Key Points
Despite increased incidence of CV events, relative risk of CV AEs with carfilzomib is low and manageable; risk of fatal AEs is not elevated.
Carfilzomib-based regimens have a favorable benefit-risk profile in RRMM; monitoring/management of CV risk is recommended.
Abstract
Carfilzomib is a selective proteasome inhibitor approved for the treatment of relapsed and/or refractory multiple myeloma (RRMM). It has significantly improved outcomes, including overall survival (OS), and shown superiority vs standard treatment with lenalidomide plus dexamethasone and bortezomib plus dexamethasone. The incidence rate of cardiovascular (CV) events with carfilzomib treatment has varied across trials. This analysis evaluated phase 1-3 trials with >2000 RRMM patients exposed to carfilzomib to describe the incidence of CV adverse events (AEs). In addition, the individual CV safety data of >1000 patients enrolled in the carfilzomib arm of phase 3 studies were compared with the control arms to assess the benefit-risk profile of carfilzomib. Pooling data across carfilzomib trials, the CV AEs (grade ≥3) noted included hypertension (5.9%), dyspnea (4.5%), and cardiac failure (4.4%). Although patients receiving carfilzomib had a numeric increase in the rates of any-grade and grade ≥3 cardiac failure, dyspnea, and hypertension, the frequency of discontinuation or death due to these cardiac events was low and comparable between the carfilzomib and control arms. Serial echocardiography in a blinded cardiac substudy showed no objective evidence of cardiac dysfunction in the carfilzomib and control arms. Moreover, carfilzomib had no significant effect on cardiac repolarization. Our results, including the OS benefit, showed that the benefit of carfilzomib treatment in terms of reducing progression or death outweighed the risk for developing cardiac failure or hypertension in most patients. Appropriate carfilzomib administration and risk factor management are recommended for elderly patients and patients with underlying risk factors.
Introduction
Multiple myeloma (MM) patients are at higher risk for cardiovascular (CV) events due to host factors (eg, age and concurrent CV risk factors),1,2 disease factors (eg, renal failure, chronic anemia, concurrent AL amyloidosis, hyperviscosity, A-V shunting),3-6 or toxicity associated with anti-MM treatment. A retrospective cohort study of >23 000 MM patients indicated that 72% of newly diagnosed MM (NDMM) patients and 71% of relapsed and/or refractory MM (RRMM) patients developed cardiac events; arrhythmias (NDMM, 24%; RRMM, 29%) and heart failure (NDMM, 15%; RRMM, 15%) were the 2 most common.7 To better understand the reason for high cardiac event rates, a study of 7895 NDMM patients revealed that they have a significantly higher incidence of hypertension, ischemic heart disease, diabetes mellitus, and hyperlipidemia compared with age- and gender-matched controls.8 Older age (≥75 years) and CV event history are strongly associated with subsequent cardiac adverse events (AEs) in MM patients.
Carfilzomib is a second-generation proteasome inhibitor that irreversibly binds to the proteasome.9 In the head-to-head ENDEAVOR study comparing carfilzomib plus dexamethasone (Kd) with bortezomib plus dexamethasone (Vd), Kd improved the complete response rate, doubled progression-free survival (PFS), and reduced the risk for death by 21%.10,11 Carfilzomib-based combinations have significantly improved PFS and overall survival (OS) by ∼9 and 8 months, respectively, compared with standard treatments (lenalidomide plus dexamethasone [Rd] and Vd) in RRMM patients. Moreover, at first relapse, Kd and carfilzomib, lenalidomide, and dexamethasone (KRd) have improved PFS by 12 months compared with Rd and Vd.12 In pivotal phase 2 and 3 studies, there has been a reported increase in CV AEs.10,13-15 In the phase 3 ASPIRE and ENDEAVOR studies, the grade ≥3 cardiac failure incidence was 3.8% (KRd) vs 1.8% (Rd) and 4.8% (Kd) vs 1.8% (Vd).10,15 Post hoc analyses of ASPIRE and ENDEAVOR data according to age showed increased cardiac failure risk in elderly patients. For KRd, the incidence of grade ≥3 cardiac failure was higher in patients aged >70 years vs <70 years (8.7% vs 2.1%).16 For Kd, grade ≥3 cardiac failure occurred more frequently in patients ≥75 years of age compared with patients 65 to 74 years of age (10.4% vs 4.9%) or those <65 years of age (10.4% vs 2.7%).17
Because carfilzomib is approved and commonly used for RRMM, a clear understanding of its CV safety and benefit-risk profile is important. Herein, we describe CV AE incidences in >2000 carfilzomib-treated patients across phase 1-3 clinical trials. Given the complex interplay among host, disease, and treatment factors leading to CV events, it is difficult to isolate the role of treatment factors in prospective single-arm phase 1/2 studies and retrospective studies. We compare CV events in the carfilzomib and control arms of the ASPIRE, ENDEAVOR, and FOCUS phase 3 trials, in which randomized design facilitates control for confounding host and disease factors. Furthermore, we conducted a benefit-risk analysis comparing the cumulative incidence of cardiac failure with the cumulative incidence of disease progression or death in carfilzomib-treated patients.
Methods
We evaluated 11 phase 1-3 carfilzomib clinical trials with a cumulative enrollment of 2044 patients to describe the incidence of CV AEs. This CV safety analysis was based on phase 1/1b trials (PX-171-001,18 PX-171-002 [NCT00150462],19 PX-171-006 [NCT00603447],20 and PX-171-008),21 phase 2 trials (PX-171-003-A0/A1 [NCT00511238],22,23 PX-171-004 [NCT00530816],24 PX-171-005 [NCT00721734],25 and 2011-002 [NCT01410500]), the pivotal phase 3 trials ASPIRE (PX-171-009 [NCT01080391])15 and ENDEAVOR (2011-003 [NCT01568866])10 that led to the approval of Kd or KRd for RRMM, and the phase 3 FOCUS trial (PX-171-011 [NCT01302392])26 (supplemental Figure 1). Eligibility criteria for phase 1 and 2 studies were presented previously. Key eligibility criteria for FOCUS, ASPIRE, and ENDEAVOR are presented in supplemental Table 1. A majority of phase 1-2 trial patients and all phase 3 trial patients had RRMM. Each study protocol was approved by institutional review boards of all participating institutions. Investigators obtained written informed consent from all patients.
For this extended cardiac safety profile analysis (CV AE incidence) in the ASPIRE, ENDEAVOR, and FOCUS trials and the benefit-risk analysis for CV events (cumulative incidence of CV AEs vs cumulative incidence of progression or death) in the ASPIRE and ENDEAVOR trials, RRMM patients who received KRd (n = 392), Kd (n = 463), and carfilzomib (n = 157) in the ASPIRE, ENDEAVOR, and FOCUS trials, respectively, were designated the “carfilzomib” group. Patients who received Rd (n = 389), Vd (n = 456), and best supportive care (BSC; n = 153) in the ASPIRE, ENDEAVOR, and FOCUS trials, respectively, were designated the “control” group.
Analysis of CV AEs
The cardiac AEs of interest included in this study were cardiac failure, hypertension, dyspnea, and ischemic heart disease. These AEs were further characterized as any grade and grade ≥3. AEs leading to dose reduction, discontinuation, or death were reported. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA; version 15.1) and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03),27 as reported by principal investigators. Cardiac failure and hypertension were grouped per standardized MedDRA query, narrow scope; dyspnea was based on preferred term; and ischemic heart disease was grouped per standardized MedDRA query, broad scope. Dyspnea events were not specifically adjudicated for cardiac or pulmonary causes. AEs were collected from informed consent through 30 days following last study treatment dose. Treatment-emergent AEs were events that began on or after first day of treatment or were conditions present at baseline but worsened in severity after treatment.
All patients who received ≥1 dose of study treatment were included in the safety analysis. Patient-level data were used for the CV analysis. Cardiac AEs were reported by the principal investigator. In the ENDEAVOR trial, AEs were reviewed by an independent committee of 3 cardiologists for cardiac event adjudication based on information supplied by trial sites. All study drug–related cardiac AEs were followed to resolution or stabilization.
