidote-treated group to the buffer control using a Kruskal-Wallis test followed by a Dunn post-test. Asterisks indicate significant differences in comparison with the buffer control. Fibers formed in the presence of 500 µg/mL andexanet alfa are significantly thinner than those in the control clot (**P < .005). ns, not significant.
SEM analysis of fibrin fiber development in edoxaban-anticoagulated whole blood
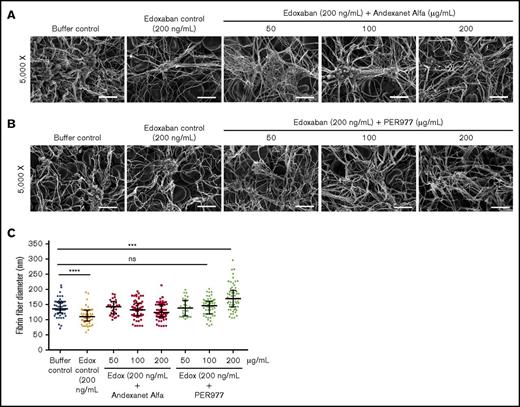
SEM analysis of the fibrin clot structure of whole blood could be a useful method for determining the reversal activity as the clotting ability of anticoagulated blood is restored by the antidotes and the fibrin diameter could be normalized. Recently, such analysis has been used by Ansell et al to determine the ability of PER977 to reverse the anticoagulation activity of edoxaban.19 In light of our ITC data that shows PER977 does not bind to either edoxaban or rivaroxaban and andexanet binds strongly to these agents, we explored the restoration of impaired clot fiber formation in edoxaban-anticoagulated blood using SEM analysis to determine the reversal activity of these agents. Edoxaban (200 ng/mL, final) inhibited blood clotting, as a tiny clot was observed in comparison with the buffer control (supplemental Figure 7). Neutralization of edoxaban (200 ng/mL, final) with andexanet at 50, 100, or 200 µg/mL normalized the impaired fibrin fiber formation and fiber diameter (Figure 3A,C). PER977 also restored fibrin formation and fiber diameter upon addition to edoxaban-anticoagulated blood (200 ng/mL, final) (Figure 3B-C). However, neutralization of edoxaban-anticoagulated blood using 200 µg/mL PER977 resulted in fibrin fibers that are significantly thicker than those observed in the buffer control; this was not observed with andexanet.
Antidotes normalize impaired fibrin formation and fiber diameter of edoxaban-treated clots. Blood clots were generated by incubating antidotes in noncitrated whole blood containing 200 ng/mL edoxaban at 37°C. (A-B) Clots formed in the presence of edoxaban (200 ng/mL, final) have fewer fibers compared with the buffer control. However, normal clot signatures can be observed in edoxaban clots, treated with 50, 100 and 200 µg/mL of Andexanet Alfa or PER977, respectively. Clot images were taken at 2 magnifications (original magnification ×2500 and ×5000, respectively. Only images from the ×5000 magnification are depicted (scale bars, 5 µm). (C) Blood clot fiber diameters in edoxaban (Edox)–treated clots formed in the absence or presence of an antidote. Fiber diameters were measured from scanning electron micrographs using ImageJ software. A total of 60 fibers were analyzed from 2 independent experiments. Fibers for size analysis were selected from 4 images by probing 4 different spots in each image. Data are median with interquartile ranges (n = 60). Black horizontal lines represent median, and error bars represent interquartile range. Statistical significance for fiber diameter was determined by comparing the antidote treated group to the buffer control using a Kruskal-Wallis test followed by a Dunn post-test. Asterisks indicate significant differences in comparison with the buffer control. A significant reduction in fiber diameter was observed in edoxaban-containing clots compared with the buffer control (****P < .0001). The diameter of fibrin fibers formed in edoxaban clots containing 50, 100, and 200 µg/mL Andexanet Alfa is comparable to that of the buffer control. A similar effect was observed with 50 and 100 µg/mL PER977. However, fiber diameters formed in the presence of 200 µg/mL PER977 were significantly larger than the buffer control clot (***P = .0004).
