Key Points
EMCN is a novel marker of human HSCs.
EMCN is a more specific marker of HSCs than CD34 as it can discriminate HSCs from lineage-committed HPCs.
Introduction
Hematopoietic stem cells (HSCs) promote the lifelong production of all mature blood cell lineages through their unique capabilities of durable self-renewal and multilineage differentiation.1 Given the extensive regenerative property of HSCs, various types of stem-cell products have been successfully used in the clinic for cellular and genetic therapies for >4 decades.2 These include donor-derived bone marrow (BM), cord blood (CB), and mobilized peripheral blood stem-cell products for allogeneic stem-cell transplantation (SCT) in patients with hematological malignancies and monogenic diseases. Moreover, patient-derived peripheral blood stem-cell products are extensively used for autologous SCT supporting hematopoietic rescue after high-dose chemotherapy for various types of hematological malignancies, solid tumors, and autoimmune diseases.3 Significantly, the development of improved viral gene transduction protocols targeting HSCs has led to successful outcomes of gene therapies for monogenic diseases of the hematopoietic system, such as severe combined immunodeficiency, β-thalassemia, Wiskott-Aldrich syndrome, and leukodystrophy.4
Despite the outstanding clinical success of cellular and genetic HSC therapies, much of our knowledge of human adult HSC biology derives from mouse studies and studies of CB HSCs, because HSCs can only be identified and quantified operationally by functional transplantation assays, raising an obvious obstacle for studies of human adult HSCs, which, compared with murine HSCs and human CB HSCs, engraft relatively poorly in syngenic or xenotransplantation assays, respectively.5
As evidenced by clinical and xenotransplantation studies, all human HSCs as well as lineage-committed hematopoietic progenitor cells (HPCs) express CD34, although they lack expression of mature blood-cell lineage markers (L-34+).6 Significantly, L-34+ cells can be further subdivided by a series of surface markers into L-34+38+ populations enriched in HPCs committed to the myeloid and lymphoid lineages, as well as L-34+38− populations enriched in progenitors with mixed lymphocyte and myeloid (monocytic) potential (LMPPs; L-34+38−45RA+90−), multipotent progenitors (MPPs; L-34+38−45RA−90−) with limited multilineage engraftment potential, and functional human HSCs (L-34+38−45RA−90+) with the capability of long-term multilineage engraftment.7,8 Given these premises, identification of novel HSC markers allowing for further refinement of the immunophenotype and an improved purification of adult HSCs is instrumental to gain new insights into human HSC biology and improve both HSC quantification and HSC graft engineering in the clinical setting.
Methods
A detailed description of multicolor flow cytometric analyses and cell sorting, western blot analyses, and NOG xenotransplantation experiments is given in the in the supplemental Materials and methods.
Results and discussion
To identify novel human HSC surface markers, we conducted a screen of microarray gene expression profiles (GEP) of BM populations with the classic immunophenotypes of HSCs, MPPs, committed HPCs, and mature blood cells purified by flow cytometry from healthy adults as described previously by our group.9,10 Significantly, this screen identified endomucin (EMCN) as a candidate HSC marker that is highly expressed by human HSCs and progressively downregulated as HSCs differentiate via MPPs toward lineage-committed common myeloid progenitors (CMPs), granulocyte-monocyte progenitors (GMPs), megakaryocyte-erythrocyte progenitors (MEPs), and mature monocytes and neutrophils (Figure 1A). Complementary flow cytometric analysis of adult BM samples from healthy subjects combining EMCN with current standard markers for the identification of lineage-committed HPCs, LMPPs, MPPs, and HSCs demonstrated that EMCN is highly expressed on the surface of immunophenotypic HSCs (± standard deviation; 65% ± 7%) and to some extent on MPPs (27% ± 9.5%), but almost