ENDEAVOR cardiopulmonary substudy
A subset of the overall ENDEAVOR trial population was enrolled in the substudy. The cardiopulmonary-evaluable subgroup was defined as all randomized patients who enrolled in the substudy before randomization who had evaluable baseline echocardiograms. All patients provided substudy participation consent. The prespecified primary end point of significant change in left ventricular ejection fraction (LVEF) within 24 weeks from baseline was defined as ≥10% absolute reduction in LVEF from baseline for baseline LVEF ≤ 55% or a decrease to <45% from baseline LVEF > 55%.
Echocardiograms were performed at baseline, every 12 weeks (pre-dose on day 1 of Kd cycles 3, 6, 9, and so forth), and at the end of treatment to evaluate LVEF, right ventricular (RV) function, and pulmonary artery systolic pressure (PASP). Echocardiogram results are from the January 2017 data cutoff for the ENDEAVOR trial. Patients were followed for >3 years. Diastolic to systolic RV fractional area change was used as an RV function measurement. A cardiologist at the central echocardiography vendor read the echocardiograms in a blinded manner. The cardiac events adjudication committee determined whether a cardiac failure or pulmonary hypertension–type outcome occurred based on clinically significant changes in echocardiograms over time.
The mean ± standard deviation for LVEF, RV function, and PASP were calculated at each time point. A mixed model for repeated measures under the assumption of missing-at-random was used as the primary analysis to compare absolute values of LVEF, RV function, and PASP between treatment arms. Least squares mean differences between treatment arms and P values at each time point were reported.
The description of exposure-adjusted AE rate and methods for corrected QT interval (QTc) analysis and benefit-risk analysis are presented in the supplemental Methods.
Results
Incidence of CV AEs in pooled analysis
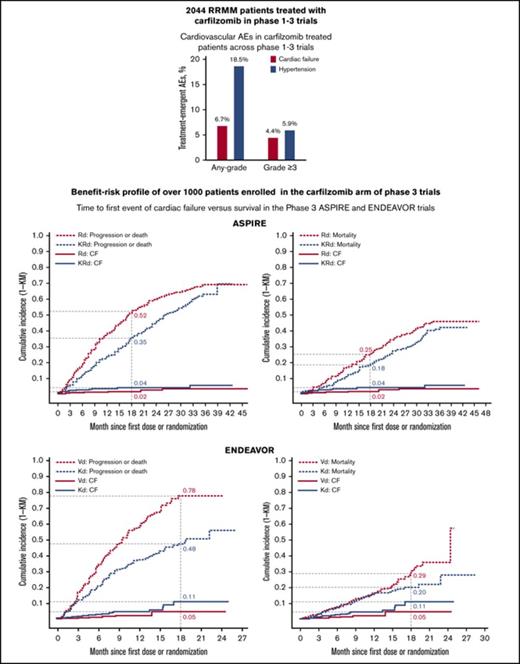
In a pooled analysis of 2044 carfilzomib-exposed patients across phase 1-3 clinical trials, the most commonly reported CV AEs were cardiac failure, hypertension, and dyspnea, with any-grade incidences of 6.7%, 18.5%, and 31.9%, respectively, and grade ≥3 incidences of 4.4%, 5.9%, and 4.5%, respectively (Table 1).
Treatment-emergent CV AEs in carfilzomib-treated patients across RRMM trials (N = 2044)
| . | Any-grade AEs . | Grade ≥3 AEs . | Serious-grade AEs . |
|---|---|---|---|
| Cardiac failure | 137 (6.7) | 90 (4.4) | 79 (3.9) |
| Cardiac failure congestive | 51 (2.5) | 36 (1.8) | 34 (1.7) |
| Cardiac failure | 38 (1.9) | 20 (1.0) | 19 (0.9) |
| Pulmonary edema | 31 (1.5) | 19 (0.9) | 17 (0.8) |
| Ejection fraction decreased | 16 (0.8) | 6 (0.3) | 0 |
| Cardiac failure acute | 7 (0.3) | 5 (0.2) | 4 (0.2) |
| Acute pulmonary edema | 6 (0.3) | 6 (0.3) | 5 (0.2) |
| RV failure | 3 (0.1) | 3 (0.1) | 2 (<0.1) |
| Acute left ventricular failure | 2 (<0.1) | 1 (<0.1) | 2 (<0.1) |
| Cardiopulmonary failure | 2 (<0.1) | 1 (<0.1) | 1 (<0.1) |
| Hepatic congestion | 2 (<0.1) | 2 (<0.1) | 0 |
| Cardiac failure chronic | 1 (<0.1) | 1 (<0.1) | 0 |
| Cardiogenic shock | 1 (<0.1) | 1 (<0.1) | 0 |
| Hepatojugular reflux | 1 (<0.1) | 0 | 0 |
| Hypertension | 378 (18.5) | 120 (5.9) | 13 (0.6) |
| Dyspnea | 653 (31.9) | 92 (4.5) | 48 (2.3) |
| Ischemic heart disease | 75 (3.7) | 40 (2.0) | 36 (1.8) |
| . | Any-grade AEs . | Grade ≥3 AEs . | Serious-grade AEs . |
|---|---|---|---|
| Cardiac failure | 137 (6.7) | 90 (4.4) | 79 (3.9) |
| Cardiac failure congestive | 51 (2.5) | 36 (1.8) | 34 (1.7) |
| Cardiac failure | 38 (1.9) | 20 (1.0) | 19 (0.9) |
| Pulmonary edema | 31 (1.5) | 19 (0.9) | 17 (0.8) |
| Ejection fraction decreased | 16 (0.8) | 6 (0.3) | 0 |
| Cardiac failure acute | 7 (0.3) | 5 (0.2) | 4 (0.2) |
| Acute pulmonary edema | 6 (0.3) | 6 (0.3) | 5 (0.2) |
| RV failure | 3 (0.1) | 3 (0.1) | 2 (<0.1) |
| Acute left ventricular failure | 2 (<0.1) | 1 (<0.1) | 2 (<0.1) |
| Cardiopulmonary failure | 2 (<0.1) | 1 (<0.1) | 1 (<0.1) |
| Hepatic congestion | 2 (<0.1) | 2 (<0.1) | 0 |
| Cardiac failure chronic | 1 (<0.1) | 1 (<0.1) | 0 |
| Cardiogenic shock | 1 (<0.1) | 1 (<0.1) | 0 |
| Hepatojugular reflux | 1 (<0.1) | 0 | 0 |
| Hypertension | 378 (18.5) | 120 (5.9) | 13 (0.6) |
| Dyspnea | 653 (31.9) | 92 (4.5) | 48 (2.3) |
| Ischemic heart disease | 75 (3.7) | 40 (2.0) | 36 (1.8) |
Pooled analysis is from the phase 1 trials (PX-171-001, PX-171-002, PX-171-006, PX-171-008), the phase 2 trials (PX-171-003-A0, PX-171-003-A1, PX-171-004, PX-171-005, and 2011-002), and the phase 3 ASPIRE (PX-171-009), ENDEAVOR (2011-003), and FOCUS trials (PX-171-011). All data are n (%). Cardiac failure and hypertension events are listed as standardized MedDRA query, narrow scope, and ischemic heart disease event is listed as standardized MedDRA query, broad scope.
Patient and study characteristics in phase 3 trials
To better understand carfilzomib’s CV safety and benefit-risk profile, a more detailed comparative analysis was performed with 1012 carfilzomib-treated patients across the phase 3 ASPIRE, ENDEAVOR, and FOCUS trials (KRd, n = 392; Kd, n = 463; carfilzomib, n = 157). In the control arms, 389, 456, and 153 patients received Rd, Vd, and BSC, respectively. Baseline and disease characteristics were generally balanced between treatment arms, although a higher proportion had a history of cardiac failure in the KRd arm (4.3%) vs the Rd arm (1.3%) and in the BSC arm (6.5%) vs the carfilzomib arm (3.8%) (Table 2).