Antidotes normalize impaired fibrin formation and fiber diameter of edoxaban-treated clots. Blood clots were generated by incubating antidotes in noncitrated whole blood containing 200 ng/mL edoxaban at 37°C. (A-B) Clots formed in the presence of edoxaban (200 ng/mL, final) have fewer fibers compared with the buffer control. However, normal clot signatures can be observed in edoxaban clots, treated with 50, 100 and 200 µg/mL of Andexanet Alfa or PER977, respectively. Clot images were taken at 2 magnifications (original magnification ×2500 and ×5000, respectively. Only images from the ×5000 magnification are depicted (scale bars, 5 µm). (C) Blood clot fiber diameters in edoxaban (Edox)–treated clots formed in the absence or presence of an antidote. Fiber diameters were measured from scanning electron micrographs using ImageJ software. A total of 60 fibers were analyzed from 2 independent experiments. Fibers for size analysis were selected from 4 images by probing 4 different spots in each image. Data are median with interquartile ranges (n = 60). Black horizontal lines represent median, and error bars represent interquartile range. Statistical significance for fiber diameter was determined by comparing the antidote treated group to the buffer control using a Kruskal-Wallis test followed by a Dunn post-test. Asterisks indicate significant differences in comparison with the buffer control. A significant reduction in fiber diameter was observed in edoxaban-containing clots compared with the buffer control (****P < .0001). The diameter of fibrin fibers formed in edoxaban clots containing 50, 100, and 200 µg/mL Andexanet Alfa is comparable to that of the buffer control. A similar effect was observed with 50 and 100 µg/mL PER977. However, fiber diameters formed in the presence of 200 µg/mL PER977 were significantly larger than the buffer control clot (***P = .0004).
Reversal of rivaroxaban and edoxaban anticoagulant activity
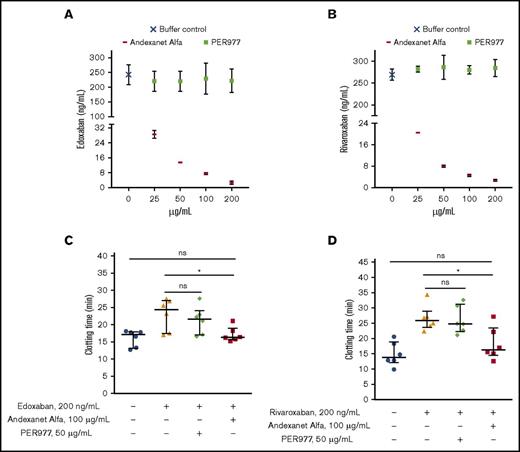
Next, we performed a chromogenic anti-FXa assay to assess the ability of andexanet or PER977 to reverse the anti-FXa activity of edoxaban and rivaroxaban. Results (Figure 4A-B) show that andexanet reduced the anti-FXa activity of edoxaban and rivaroxaban in a dose-dependent manner. When the concentration of andexanet reached 200 µg/mL, we observed that the anti-FXa activity of edoxaban and rivaroxaban decreased by ∼99% compared with the buffer control. However, PER977 at any studied concentration did not reduce the anti-FXa activity of tested DOACs, which suggests that PER977 is not able to bind or reverse the anti-FXa activity of DOACs. The data are consistent with the ITC data described earlier.