entirely absent on LMPPs (11% ± 8%) and lineage-committed HPCs (0.7% ± 0.5%; Figure 1B-C).9,10 Consistently, fractionation of lineage-negative adult BM cells into subpopulations lacking expression of EMCN (L−E−) or expressing EMCN at low or high levels (L−Elo and L−Ehi, respectively) demonstrated a >200-fold enrichment of immunophenotypic HSCs in L−Ehi populations as compared with L−E− populations in BM (supplemental Figure 1). Moreover, the L−E+ population was significantly enriched for immunophenotypic HSCs and almost completely devoid of lineage-committed HPCs when compared with the L−34+ population (± standard deviation; HSCs: L−E+, 59% ± 16% vs L−34+, 11% ± 5%; P = .0003; HPCs: L−E+, 7% ± 6% vs L−34+, 56% ± 9%; P < .001; n = 7; supplemental Figure 2). Significantly, the latter indicates that EMCN is a more specific marker of HSCs than CD34, which is currently used routinely in the clinic for the monitoring of stem-cell mobilization in patients and healthy donors and quality assessment of their stem-cell products.11
EMCN is highly expressed by HSCs in human adult BM. (A) Microarray GEPs of EMCN in highly purified human hematopoietic BM cell populations including immunophenotypic HSCs (n = 5), MPPs (n = 3), CMPs (n = 7), GMPs (n = 9), MEPs (n = 6), mature neutrophils (polymorphonuclear neutrophils [PMN]; n = 3), and monocytes (Mono; n = 4). One-way analysis of variance (ANOVA) of EMCN expression in HSCs vs MPPs, CMPs, GMPs, MEPs, PMNs, and Monos: P ≤ .0001. (B) Representative gating strategy of viable (7AAD−) human BM populations including all lineage-committed HPCs (L−34+38+; black), HSCs (L−34+38−45RA−90+; green), MPPs (L−34+38−45RA−90−; red), and LMPPs (L−34+38−45RA+90−; blue). (C) Average frequencies (± standard deviation; n = 7) of EMCN+ cells among HSCs (65.1% ± 6.8%), MPPs (26.8% ± 9.5%), LMPPs (11.1% ± 8.1%), HPCs (0.7% ± 0.5%). One-way ANOVA of EMCN+ cells in HSCs vs MPPs, LMPPs, and HPCs: P ≤ .0001.
EMCN is highly expressed by HSCs in human adult BM. (A) Microarray GEPs of EMCN in highly purified human hematopoietic BM cell populations including immunophenotypic HSCs (n = 5), MPPs (n = 3), CMPs (n = 7), GMPs (n = 9), MEPs (n = 6), mature neutrophils (polymorphonuclear neutrophils [PMN]; n = 3), and monocytes (Mono; n = 4). One-way analysis of variance (ANOVA) of EMCN expression in HSCs vs MPPs, CMPs, GMPs, MEPs, PMNs, and Monos: P ≤ .0001. (B) Representative gating strategy of viable (7AAD−) human BM populations including all lineage-committed HPCs (L−34+38+; black), HSCs (L−34+38−45RA−90+; green), MPPs (L−34+38−45RA−90−; red), and LMPPs (L−34+38−45RA+90−; blue). (C) Average frequencies (± standard deviation; n = 7) of EMCN+ cells among HSCs (65.1% ± 6.8%), MPPs (26.8% ± 9.5%), LMPPs (11.1% ± 8.1%), HPCs (0.7% ± 0.5%). One-way ANOVA of EMCN+ cells in HSCs vs MPPs, LMPPs, and HPCs: P ≤ .0001.
More recently, Notta et al8 elegantly demonstrated that in CB, HSCs express CD49f (ITGA6) at intermediate levels, allowing for the sorting of a L−34+38−45RA−90+49f+ population containing 10% functional HSCs. We therefore investigated whether CD49f, similar to EMCN, could be used to discriminate immunophenotypic HSCs and HPCs in CB as well as adult BM. Microarray GEP demonstrated a minor but still significant increase of CD49f expression in CB but not adult BM HSCs compared with HPCs (supplemental Figure 3). However, subsequent flow cytometric analyses did not demonstrate significant differences in CD49f surface expression, either on HSCs or HPCs from CB or adult BM (supplemental Figure 4).10,12 Hence, in contrast to EMCN, CD49f cannot discriminate between HSCs and HPCs, despite its previously shown ability to enrich for functional HSCs in combination with other HSCs markers (L−34+38−45RA−90+49f+).8
To rule out off-target binding, we tested 2 independent rat–anti-human EMCN antibodies (SDBC0008N-G, L6H10) on human BM cells and recombinant human EMCN.13 Significantly, both monoclonal antibodies stained immunophenotypic HSCs and recombinant human EMCN protein in flow cytometric (supplemental Figure 5) and western blot analyses (supplemental Figure 6), respectively.