Patients’ baseline and disease characteristics
| Characteristic . | ASPIRE . | ENDEAVOR . | FOCUS . | |||
|---|---|---|---|---|---|---|
| Carfilzomib (KRd) . | Control (Rd) . | Carfilzomib (Kd) . | Control (Vd) . | Carfilzomib (carfilzomib) . | Control (BSC) . | |
| n | 392 | 389 | 463 | 456 | 157 | 153 |
| Age, median (range), y | 64.0 (38-87) | 65.0 (31-91) | 65.0 (35-89) | 65.0 (30-88) | 63.0 (32-85) | 66.0 (43-81) |
| Age ≥75 y, n (%) | 43 (11.0) | 50 (12.9) | 77 (16.6) | 65 (14.3) | 25 (15.9) | 23 (15.0) |
| Sex, male/female, % | 53.8/46.2 | 58.6/41.4 | 51.8/48.2 | 48.9/51.1 | 52.2/47.8 | 60.1/39.9 |
| Eastern Cooperative Oncology Group performance status, n (%) | ||||||
| 0 | 164 (41.8) | 174 (44.7) | 220 (47.5) | 228 (50.0) | 49 (31.2) | 30 (19.6) |
| 1 | 189 (48.2) | 181 (46.5) | 211 (45.6) | 199 (43.6) | 78 (49.7) | 89 (58.2) |
| 2 | 39 (9.9) | 34 (8.7) | 32 (6.9) | 29 (6.4) | 29 (18.5) | 33 (21.6) |
| International Staging System, n (%) | ||||||
| I | 167 (42.6) | 152 (39.1) | 204 (44.1) | 200 (43.9) | 29 (18.5) | 31 (20.3) |
| II-III | 217 (55.4) | 230 (59.1) | 259 (55.9) | 256 (56.1) | 125 (79.6) | 120 (78.4) |
| Serum β2 microglobulin, median (range), mg/L | 3.5 (1.3-13.0) | 3.6 (1.5-31.7) | 3.6 (1.4-24.2) | 3.7 (1.2-31.6) | 5.3 (2.0-35.7) | 5.9 (1.8-49.6) |
| Creatinine clearance, n (%), mL/min | ||||||
| <30 | 0 | 1 (0.3) | 28 (6.0) | 28 (6.1) | 16 (10.2) | 13 (8.5) |
| 30 to <50 | 24 (6.1) | 30 (7.7) | 57 (12.3) | 69 (15.1) | 29 (18.5) | 40 (26.1) |
| 50 to <80 | 170 (43.4) | 150 (38.6) | 186 (40.2) | 174 (38.2) | 64 (40.8) | 58 (37.9) |
| ≥80 | 197 (50.3) | 203 (52.2) | 192 (41.5) | 185 (40.6) | 48 (30.6) | 41 (26.8) |
| Medical history, n (%) | ||||||
| History of hypertension | 194 (49.5) | 178 (45.8) | 233 (50.3) | 221 (48.5) | 60 (38.2) | 75 (49.0) |
| History of cardiac failure | 17 (4.3) | 5 (1.3) | 14 (3.0) | 13 (2.9) | 6 (3.8) | 10 (6.5) |
| History of cardiac arrhythmias | 44 (11.2) | 38 (9.8) | 27 (5.8) | 33 (7.2) | 18 (11.5) | 17 (11.1) |
| Characteristic . | ASPIRE . | ENDEAVOR . | FOCUS . | |||
|---|---|---|---|---|---|---|
| Carfilzomib (KRd) . | Control (Rd) . | Carfilzomib (Kd) . | Control (Vd) . | Carfilzomib (carfilzomib) . | Control (BSC) . | |
| n | 392 | 389 | 463 | 456 | 157 | 153 |
| Age, median (range), y | 64.0 (38-87) | 65.0 (31-91) | 65.0 (35-89) | 65.0 (30-88) | 63.0 (32-85) | 66.0 (43-81) |
| Age ≥75 y, n (%) | 43 (11.0) | 50 (12.9) | 77 (16.6) | 65 (14.3) | 25 (15.9) | 23 (15.0) |
| Sex, male/female, % | 53.8/46.2 | 58.6/41.4 | 51.8/48.2 | 48.9/51.1 | 52.2/47.8 | 60.1/39.9 |
| Eastern Cooperative Oncology Group performance status, n (%) | ||||||
| 0 | 164 (41.8) | 174 (44.7) | 220 (47.5) | 228 (50.0) | 49 (31.2) | 30 (19.6) |
| 1 | 189 (48.2) | 181 (46.5) | 211 (45.6) | 199 (43.6) | 78 (49.7) | 89 (58.2) |
| 2 | 39 (9.9) | 34 (8.7) | 32 (6.9) | 29 (6.4) | 29 (18.5) | 33 (21.6) |
| International Staging System, n (%) | ||||||
| I | 167 (42.6) | 152 (39.1) | 204 (44.1) | 200 (43.9) | 29 (18.5) | 31 (20.3) |
| II-III | 217 (55.4) | 230 (59.1) | 259 (55.9) | 256 (56.1) | 125 (79.6) | 120 (78.4) |
| Serum β2 microglobulin, median (range), mg/L | 3.5 (1.3-13.0) | 3.6 (1.5-31.7) | 3.6 (1.4-24.2) | 3.7 (1.2-31.6) | 5.3 (2.0-35.7) | 5.9 (1.8-49.6) |
| Creatinine clearance, n (%), mL/min | ||||||
| <30 | 0 | 1 (0.3) | 28 (6.0) | 28 (6.1) | 16 (10.2) | 13 (8.5) |
| 30 to <50 | 24 (6.1) | 30 (7.7) | 57 (12.3) | 69 (15.1) | 29 (18.5) | 40 (26.1) |
| 50 to <80 | 170 (43.4) | 150 (38.6) | 186 (40.2) | 174 (38.2) | 64 (40.8) | 58 (37.9) |
| ≥80 | 197 (50.3) | 203 (52.2) | 192 (41.5) | 185 (40.6) | 48 (30.6) | 41 (26.8) |
| Medical history, n (%) | ||||||
| History of hypertension | 194 (49.5) | 178 (45.8) | 233 (50.3) | 221 (48.5) | 60 (38.2) | 75 (49.0) |
| History of cardiac failure | 17 (4.3) | 5 (1.3) | 14 (3.0) | 13 (2.9) | 6 (3.8) | 10 (6.5) |
| History of cardiac arrhythmias | 44 (11.2) | 38 (9.8) | 27 (5.8) | 33 (7.2) | 18 (11.5) | 17 (11.1) |
Cardiac failure and hypertension events are listed as standardized MedDRA query, narrow scope and ischemic heart disease event is listed as standardized MedDRA query, broad scope.
Treatment exposure was longer in the carfilzomib arm vs the control arms of the phase 3 trials, with a median duration of 72, 39.9, and 16.3 weeks of KRd, Kd, and carfilzomib, respectively, and 56.7 weeks of lenalidomide in the Rd arm, 26.8 weeks of bortezomib in the Vd arm, and 10.7 and 10.1 weeks of corticosteroid and cyclophosphamide, respectively, in the BSC arm (Table 3).