Andexanet alfa reverses the anticoagulation activity of edoxaban and rivaroxaban. Andexanet alfa dose-dependently reduces plasma concentration of rivaroxaban and edoxaban, respectively. Chromogenic FXa assay was performed to determine the anti-FXa activity of rivaroxaban or edoxaban in plasma treated with antidotes. (A-B) Andexanet alfa reduced plasma concentration of edoxaban; no change in plasma concentration of edoxaban was observed with PER977. At 200 µg/mL andexanet, the plasma concentration of edoxaban decreased by ∼99%. Andexanet alfa reduced plasma concentration of rivaroxaban; no change in plasma concentration of edoxaban was observed with PER977. At 200 µg/mL andexanet, the plasma concentration of rivaroxaban decreased by ∼99%. Results are expressed as the mean ± SE of 6 measurements from 2 independent experiments (n = 2). TEG assay was performed in noncitrated human blood. Andexanet alfa neutralized the anticoagulation activity of edoxaban. However, PER977 did not neutralize the anticoagulation activity of edoxaban. (C-D) 100 µg/mL andexanet alfa normalized the augmented clotting time induced by edoxaban and rivaroxaban. However, 50 µg/mL PER977 did not show edoxaban and rivaroxaban anticoagulation reversal activity. Data are median with interquartile ranges (n = 6 donors). Black horizontal lines represent median, and error bars represent interquartile range. Asterisks indicate significant differences in comparison with the edoxaban control. Mann-Whitney (unpaired, 2-tailed) U tests were performed to determine significance, with P < .05 indicating a statistically significant change (*P = .026 for the edoxaban control vs andexanet alfa–treated groups; *P = .041 for the rivaroxaban control vs andexanet alfa–treated groups).
Andexanet alfa reverses the anticoagulation activity of edoxaban and rivaroxaban. Andexanet alfa dose-dependently reduces plasma concentration of rivaroxaban and edoxaban, respectively. Chromogenic FXa assay was performed to determine the anti-FXa activity of rivaroxaban or edoxaban in plasma treated with antidotes. (A-B) Andexanet alfa reduced plasma concentration of edoxaban; no change in plasma concentration of edoxaban was observed with PER977. At 200 µg/mL andexanet, the plasma concentration of edoxaban decreased by ∼99%. Andexanet alfa reduced plasma concentration of rivaroxaban; no change in plasma concentration of edoxaban was observed with PER977. At 200 µg/mL andexanet, the plasma concentration of rivaroxaban decreased by ∼99%. Results are expressed as the mean ± SE of 6 measurements from 2 independent experiments (n = 2). TEG assay was performed in noncitrated human blood. Andexanet alfa neutralized the anticoagulation activity of edoxaban. However, PER977 did not neutralize the anticoagulation activity of edoxaban. (C-D) 100 µg/mL andexanet alfa normalized the augmented clotting time induced by edoxaban and rivaroxaban. However, 50 µg/mL PER977 did not show edoxaban and rivaroxaban anticoagulation reversal activity. Data are median with interquartile ranges (n = 6 donors). Black horizontal lines represent median, and error bars represent interquartile range. Asterisks indicate significant differences in comparison with the edoxaban control. Mann-Whitney (unpaired, 2-tailed) U tests were performed to determine significance, with P < .05 indicating a statistically significant change (*P = .026 for the edoxaban control vs andexanet alfa–treated groups; *P = .041 for the rivaroxaban control vs andexanet alfa–treated groups).
Given these results, we performed TEG in noncitrated whole blood without adding clotting initiators to verify the ability of andexanet or PER977 to reverse the anticoagulant activity of edoxaban and rivaroxaban. The TEG data show that andexanet normalizes the prolonged clotting time induced by edoxaban and rivaroxaban, respectively (Figure 4C-D; supplemental Figure 8A-D). In contrast, we observed no reversal of edoxaban and rivaroxaban anticoagulation activity with PER977. The concentration of PER977 and andexanet used in the TEG study falls within the therapeutic range.19-23
Reversal of anticoagulant activity of UFH and enoxaparin
Our ITC data show that UHRA, andexanet, and PER977 have 2 common binding partners: UFH and enoxaparin. Indeed, although andexanet and PER977 are principally designed to neutralize DOACs, activity against indirect FXa inhibitors has been indicated for both.20,23 To determine if binding of each of these antidotes to UFH and enoxaparin correlates with anticoagulant neutralization, we performed clotting assays in noncitrated plasma and noncitrated whole blood, respectively.