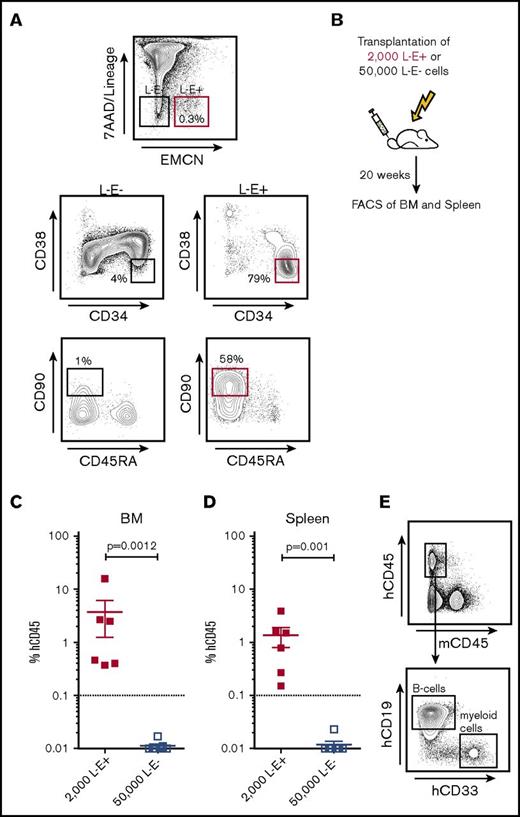
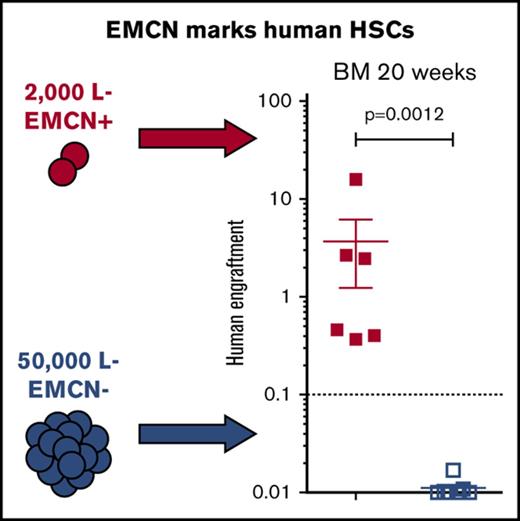
Given these promising immunophenotypic analyses, we further assessed whether EMCN actually marks functional HSCs without additional use of CD34 or any other known HSC markers. For this, we transplanted 50 000 L−E− and 2000 L−E+ highly purified BM cells into sublethally irradiated NOG mice (Figure 2A-B) and assessed their capability for human long-term multilineage engraftment 20 weeks after transplantation (Figure 2C-D; supplemental Figure 7).14 Significantly, only NOG mice transplanted with 2000 L−E+ cells demonstrated significant human multilineage engraftment in BM and spleen, including CD19+ B cells and CD33+ myeloid cells, after 20 weeks (Figure 2C-E). In contrast, mice transplanted with 50 000 L−E− cells demonstrated no human engraftment (Figure 2C-E). These findings establish EMCN as a marker expressed by a majority, if not all, bona fide functional human adult HSCs.