Exposure to study treatment in phase 3 trials
| . | ASPIRE . | ENDEAVOR . | FOCUS . | |||||
|---|---|---|---|---|---|---|---|---|
| Carfilzomib . | Lenalidomide . | Carfilzomib . | Bortezomib . | Carfilzomib . | BSC . | |||
| KRd . | KRd . | Rd (control) . | Kd . | Vd (control) . | Carfilzomib . | Corticosteroid . | Cyclophosphamide . | |
| n | 392 | 391 | 392 | 463 | 456 | 157 | 153 | 145 |
| Treatment duration, median (range), wk | 72.0 (1.0-93.1) | 85.0 (0.1-185.0) | 56.7 (0.4-200.7) | 39.9 (1.0-108.1) | 26.8 (1.0-106.1) | 16.3 (0.3-138.4) | 10.7 (0.4-138.3) | 10.1 (0.10-138.3) |
| Average dose per administration, median (range)*† | 26.8 mg/m2 (15.3-26.9) | 25.0 mg (6.3-25.1) | 25.0 mg (6.6-25.0) | 55.9 mg/m2 (19.5-59.9) | 1.2 mg/m2 (0.7-1.7) | 26.4 mg/m2 (20.0-26.9) | 65.0 mg (6.6-200.0) | 1 083 mg (36.2-2 567.0) |
| Total dose received during study, median (range) | 2362 mg/m2 (20.0-2902.0) | 7875 mg (25.0-23400.0) | 5825 mg (75.0-26375.0) | 2898 mg/m2 (39.6-8288.0) | 36.3 mg/m2 (1.2-190.9) | 661.0 mg/m2 (40.0-4711.0) | 204 mg (9.0-2868.0) | 3 200 mg (46.1-48 400.0) |
| . | ASPIRE . | ENDEAVOR . | FOCUS . | |||||
|---|---|---|---|---|---|---|---|---|
| Carfilzomib . | Lenalidomide . | Carfilzomib . | Bortezomib . | Carfilzomib . | BSC . | |||
| KRd . | KRd . | Rd (control) . | Kd . | Vd (control) . | Carfilzomib . | Corticosteroid . | Cyclophosphamide . | |
| n | 392 | 391 | 392 | 463 | 456 | 157 | 153 | 145 |
| Treatment duration, median (range), wk | 72.0 (1.0-93.1) | 85.0 (0.1-185.0) | 56.7 (0.4-200.7) | 39.9 (1.0-108.1) | 26.8 (1.0-106.1) | 16.3 (0.3-138.4) | 10.7 (0.4-138.3) | 10.1 (0.10-138.3) |
| Average dose per administration, median (range)*† | 26.8 mg/m2 (15.3-26.9) | 25.0 mg (6.3-25.1) | 25.0 mg (6.6-25.0) | 55.9 mg/m2 (19.5-59.9) | 1.2 mg/m2 (0.7-1.7) | 26.4 mg/m2 (20.0-26.9) | 65.0 mg (6.6-200.0) | 1 083 mg (36.2-2 567.0) |
| Total dose received during study, median (range) | 2362 mg/m2 (20.0-2902.0) | 7875 mg (25.0-23400.0) | 5825 mg (75.0-26375.0) | 2898 mg/m2 (39.6-8288.0) | 36.3 mg/m2 (1.2-190.9) | 661.0 mg/m2 (40.0-4711.0) | 204 mg (9.0-2868.0) | 3 200 mg (46.1-48 400.0) |
Median PFS (months) for ASPIRE (Kd, 26.3; Vd, 17.6), ENDEAVOR (Kd, 18.7; Vd, 9.4) and FOCUS (K, 3.7; BSC; 3.3).
Values for average dose per administration are for the time period beyond cycle 1 in the ENDEAVOR trial.
Values represent average dose per cycle (in milligrams) for corticosteroid and cyclophosphamide in the FOCUS trial.
CV treatment-emergent AEs, treatment discontinuations, and deaths in phase 3 trials
The rates of any-grade and grade ≥3 CV AEs (cardiac failure, hypertension, dyspnea, and ischemic heart disease) for the phase 3 ASPIRE, ENDEAVOR, and FOCUS trials are summarized in Table 4. The exposure-adjusted cardiac failure incidence rate, which specified the number of patients with a cardiac failure event over exposure time, was similar between the KRd and Rd arms in the ASPIRE trial (3.94 and 3.14, respectively) and was higher for the Kd arm than for the Vd arm in the ENDEAVOR trial (11.23 and 5.17, respectively) (Table 5). Exposure-adjusted risk for hypertension was higher in the carfilzomib arm vs the control arm in the ASPIRE and ENDEAVOR trials (Table 5). Cardiac failure event outcomes in the phase 3 trials are summarized in Table 6.
Treatment-emergent CV AEs, treatment reduction, discontinuation, and deaths in the ASPIRE, ENDEAVOR, and FOCUS phase 3 trials
| . | ASPIRE . | ENDEAVOR . | FOCUS . | |||
|---|---|---|---|---|---|---|
| Carfilzomib (Krd) (n = 392) . | Control (Rd) (n = 389) . | Carfilzomib (Kd) (n = 463) . | Control (Vd) (n = 456) . | Carfilzomib (carfilzomib) (n = 157) . | Control (BSC) (n = 153) . | |
| Carfilzomib dose, mg/m2 | 20/27 | 20/56 | 20/27 | |||
| Treatment-emergent cardiac AEs of interest, any-grade | ||||||
| Cardiac failure | 25 (6.4) | 16 (4.1) | 38 (8.2) | 13 (2.9) | 12 (7.6) | 7 (4.6) |
| Hypertension | 62 (15.8) | 32 (8.2) | 120 (25.9) | 44 (9.6) | 25 (15.9) | 9 (5.9) |
| Dyspnea | 89 (22.7) | 70 (18.0) | 143 (30.9) | 78 (17.1) | 25 (15.9) | 19 (12.4) |
| Ischemic heart disease | 23 (5.9) | 18 (4.6) | 13 (2.8) | 9 (2.0) | 3 (1.9) | 0 |
| Treatment-emergent cardiac AEs of interest, grade ≥3 | ||||||
| Cardiac failure | 15 (3.8) | 7 (1.8) | 22 (4.8) | 8 (1.8) | 9 (5.7) | 5 (3.3) |
| Hypertension | 22 (5.6) | 8 (2.1) | 44 (9.5) | 12 (2.6) | 6 (3.8) | 0 |
| Dyspnea | 12 (3.1) | 8 (2.1) | 26 (5.6) | 10 (2.2) | 3 (1.9) | 0 |
| Ischemic heart disease | 13 (3.3) | 8 (2.1) | 8 (1.7) | 7 (1.5) | 1 (0.6) | 0 |
| Reason for treatment reduction | ||||||
| Cardiac failure | 4 (1.0) | 3 (0.8) | 7 (1.5) | 0 | 0 | 0 |
| Hypertension | 3 (0.8) | 3 (0.8) | 15 (3.2) | 3 (0.7) | 0 | 0 |
| Dyspnea | 5 (1.3) | 1 (0.3) | 13 (2.8) | 5 (1.1) | 0 | 0 |
| Ischemic heart disease | 1 (0.3) | 2 (0.5) | 3 (0.6) | 0 | 0 | 0 |
| Reason for treatment discontinuation | ||||||
| Cardiac failure | 2 (0.5) | 3 (0.8) | 13 (2.8) | 4 (0.9) | 4 (2.5) | 2 (1.3) |
| Hypertension | 1 (0.3) | 1 (0.3) | 2 (0.4) | 0 | 0 | 1 (0.7) |
| Dyspnea | 0 | 1 (0.3) | 5 (1.1) | 6 (1.3) | 0 | 2 (1.3) |
| Ischemic heart disease | 5 (1.3) | 2 (0.5) | 5 (1.1) | 3 (0.7) | 0 | 0 |
| Cause of death | ||||||
| Cardiac failure | 3 (0.8) | 4 (1.0) | 2 (0.4) | 2 (0.4) | 2 (1.3) | 2 (1.3) |
| Hypertension | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 |
| Ischemic heart disease | 3 (0.8) | 2 (0.5) | 0 | 3 (0.7) | 0 | 0 |
| . | ASPIRE . | ENDEAVOR . | FOCUS . | |||
|---|---|---|---|---|---|---|
| Carfilzomib (Krd) (n = 392) . | Control (Rd) (n = 389) . | Carfilzomib (Kd) (n = 463) . | Control (Vd) (n = 456) . | Carfilzomib (carfilzomib) (n = 157) . | Control (BSC) (n = 153) . | |
| Carfilzomib dose, mg/m2 | 20/27 | 20/56 | 20/27 | |||
| Treatment-emergent cardiac AEs of interest, any-grade | ||||||
| Cardiac failure | 25 (6.4) | 16 (4.1) | 38 (8.2) | 13 (2.9) | 12 (7.6) | 7 (4.6) |
| Hypertension | 62 (15.8) | 32 (8.2) | 120 (25.9) | 44 (9.6) | 25 (15.9) | 9 (5.9) |