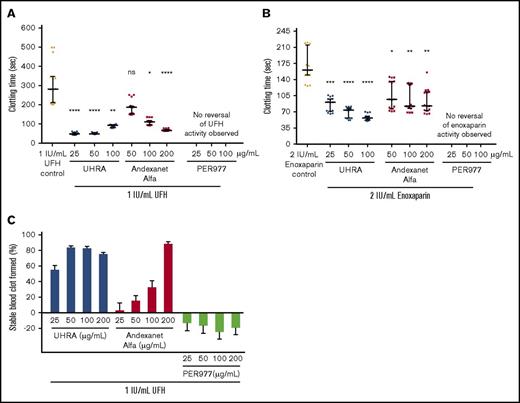
The aPTT assay (Figure 5A-B) shows the reversal of UFH (1 IU/mL) or enoxaparin (2 IU/mL) in noncitrated human plasma. As expected, UHRA was able to neutralize the anticoagulation activity of UFH or enoxaparin at a range of concentrations. Similarly, andexanet was also able to neutralize the anticoagulation activity of UFH or enoxaparin over a range of antidote concentrations; however, neutralization of these heparins by PER977 was not observed at any concentrations studied. In this assay, UHRA was shown to be superior in neutralizing the activity of UFH and enoxaparin.
Andexanet alfa and UHRA reverse the anticoagulation activity UFH and enoxaparin. An aPTT assay was performed in heparinized, noncitrated human platelet–poor plasma. (A-B) Andexanet alfa and UHRA neutralized UFH and enoxaparin anticoagulation activity. However, PER977 at all tested concentrations showed no anticoagulation reversal activity. Experiments were performed in triplicate. Results are expressed as the median with interquartile ranges of 15 measurements from 5 independent experiments (n = 5 donors). Black horizontal lines represent median, and error bars represent interquartile range. Asterisks indicate significant differences. Statistical significance was determined by comparing the antidote treated group to the UFH/enoxaparin control using a Kruskal-Wallis test followed by a Dunn post-test (*P < .05, **P < .02, ***P < .0006, ****P < .0001). (C) Microplate whole-blood clotting assay was performed in heparinized and noncitrated human whole blood. Neutralization of UFH activity was verified by measuring changes in absorbance at 510 nm. Neutralization of anticoagulation activity of UFH and subsequent stable clot generation reduces absorbance. Stable clot formation was observed in heparinized blood containing UHRA (50, 100, and 200 µg/mL) or andexanet alfa (200 µg/mL), but not with PER977. UHRA and andexanet alfa can reverse the anticoagulation activity of UFH. However, we observed an increase in absorbance in PER977-treated samples in comparison with UFH control itself, possibly due to hemolysis. Experiments were performed in triplicate. Results are expressed as the mean ± SE of 15 measurements from 5 independent experiments (n = 5 donors).
Andexanet alfa and UHRA reverse the anticoagulation activity UFH and enoxaparin. An aPTT assay was performed in heparinized, noncitrated human platelet–poor plasma. (A-B) Andexanet alfa and UHRA neutralized UFH and enoxaparin anticoagulation activity. However, PER977 at all tested concentrations showed no anticoagulation reversal activity. Experiments were performed in triplicate. Results are expressed as the median with interquartile ranges of 15 measurements from 5 independent experiments (n = 5 donors). Black horizontal lines represent median, and error bars represent interquartile range. Asterisks indicate significant differences. Statistical significance was determined by comparing the antidote treated group to the UFH/enoxaparin control using a Kruskal-Wallis test followed by a Dunn post-test (*P < .05, **P < .02, ***P < .0006, ****P < .0001). (C) Microplate whole-blood clotting assay was performed in heparinized and noncitrated human whole blood. Neutralization of UFH activity was verified by measuring changes in absorbance at 510 nm. Neutralization of anticoagulation activity of UFH and subsequent stable clot generation reduces absorbance. Stable clot formation was observed in heparinized blood containing UHRA (50, 100, and 200 µg/mL) or andexanet alfa (200 µg/mL), but not with PER977. UHRA and andexanet alfa can reverse the anticoagulation activity of UFH. However, we observed an increase in absorbance in PER977-treated samples in comparison with UFH control itself, possibly due to hemolysis. Experiments were performed in triplicate. Results are expressed as the mean ± SE of 15 measurements from 5 independent experiments (n = 5 donors).