| Dyspnea | 89 (22.7) | 70 (18.0) | 143 (30.9) | 78 (17.1) | 25 (15.9) | 19 (12.4) |
| Ischemic heart disease | 23 (5.9) | 18 (4.6) | 13 (2.8) | 9 (2.0) | 3 (1.9) | 0 |
| Treatment-emergent cardiac AEs of interest, grade ≥3 | ||||||
| Cardiac failure | 15 (3.8) | 7 (1.8) | 22 (4.8) | 8 (1.8) | 9 (5.7) | 5 (3.3) |
| Hypertension | 22 (5.6) | 8 (2.1) | 44 (9.5) | 12 (2.6) | 6 (3.8) | 0 |
| Dyspnea | 12 (3.1) | 8 (2.1) | 26 (5.6) | 10 (2.2) | 3 (1.9) | 0 |
| Ischemic heart disease | 13 (3.3) | 8 (2.1) | 8 (1.7) | 7 (1.5) | 1 (0.6) | 0 |
| Reason for treatment reduction | ||||||
| Cardiac failure | 4 (1.0) | 3 (0.8) | 7 (1.5) | 0 | 0 | 0 |
| Hypertension | 3 (0.8) | 3 (0.8) | 15 (3.2) | 3 (0.7) | 0 | 0 |
| Dyspnea | 5 (1.3) | 1 (0.3) | 13 (2.8) | 5 (1.1) | 0 | 0 |
| Ischemic heart disease | 1 (0.3) | 2 (0.5) | 3 (0.6) | 0 | 0 | 0 |
| Reason for treatment discontinuation | ||||||
| Cardiac failure | 2 (0.5) | 3 (0.8) | 13 (2.8) | 4 (0.9) | 4 (2.5) | 2 (1.3) |
| Hypertension | 1 (0.3) | 1 (0.3) | 2 (0.4) | 0 | 0 | 1 (0.7) |
| Dyspnea | 0 | 1 (0.3) | 5 (1.1) | 6 (1.3) | 0 | 2 (1.3) |
| Ischemic heart disease | 5 (1.3) | 2 (0.5) | 5 (1.1) | 3 (0.7) | 0 | 0 |
| Cause of death | ||||||
| Cardiac failure | 3 (0.8) | 4 (1.0) | 2 (0.4) | 2 (0.4) | 2 (1.3) | 2 (1.3) |
| Hypertension | 0 | 0 | 0 | 0 | 0 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 |
| Ischemic heart disease | 3 (0.8) | 2 (0.5) | 0 | 3 (0.7) | 0 | 0 |
Cardiac failure and hypertension events are listed as standardized MedDRA query, narrow scope, and ischemic heart disease is listed as standardized MedDRA query, broad scope. All data are n (%), unless indicated otherwise.
Exposure-adjusted incidence of treatment-emergent CV AEs in the ASPIRE and ENDEAVOR trials
| . | ASPIRE . | ENDEAVOR . | ||||||
|---|---|---|---|---|---|---|---|---|
| KRd . | Rd . | Kd . | Vd . | |||||
| Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | |
| Cardiac failure | 6.3 | 3.94 (2.66-5.83) | 5.1 | 3.14 (1.92-5.12) | 3.4 | 11.23 (8.17-15.44) | 2.5 | 5.17 (3.00-8.90) |
| Hypertension | 5.7 | 10.62 (8.26-13.65) | 5.0 | 6.05 (4.23-8.66) | 2.9 | 41.97 (35.09-50.19) | 2.3 | 19.04 (14.17-25.59) |
| Dyspnea | 5.6 | 15.82 (12.84-19.50) | 4.5 | 15.66 (12.39-19.79) | 2.6 | 54.37 (46.15-64.05) | 2.2 | 36.02 (28.85-44.97) |
| Ischemic heart disease | 6.3 | 3.63 (2.41-5.46) | 5.1 | 3.53 (2.22-5.60) | 3.4 | 3.79 (2.20-6.53) | 2.5 | 3.59 (1.87-6.90) |
| . | ASPIRE . | ENDEAVOR . | ||||||
|---|---|---|---|---|---|---|---|---|
| KRd . | Rd . | Kd . | Vd . | |||||
| Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | Total person time (100 subject years)* . | Exposure adjusted risk estimate (95% CI) . | |
| Cardiac failure | 6.3 | 3.94 (2.66-5.83) | 5.1 | 3.14 (1.92-5.12) | 3.4 | 11.23 (8.17-15.44) | 2.5 | 5.17 (3.00-8.90) |
| Hypertension | 5.7 | 10.62 (8.26-13.65) | 5.0 | 6.05 (4.23-8.66) | 2.9 | 41.97 (35.09-50.19) | 2.3 | 19.04 (14.17-25.59) |
| Dyspnea | 5.6 | 15.82 (12.84-19.50) | 4.5 | 15.66 (12.39-19.79) | 2.6 | 54.37 (46.15-64.05) | 2.2 | 36.02 (28.85-44.97) |
| Ischemic heart disease | 6.3 | 3.63 (2.41-5.46) | 5.1 | 3.53 (2.22-5.60) | 3.4 | 3.79 (2.20-6.53) | 2.5 | 3.59 (1.87-6.90) |
Cardiac failure and hypertension events are listed as standardized MedDRA query, narrow scope, and ischemic heart disease is listed as standardized MedDRA query, broad scope. Dyspnea is a high-level term.
Total person time is the sum of the time to first treatment-emergent AE for all patients in each treatment group.
Outcome of cardiac failure events in the ASPIRE, ENDEAVOR, and FOCUS trials
| . | ASPIRE . | ENDEAVOR . | FOCUS . | |||
|---|---|---|---|---|---|---|
| Carfilzomib (KRd) . | Control (Rd) . | Carfilzomib (Kd) . | Control (Vd) . | Carfilzomib (carfilzomib) . | Control (BSC) . | |
| Patients with cardiac failure, n | 25 | 16 | 38 | 13 | 12 | 7 |
| Any-grade (standardized MedDRA query, narrow scope) cardiac failure outcome, n (%) | ||||||
| Resolved | 15 (60.0) | 6 (37.5) | 14 (36.8) | 8 (61.5) | 6 (50.0) | 1 (14.3) |
| Resolved with sequelae | 2 (8) | 0 | 0 | 0 | 0 | 0 |
| Not resolved | 10 (40) | 10 (62.5) | 23 (60.5) | 5 (38.5) | 6 (50) | 5 (71.4) |
| Deaths | 3 (12.0) | 4 (25.0) | 2 (5.3) | 2 (15.4) | 2 (16.7) | 2 (28.6) |
| . | ASPIRE . | ENDEAVOR . | FOCUS . | |||
|---|---|---|---|---|---|---|
| Carfilzomib (KRd) . | Control (Rd) . | Carfilzomib (Kd) . | Control (Vd) . | Carfilzomib (carfilzomib) . | Control (BSC) . | |
| Patients with cardiac failure, n | 25 | 16 | 38 | 13 | 12 | 7 |
| Any-grade (standardized MedDRA query, narrow scope) cardiac failure outcome, n (%) | ||||||
| Resolved | 15 (60.0) | 6 (37.5) | 14 (36.8) | 8 (61.5) | 6 (50.0) | 1 (14.3) |
| Resolved with sequelae | 2 (8) | 0 | 0 | 0 | 0 | 0 |
| Not resolved | 10 (40) | 10 (62.5) | 23 (60.5) | 5 (38.5) | 6 (50) | 5 (71.4) |
| Deaths | 3 (12.0) | 4 (25.0) | 2 (5.3) | 2 (15.4) | 2 (16.7) | 2 (28.6) |
To better understand the significance of AEs, we compared common CV AEs leading to treatment reduction. In the ASPIRE and ENDEAVOR trials, these were dyspnea (KRd, 1.3%; Rd, 0.3%; Kd, 2.8%; Vd, 1.1%), cardiac failure (KRd, 1.0%; Rd, 0.8%; Kd, 1.5%; Vd, 0%), and hypertension (KRd, 0.8%; Rd, 0.8%; Kd, 3.2%; Vd, 0.7%) (Table 4). No dose reduction due to CV AEs was reported in the carfilzomib or control arm of the FOCUS trial. Cardiac failure resulted in treatment discontinuation in 2 KRd patients (0.5%) and 3 Rd patients (0.8%) in the ASPIRE trial, 13 Kd patients (2.8%) and 4 Vd patients (0.9%) in the ENDEAVOR trial, and 4 carfilzomib patients (2.5%) and 2 BSC patients (1.3%) in the FOCUS trial (Table 4). Overall, 2.6%, 3.5%, and 3.2% of patients in the carfilzomib arm of the ASPIRE, ENDEAVOR, and FOCUS phase 3 studies, respectively, experienced cardiac failure events that led to missed doses of carfilzomib. Carfilzomib was reinstated in 70% (ASPIRE), 75% (ENDEAVOR), and 80% (FOCUS) of patients following a missed dose.