A microplate-based clotting assay in noncitrated whole blood was further performed to confirm UFH neutralization activity of each antidote at different concentrations (25-200 µg/mL). In this assay, the end point is the formation of stable blood clots, assessed on the basis of absorbance (510 nm) of the released red blood cells from blood clots. The buffer control blood clots are considered as 100% stable, whereas UFH control blood clots are considered as 0% stable. The formation of stable blood clots in UFH anticoagulated blood treated with each antidote is correlated to the UFH neutralization activity. A dose-dependent UFH neutralization activity was observed for UHRA reaching close to 84% stable clot at optimal doses. Andexanet demonstrated a dose-dependent and near-complete reversal of UFH at 200 µg/mL (Figure 5C; supplemental Figure 9). In the case of PER977, no UFH neutralization activity was observed, as there were no stable clots formed at any concentration studied (Figure 5C). Surprisingly, negative values were obtained with PER977, as there was an increase in absorbance values in comparison with UFH control itself, possibly due to hemolysis (supplemental Figure 9). These findings, along with our previous reports on UHRA antidote activity,17,18,28 suggest that UHRA is a superior antidote for heparin-based anticoagulants compared with andexanet or PER977.
Discussion
Worldwide, millions of patients receive anticoagulants for the treatment and prevention of thromboembolic disorders.42 Anticoagulant-related bleeding remains a serious concern, and improved reversal strategies with safe and effective antidotes that specifically target anticoagulants are needed.43 Recent studies report that the development of newer antidotes (andexanet, PER977, and UHRA) for both DOACs and heparins is approved, in advanced human clinical trials, or undergoing preclinical studies.17-24 For each of these antidotes under development, binding affinities to their putative targets have not been directly compared. The aim of this study was therefore to provide direct comparison and further insight into the mechanisms of the anticoagulant reversal activities by probing the binding affinities of these antidotes to their presumed targets. Neutralization activities and effects on clot structure in the absence or presence of anticoagulants were also investigated using a panel of orthogonal assays to connect the binding thermodynamics data to function.
UHRA is specifically designed to neutralize the activity of heparin-based anticoagulants,17,18 and the ITC data reported here show that UHRA directly binds heparin-based anticoagulants with micromolar affinity. Moreover, we show that UHRA does not bind relevant coagulation proteins, such as FXa, AT, thrombin, or fibrinogen. As intended, the methoxy-capped polyethylene glycol chains present on the exterior of the UHRA molecule effectively prevent nonspecific interactions with clotting proteins and provide specificity toward anionic heparin anticoagulants. Our clotting assays confirm the heparin neutralization activity of UHRA and are consistent with ITC data. These results collectively suggest that the previously demonstrated ability of UHRA to reverse the activity of all clinically available heparin-based anticoagulants17,18,28 is established through its selective affinity for heparin, which enables UHRA to disrupt heparin-activated AT to form a UHRA–heparin complex.