The incidence of death due to cardiac failure was similar across the phase 3 trial treatments. Deaths due to cardiac failure AEs occurred in 3 patients (0.8%) receiving KRd and 4 patients (1.0%) receiving Rd, 2 patients (0.4%) receiving Kd or Vd, and 2 patients (1.3%) receiving carfilzomib or BSC (Table 4). Ischemic heart disease AEs leading to death occurred in 3 patients (0.8%) in the KRd arm, 2 patients (0.5%) in the Rd arm, 3 patients (0.7%) in the Vd arm, and no patient in the Kd arm. No deaths from hypertension or dyspnea AEs were reported in the ASPIRE, ENDEAVOR, and FOCUS trials.
QTc analysis
Electrocardiographic data were analyzed to determine the impact of carfilzomib on cardiac function in patients with advanced malignancies, including MM. Carfilzomib plasma concentration had no effect on QT interval with Fridericia's correction (QTcF interval) (supplemental Figure 2). The upper bounds of 1-sided 95% confidence intervals (CIs) for predicted effects on QTcF and QT interval with Bazett’s correction at maximum concentration were 4.8 and 5.9 milliseconds, respectively.
ENDEAVOR cardiac substudy
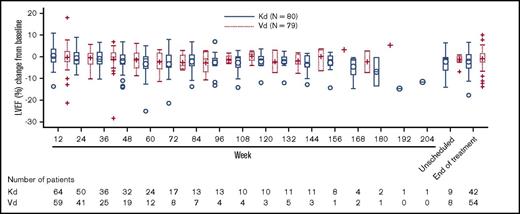
Of 929 patients in the ENDEAVOR trial, 159 patients (80 Kd and 79 Vd) enrolled in the substudy. For the primary end point, only 1 patient (Vd) had a significant reduction in LVEF within the first 24 weeks. Overall, 6 patients (3 Kd and 3 Vd) had a significant decrease in LVEF at any time during the study that was mostly reversible. Serial echocardiograms performed at baseline, every 3 months (for ∼45 months), and at the end of the study indicated that change in LVEF from baseline values for each visit was similar across groups throughout the study (Figure 1). The mixed model for repeated measures indicated no significant treatment or treatment-by-time interactions and no significant difference between the Kd and Vd arms for LVEF and RV function at any time point (Table 7).
Change in LVEF from baseline: results from the ENDEAVOR cardiopulmonary substudy.
Change in LVEF from baseline: results from the ENDEAVOR cardiopulmonary substudy.
LVEF, RV function, and PASP estimation by mixed-model for repeated measures: data from the ENDEAVOR cardiopulmonary substudy
| Visit . | LVEF estimation . | RV function estimation . | PASP estimation . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean estimate (Kd), % . | Mean estimate (Vd), % . | P . | Mean estimate (Kd), % . | Mean estimate (Vd), % . | P . | Mean estimate (Kd), mm Hg . | Mean estimate (Vd), mm Hg . | P . | |
| Baseline | 63.3 | 64.2 | — | 42.8 | 43.4 | — | 26.4 | 23.2 | — |
| Week 12 | 63.38 | 63.70 | 0.6106 | 41.91 | 42.92 | 0.1025 | 28.72 | 25.30 | 0.0288 |
| Week 24 | 62.84 | 63.22 | 0.5279 | 42.08 | 42.92 | 0.0936 | 28.00 | 26.23 | 0.2154 |
| Week 36 | 62.29 | 62.75 | 0.5223 | 42.24 | 42.93 | 0.1804 | 27.27 | 27.15 | 0.9409 |
| Week 48 | 61.75 | 62.27 | 0.5554 | 42.41 | 42.94 | 0.4046 | 26.55 | 28.08 | 0.4645 |
| Week 60 | 61.21 | 61.79 | 0.5925 | 42.57 | 42.94 | 0.6508 | 25.82 | 29.00 | 0.2349 |
| Week 72 | 60.66 | 61.32 | 0.6239 | 42.74 | 42.95 | 0.8363 | 25.10 | 29.92 | 0.1464 |
| Week 84 | 60.12 | 60.84 | 0.6487 | 42.90 | 42.96 | 0.9641 | 24.37 | 30.85 | 0.1058 |
| Week 96 | 59.58 | 60.36 | 0.6683 | 43.07 | 42.96 | 0.9471 | 23.65 | 31.77 | 0.0840 |
| Visit . | LVEF estimation . | RV function estimation . | PASP estimation . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean estimate (Kd), % . | Mean estimate (Vd), % . | P . | Mean estimate (Kd), % . | Mean estimate (Vd), % . | P . | Mean estimate (Kd), mm Hg . | Mean estimate (Vd), mm Hg . | P . | |
| Baseline | 63.3 | 64.2 | — | 42.8 | 43.4 | — | 26.4 | 23.2 | — |
| Week 12 | 63.38 | 63.70 | 0.6106 | 41.91 | 42.92 | 0.1025 | 28.72 | 25.30 | 0.0288 |
| Week 24 | 62.84 | 63.22 | 0.5279 | 42.08 | 42.92 | 0.0936 | 28.00 | 26.23 | 0.2154 |
| Week 36 | 62.29 | 62.75 | 0.5223 | 42.24 | 42.93 | 0.1804 | 27.27 | 27.15 | 0.9409 |
| Week 48 | 61.75 | 62.27 | 0.5554 | 42.41 | 42.94 | 0.4046 | 26.55 | 28.08 | 0.4645 |
| Week 60 | 61.21 | 61.79 | 0.5925 | 42.57 | 42.94 | 0.6508 | 25.82 | 29.00 | 0.2349 |
| Week 72 | 60.66 | 61.32 | 0.6239 | 42.74 | 42.95 | 0.8363 | 25.10 | 29.92 | 0.1464 |
| Week 84 | 60.12 | 60.84 | 0.6487 | 42.90 | 42.96 | 0.9641 | 24.37 | 30.85 | 0.1058 |
| Week 96 | 59.58 | 60.36 | 0.6683 | 43.07 | 42.96 | 0.9471 | 23.65 | 31.77 | 0.0840 |
Data are least squares mean estimates.
—, P values were not reported at baseline for LVEF, RV function, and PASP estimations.
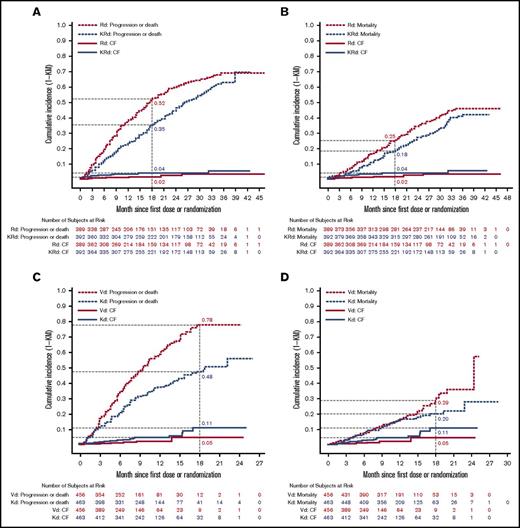
Benefit-risk analysis: time to first event of cardiac failure and PFS or OS in the ASPIRE and ENDEAVOR trials
In the ASPIRE trial, the cumulative grade ≥3 cardiac failure incidence was slightly higher for KRd than for Rd. At 18 months from randomization, the cumulative grade ≥3 cardiac failure incidence was 4% for KRd and 2% for Rd (Figure 2A-B). The cumulative incidence of MM disease progression or death at 18 months was 35% (KRd) vs 52% (Rd) (Figure 2A), and the cumulative incidence of death due to any cause was 18% (KRd) vs 25% (Rd) (Figure 2B). The cumulative incidence of grade ≥3 cardiac failure for the ENDEAVOR trial was greater for Kd than for Vd at 18 months from randomization (11% vs 5%) (Figure 2C-D). The cumulative incidence of MM disease progression or death at 18 months was 48% (Kd) vs 78% (Vd) (Figure 2C), and the cumulative incidence of death due to any cause was 20% (Kd) vs 29% (Vd) (Figure 2D). The fact that the AE incidence was based on the number of patients at risk and that the duration of therapy was longer in the carfilzomib treatment arms (due to longer PFS) could have contributed to the increased reported AEs. Exposure-adjusted incidence rates for CV AEs are included in Table 5. Overall, the difference in the cumulative incidence of progression was greater than the difference in cumulative incidence of grade ≥3 cardiac failure in the ASPIRE trial (eg, 17% vs 2% at month 18; Figure 2A) and in the ENDEAVOR trial (eg, 30% vs 6% at month 18; Figure 2C). More importantly, the difference in cumulative incidence of death was greater than that for grade ≥3 cardiac failure in both trials (Figure 2B,D) showing the favorable benefit-risk profile in RRMM.
Time to first event of cardiac failure (CF) and survival analyses in the ASPIRE and ENDEAVOR trials. (A) Time to first event of grade ≥3 CF and progression or death in the ASPIRE (A) and ENDEAVOR (C) trials. Time to first event of grade ≥3 CF and mortality in the ASPIRE (B) and ENDEAVOR (D) trials. The x-axis shows months since first dose for grade ≥3 CF AEs and months since randomization for PFS or OS.
Time to first event of cardiac failure (CF) and survival analyses in the ASPIRE and ENDEAVOR trials. (A) Time to first event of grade ≥3 CF and progression or death in the ASPIRE (A) and ENDEAVOR (C) trials. Time to first event of grade ≥3 CF and mortality in the ASPIRE (B) and ENDEAVOR (D) trials. The x-axis shows months since first dose for grade ≥3 CF AEs and months since randomization for PFS or OS.
The number needed to harm and number needed to treat in the ASPIRE and ENDEAVOR trials
The number needed to harm (NNH) for the development of cardiac failure or hypertension and the number needed to treat (NNT) to prevent progression or death are presented in Table 8. The NNH for grade ≥3 cardiac failure over 1 year was 102.8 for KRd patients in the ASPIRE trial and 30.8 for Kd patients in the ENDEAVOR trial. The NNH to develop 1 grade ≥3 hypertension event was 75.1 for ASPIRE patients and 12.9 for ENDEAVOR patients.
NNH and NNT in ASPIRE and ENDEAVOR trials
| . | ASPIRE . | ENDEAVOR . |
|---|---|---|
| Unfavorable effects: NNH (subject year) | ||
| Grade ≥3 cardiac failure* | 102.8 | 30.8 |
| Grade ≥3 hypertension† | 75.1 | 12.9 |
| Favorable effect: NNT (subject year) | ||
| PFS | 8.2 | 2.5 |
| . | ASPIRE . | ENDEAVOR . |
|---|---|---|
| Unfavorable effects: NNH (subject year) | ||
| Grade ≥3 cardiac failure* | 102.8 | 30.8 |
| Grade ≥3 hypertension† | 75.1 | 12.9 |
| Favorable effect: NNT (subject year) | ||
| PFS | 8.2 | 2.5 |
Based on standardized MedDRA query, narrow scope.
Based on preferred term.
The NNT for PFS indicates that 8.2 patients needed to be treated with KRd instead of Rd and 2.5 patients needed to be treated with Kd instead of Vd to prevent 1 PFS event over 1 year.
Discussion
Carfilzomib-based regimens have produced clinically meaningful responses and shown significant improvement in the depth and duration of responses in RRMM patients.10,15 More recently, OS data showed superiority of Kd vs Vd and KRd vs Rd, with a nearly 8-month increase in median OS in patients treated with carfilzomib.11,28 Carfilzomib-based regimens have been generally well tolerated, but concerns for CV toxicity have been raised.10,13-15,29 Here, we assessed carfilzomib-associated CV AEs in phase 1-3 clinical trials. Across all RRMM phase 3 trials, treatment with carfilzomib was associated with a numeric increase in cardiac AE incidence. Specifically, the incidence of any-grade and grade ≥3 cardiac failure, hypertension, and dyspnea was higher in carfilzomib-treated patients vs control-treated patients in the phase 3 trials. In the pooled phase 1-3 trials, rates of grade ≥3 and serious-grade cardiac failure (grouped terms) were 4.4% and 3.9%, respectively. Importantly, however, the duration of exposure and safety follow-up was longer for the carfilzomib arms in the phase 3 studies; when adjusted for these differences, the differences in the AE incidence between the 2 arms were less prominent. In the ASPIRE trial, exposure-adjusted risk estimates for cardiac failure, dyspnea, and ischemic heart disease were comparable, whereas exposure-adjusted hypertension rates remained higher in the carfilzomib arms vs control arms. In the ENDEAVOR trial, exposure-adjusted risk estimates for cardiac failure, hypertension, and dyspnea were lower than the unadjusted incidence of these AEs, but were still higher in the carfilzomib arms vs control arms.
Although these are phase 3 studies, AE reporting was not performed by cardiologists. Moreover, none of these studies were double blinded or included placebo, which could introduce reporting bias. Additionally, the carfilzomib schedule in the phase 3 studies added 3-6 more visits per month to receive infusions, thereby contributing to possible ascertainment bias. Thus, we compared rates of death or treatment discontinuation due to CV events, which were low and comparable between carfilzomib and control arms across all studies.
An independent meta-analysis of all published carfilzomib clinical studies (2623 patients from 25 studies) estimated grade ≥3 cardiac failure and hypertension incidences at 2.5% and 4.3%, respectively.30 Higher cardiac event rates have been observed in some retrospective single-center studies, with doxorubicin exposure associated with increased risk.31,32 Real-world data based on the MarketScan Claims database showed an ∼3% incidence of hospitalizations due to cardiac AEs during carfilzomib treatment, validating the low rate of grade ≥3 CV AEs reported across carfilzomib clinical trials in real-world practice.33
Evaluation of the possible effects of carfilzomib on cardiac function by concentration-QTc analysis in cancer patients, including MM, revealed that carfilzomib had no clinically significant impact on cardiac repolarization. A substudy within the ENDEAVOR trial was conducted using echocardiography to further characterize cardiopulmonary carfilzomib-associated toxicities based on changes in LVEF, RV function, and PASP from baseline. Importantly, the assessment of echocardiographic results, unlike the AE reporting, was done in a blinded manner. Despite the increased incidence of cardiac failure and hypertension events in the Kd arm vs the Vd arm, the >3-year follow-up for this study revealed no objective evidence of subclinical or clinically relevant declines in cardiac function for carfilzomib vs bortezomib in terms of reductions in LVEF, RV, and PASP function. This suggests that the mechanism resulting in increased cardiac failure events in the Kd arm is not due to a direct cardiomyopathic effect, distinct from other forms of chemotherapy, such as trastuzumab or doxorubicin. The aggregate impact of an increase in blood pressure, along with hydration administered with Kd therapy, could play a significant role in the observed increase in cardiac failure AEs reported with carfilzomib. A careful pretreatment cardiac history, judicious use of hydration, and careful management of systemic hypertension are recommended as good clinical practice.