Our ITC data show that andexanet has high affinity (in the low nanomolar range) for rivaroxaban and edoxaban with a 1:1 stoichiometry. The chromogenic anti-FXa assay data confirms the ability of andexanet to bind and reverse the anti-FXa activity of edoxaban and rivaroxaban, respectively. These findings correlate with reversal of edoxaban and rivaroxaban anticoagulation activity as measured by TEG assay. Reversal of anticoagulant activity was also indicated by restoration of impaired clot fiber formation and clot morphology as measured by SEM analysis. Components of clot structure obtained after neutralization of edoxaban with andexanet were comparable to buffer control clots and suggested complete recovery of normal hemostatic mechanisms. Collectively, the data therefore support high-affinity, direct stoichiometric binding as the mechanism by which andexanet neutralizes each of these DOACs.
The ITC data also record a Kd ∼10−7 M for binding of andexanet to enoxaparin-activated AT. This affinity is ∼50-fold higher than the corresponding andexanet–UFH or andexanet–enoxaparin complex, suggesting that andexanet reverses the activity of indirect FXa inhibitors primarily by binding heparin-activated AT and thereby inhibiting the ability of that complex to interact with FXa,44 while direct sequestration of heparin possibly plays a minor role. Orthogonal plasma and whole-blood clotting assays confirm that neutralization activity.
Andexanet shows no binding to relevant coagulation proteins (Table 1), except to tissue factor pathway inhibitor.44 Moreover, andexanet does not adversely affect fibrin polymerization or alter fibrin and whole-blood clot structures at clinically relevant concentrations (up to 200 μg/mL). At the supratherapeutic concentration studied (500 μg/mL), andexanet shows subtle effects on fibrin polymerization (reduced optical density for fibrin clots) and overall clot morphology.
For PER977, although previous literature cites it as a putative mechanism of action,24 direct binding of PER977 to rivaroxaban or edoxaban was not observed by ITC. In agreement with this observation, the anti-FXa assay performed in citrated plasma shows that PER977 is unable to reverse the anti-FXa activity of edoxaban or rivaroxaban, respectively. Since it has been reported that the presence of citrate or kaolin could adversely affect PER977’s anticoagulant reversal activity,23 we performed TEG studies in noncitrated whole blood without the presence of any clotting activators and found that PER977 did not show reversal of edoxaban or rivaroxaban activity. This is consistent with the previous observation that PER977 does not reverse the anti-FXa activity of FXa inhibitors in buffer systems (performed in the absence of citrate or kaolin).37 However, we observed that clot structure and fibrin fiber diameter were normalized to that of the control when edoxaban-anticoagulated blood was treated with PER977, a result consistent with previously reported data.19 The exception was that neutralization was observed with 200 μg/mL PER977 (400 µM or ∼10X therapeutic dose), which resulted in significantly thicker fibrin fibers than those within the control clots (in analyses performed with utmost care to avoid measuring the diameter of intertwined fibers). When applied to whole blood containing no anticoagulant, PER977 did not affect fibrin polymerization or alter clot structure at any concentrations studied. Thus, if one takes restoration of impaired clot fiber diameter as a metric of reversal activity, PER977 is found to reverse anticoagulation activity induced by edoxaban. Given that direct binding of edoxaban by PER977 was not observed by ITC, the mechanism underpinning this observed restoration of impaired clot fiber diameter is unclear. One of the possible explanations may be related to the measured affinity of PER977 for FIXa (Table 1), as we did not observe an interaction between PER977 and other relevant coagulation proteins.
PER977 showed near-micromolar affinity for UFH or enoxaparin, with binding affinity to enoxaparin weakened when it is precomplexed with AT. The observed binding to enoxaparin aligns with the data indicating capture of PER977 on heparin affinity chromatography columns.23 Once again, however, the ability of PER977 to directly bind these anticoagulants did not correlate with standard measures of reversal activity, as neutralization of UFH or enoxaparin by PER977 was not observed in any clotting assays performed in this study. In our aPTT assay, kaolin was used to trigger the clotting reaction. Therefore, one could argue that the lack of PER977’s activity might be due the interaction of PER977 with kaolin. However, this potential issue was ruled out in our microplate whole-blood clotting assay performed in the absence of citrate or kaolin, where we observed no heparin reversal activity for PER977. Thus, unlike for UHRA and andexanet, a correlation between binding partners and reversal function could not be established for PER977, raising questions with regard to its mode of action depicted in a recent report indicating neutralization of enoxaparin by PER977 based on whole-blood clotting time data.23
Our data, particularly for PER977, provide several insights. First and foremost is the finding that a single metric, such as whole-blood clotting time or fibrin fiber diameter measurements,19 though informative, may not on its own provide a definitive indication of anticoagulant neutralization activity. Although PER977 displays indirect evidence of anticoagulant neutralization based on the restoration of fibrin fiber thickness by SEM analysis, direct reversal of DOACs (edoxaban or rivaroxaban) or heparin-based anticoagulants was not detected in any clotting assays or chromogenic assay performed in this study.
A panel of orthogonal tests of anticoagulant reversal activity, including biological function assessments provided by aPTT, prothrombin time/international normalized ratio, and/or anti-FXa activity,21,22 may therefore be required (or at least advised) to prove therapeutic efficacy by mitigating uncertainties in results obtained from any given assay. For example, while electron micrographs of clots can provide in-depth understanding about the impact of an anticoagulant or antidote on clot components and structure, they provide no direct information on clot formation kinetics or strengths. Conversely, the microplate-based clotting assays and TEG data most often used to characterize neutralization activity do not ensure that drug use results in the desired clot morphologies. Moreover, a degree of uncertainty is associated with each of these assays. For example, clotting end points in whole-blood clotting time, aPTT, TEG, and microplate-based clotting assays can show interindividual variation depending on several factors, including abundances of coagulation factors within the assayed plasma or blood.
Second, for drugs designed as anticoagulant neutralizing agents, verification of specific and selective binding to the intended target is critical and should be evaluated with a sufficient set of orthogonal data or metrics. As demonstrated in this study for PER977, we observe evidence of anticoagulation neutralization activity in some specialty (ie, fiber diameter) metrics, but not in other assays that are more typically used to assess anticoagulant activity.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Derrick Horne at the UBC Bioimaging facility for his assistance in SEM imaging. Iren Constantinescu, Brauna Culibrick, Scott Meixner, and Rolinda Carter at the Centre for Blood Research are thanked for their technical suggestions. Finally, the authors would like to thank all blood donors who participated in this study.
M.T.K. was supported by a Center for Blood Research graduate student award. J.N.K. and C.H. acknowledge funding from Canadian Institutes of Health Research and the Natural Sciences and Engineering Council of Canada. Additional funds for the project were provided by Portola Pharmaceuticals, Inc. J.N.K. and C.H. are recipients of a Michael Smith Foundation for Health Research Career Scholar Award and a Canada Research Chair, respectively. S.A. acknowledges Michael Smith Foundation for Health Research postdoctoral fellowship.
Authorship
Contribution: M.T.K. and A.L.C. performed all experiments, and helped analyze results and write the manuscript; and S.A., G.L., M.J.K., A.P., P.B.C., J.N.K., and C.H. helped design experiments, analyze results, and write and edit the manuscript.
Conflict-of-interest disclosure: J.N.K. and C.H. hold patents for the use of UHRA compounds as antidotes for heparin-based anticoagulants and for treating other blood disorders. G.L., M.J.K., A.P. and P.B.C. are employed by Portola Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: Charles Haynes, Michael Smith Laboratories, University of British Columbia, Vancouver, BC V6T 1Z3, Canada; e-mail: israels@mail.ubc.ca; and Jayachandran N. Kizhakkedathu, Centre for Blood Research, University of British Columbia, Vancouver, BC V6T 1Z3, Canada; e-mail: jay@pathology.ubc.ca.