Additional surveillance strategies are needed for cardiopulmonary AE monitoring. Future studies would need to explore the merit of comprehensive cardiac assessment for heart failure and other CV events to clearly define CV risk factors. Careful clinical volume status assessment may be a useful measure to proactively manage patients at risk for developing heart failure. Increasing evidence suggests a possible effect of proteasome inhibition on vascular endothelium,34-37 rather than cardiac myocytes. Therefore, vascular impact biomarkers should be investigated.
Although results from the ASPIRE and ENDEAVOR trials suggest that higher carfilzomib doses might be associated with higher incidences of cardiac AEs, conclusions drawn from cross-trial comparisons should be made cautiously. The association between carfilzomib dose and cardiac AE incidence is inconclusive.38 In 1 single-center study (n = 60), no association was found between carfilzomib dose and the incidence of cardiac events.39 Currently, 2 randomized trials are exploring the relationship between carfilzomib dose and safety in MM patients. The phase 2 SWOG1304 study compares 27 mg/m2 and 56 mg/m2 carfilzomib twice weekly. The phase 3 A.R.R.O.W. study compares 70 mg/m2 carfilzomib once weekly with carfilzomib 27 mg/m2 twice weekly.
The current benefit-risk analysis indicated that the cumulative incidence of grade ≥3 cardiac failure was slightly higher for the carfilzomib arms vs the control arms in the ASPIRE and ENDEAVOR trials. Importantly, the reduction in the incidence of progression or death between the carfilzomib and control arms was greater than the difference in the cumulative cardiac failure incidence between the 2 arms. NNT analyses indicated that a small number of patients needed to be treated with KRd or Kd vs control to prevent 1 PFS event over 1 year (NNT for KRd vs Rd, 8.2; NNT for Kd vs Vd, 2.5). With respect to cardiac failure NNH, ∼103 and 31 patients were treated with KRd and Kd, respectively, for 1 more patient who developed cardiac failure vs control over 1 year. In addition, nearly 75 and 13 patients were treated with KRd and Kd, respectively, for 1 more patient who developed hypertension vs control. The smaller NNT vs NNH values represent a larger treatment benefit than risk for KRd and Kd. Overall, the results of this benefit-risk analysis suggest that the benefit of carfilzomib treatment in terms of reducing progression or death may outweigh the potential for developing cardiac failure and hypertension.
Although CV AEs occurred throughout the course of carfilzomib administration in the ASPIRE and ENDEAVOR trials, the cumulative incidence curves (Figure 2) suggest a higher incidence of grade ≥ 3 cardiac failure earlier in treatment. Additionally, there is no evidence for a correlation between carfilzomib-associated cardiac AEs and cumulative dose. An exposure-response analysis using data from 10 phase 1-3 carfilzomib studies, aimed at evaluating carfilzomib safety at various doses (15 to 56 mg/m2) and infusion lengths (2-10 minutes or 30 minutes), showed no statistically significant relationship between these exposure parameters and rates of any-grade cardiac failure.38
Elderly patients and patients with preexisting cardiac conditions, such as cardiac failure and hypertension, could have an increased risk for developing CV events while receiving carfilzomib.40,41 A single-institution study to identify potential biomarkers in MM patients at high risk for cardiac AEs when treated with carfilzomib indicated a trend toward differential baseline expression of 8 proteins (tumor necrosis factor–related apoptosis-inducing ligand, myoglobin, matrix metalloproteinase-1, heparin-binding EGF-like growth factor, CD40 ligand, platelet-derived growth factor, heat shock protein 27, and epidermal growth factor).42 Multivariate analysis did not reveal any association between these proteins and cardiac AEs. Furthermore, a single-institution prospective observational study of patients receiving carfilzomib- or bortezomib-based therapy to assess CV risk factors and outcomes showed that elevated baseline levels of brain natriuretic peptide (>100 pg/mL) were associated with a higher risk for cardiac AEs in patients treated with a carfilzomib-based regimen (odds ratio, 35.1; 95% CI, 4.3–289.5; P < .001).43 These findings are only hypothesis generating, because this was not a randomized clinical study.
The potential for cardiac failure and hypertension must be considered in the context of significant potential for reducing MM disease progression or death. Nevertheless, closely monitoring patients with risk factors for cardiac AEs is warranted. For patients at higher risk for cardiac failure events, referral to a cardiologist with expertise in cardio-oncology for comanagement is prudent. Although it is unclear whether aggressively managing cardiac AE risk factors will result in a reduction in cardiac failure events, it is possible that controlling risk factors reduces event occurrences. Because hypertension generally increases the risk for congestive heart failure and predates it in a majority of cases,44,45 monitoring and aggressively controlling high blood pressure at the start and during treatment with carfilzomib is critical. In addition, dyspnea is a common cardiopulmonary AE in carfilzomib-treated patients and is a frequent presenting symptom for patients with cardiac failure.10,28,46,47 Dyspnea may indicate volume overload in these patients. For patients who have dyspnea while on carfilzomib, a clinically driven evaluation for dyspnea causes should be sought. Of note, patients experiencing dyspnea should be monitored, and dyspnea should be managed immediately. Carfilzomib should be withheld until dyspnea symptoms have resolved or returned to baseline. Finally, when administering higher doses of carfilzomib (>27 mg/m2), it is important to use a longer infusion time of 30 minutes. This has been shown to be well tolerated by reducing the incidence of infusion-related reactions.48,49
In conclusion, the current analysis of CV events across carfilzomib clinical trials conducted to date indicates that, although cardiac AEs are numerically higher among carfilzomib-treated patients, the relative risk remains relatively low and rarely leads to dose reductions or treatment discontinuation. Moreover, the consequences are generally manageable, with no higher risk for fatal outcomes. The results suggest that the benefit of carfilzomib treatment in reducing disease progression, and even death, outweighs CV risks for most patients. Nonetheless, careful monitoring and management of CV risk factors, including blood pressure and volume status, are recommended for all myeloma patients as good clinical practice.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Jacqueline Sayyah (Amgen Inc.) for medical writing assistance and Ashfield Healthcare Communications, part of UDG Healthcare plc, for editorial assistance. Jacqueline Sayyah was responsible for writing the first version of the manuscript, with input from the authors.
This work was supported by Onyx Pharmaceuticals Inc., a subsidiary of Amgen Inc.
Authorship
Contribution: A.C., J.B., R.H., J.G., and A.R.L. analyzed data; A.K.S. and P.M. acquired and analyzed data; S.D.R., G.N.B., S.R., and M.H. designed the study and acquired and analyzed data; J.H., K.S.I., and D.L. designed the study and analyzed data; and all authors drafted the manuscript and approved the final version.
Conflict-of-interest disclosure: A.C. has received consulting fees, research grants, and research funding from and is on the advisory board for Takeda, Celgene, Novartis, Amgen, and Janssen; grants and research funding were also provided by Pharmacyclics. A.K.S. has received honoraria and consulting fees from Abbvie, Amgen, BMS, Celgene, Janssen, Roche, Seattle Genetics, and Takeda and has received clinical trials support from Celgene, Amgen, Seattle Genetics, Karyopharm, and Roche. P.M. has received consulting fees from Amgen, Bristol-Myers Squibb, Celgene, Janssen, Novartis, and Takeda. J.H. has received consulting fees from Bristol-Myers Squibb and royalties from Elsevier. J.B. has received research grants from Astellas and is a consultant for Biomedical Systems Corporation. R.H. has received research grants from Celgene, Amgen, and Novartis and consulting fees from Janssen, Amgen, BMS, and Takeda. J.G. has received research grants from Amgen and consulting fees from Takeda. A.R.L. has acted as a consultant and is on the advisory board for Amgen, Novartis, Pfizer, Boehringer Ingelheim, Servier, Eli Lily, Stealth Peptides, and Roche. S.R., M.H., and K.S.I. and employed by and own stock in Amgen. D.L. has received research grants from Takeda and consulting fees from Roche, Amgen, BMS, Janssen, and Prothena. The remaining authors declare no competing financial interests.
Correspondence: Ajai Chari, Mount Sinai School of Medicine, 1 Gustave Levy Pl, Box 1185, New York, NY 10029; e-mail: ajai.chari@mountsinai.org.