All functional HSCs in adult BM express EMCN. (A) Sorting strategy for the purification of 7AAD−L−EMCN+ (L−E+) and 7AAD−Lin-EMCN− (L−E−) cells from human BM and representative plot demonstrating a significant enrichment of immunophenotypic HSCs in L−E+ as compared with L−E− populations. (B) Transplantation of 50 000 L−E− and 2000 L−E+ highly purified BM cells into sublethally irradiated NOG mice and assessment of their capability for human long-term multilineage engraftment in BM and spleen 20 weeks after transplantation. (C-D) NOG mice transplanted with 2000 L−E+ (red squares) cells demonstrated marked human multilineage engraftment after 20 weeks (2 independent experiments; 3-4 replicates each, error bars shown as standard error of the mean), whereas mice transplanted with 50 000 L−E− (blue squares) cells demonstrated human engraftment at very low levels without detection of multilineage engraftment. (E) Representative flow cytometric analysis of human multilineage engraftment in the BM of NOG mice including human CD45+CD19+ B cells and human CD45+CD33+ myeloid cells. FACS, fluorescence-activated cell sorting.
All functional HSCs in adult BM express EMCN. (A) Sorting strategy for the purification of 7AAD−L−EMCN+ (L−E+) and 7AAD−Lin-EMCN− (L−E−) cells from human BM and representative plot demonstrating a significant enrichment of immunophenotypic HSCs in L−E+ as compared with L−E− populations. (B) Transplantation of 50 000 L−E− and 2000 L−E+ highly purified BM cells into sublethally irradiated NOG mice and assessment of their capability for human long-term multilineage engraftment in BM and spleen 20 weeks after transplantation. (C-D) NOG mice transplanted with 2000 L−E+ (red squares) cells demonstrated marked human multilineage engraftment after 20 weeks (2 independent experiments; 3-4 replicates each, error bars shown as standard error of the mean), whereas mice transplanted with 50 000 L−E− (blue squares) cells demonstrated human engraftment at very low levels without detection of multilineage engraftment. (E) Representative flow cytometric analysis of human multilineage engraftment in the BM of NOG mice including human CD45+CD19+ B cells and human CD45+CD33+ myeloid cells. FACS, fluorescence-activated cell sorting.
During development, HSCs are generated from hemogenic endothelium before they seed and expand in the fetal liver (FL) and ultimately migrate to their lifelong niches in the BM. Notably, this origin of HSCs is reflected by expression of common surface markers on endothelial cells and adult HSCs, such as CD34 and CD90.15 In a previous study, Matsubara et al16 demonstrated that the endothelial marker EMCN is expressed on murine HSCs but not erythroid HPCs during embryonic development. Consistent with this murine study, analysis of a series of publicly available microarray GEPs of sorted human CB and FL HSCs (L−34+38−45RA−90+) and HPCs (L−34+38+), which were conducted on the same microarray platform as our own GEPs of sorted BM HSCs (L−34+38−45RA−90+) and HPCs (L−34+38+), demonstrated high expression of EMCN transcripts in HSCs compared with their respective lineage-committed HPCs (supplemental Figure 3).10,12,17 Complementary flow cytometric analyses of immunophenotypic CB HSCs (L−34+38−45RA−90+), CB MPPs (L−34+38−45RA−90−), CB LMPPs (L−34+38−45RA+90−), and CB HPCs (L−34+38+) demonstrated that EMCN is expressed on immunophenotypic CB-derived HSCs and is progressively downregulated as HSCs differentiate into lineage-committed HPCs (supplemental Figures 4 and 8). Hence, our study extends the previous report by Matsubara et al on EMCN expression during murine embryonic development and substantiates a common developmental origin of endothelial cells and adult HSCs by demonstrating that EMCN is a novel marker the expression of which is conserved on human HSCs throughout development on FL, CB, and adult BM HSCs.
Our findings further highlight the use of EMCN in future studies in combination with classic HSC markers for refinement of the current human HSC immunophenotypes to gain new insights into human HSC biology. Such studies might include single-cell RNA sequencing and limiting-dilution transplantation experiments to characterize EMCN-expressing HSCs and determine whether addition of EMCN to standard HSC marker combinations such as L−34+38− or rather L−34+38−45RA−90+ can improve enrichment of HSCs in prospectively FACS-purified BM and CB populations.6-10,12
With respect to the clinical applications, EMCN may be used complementarily with the current standard marker CD34 for routine assessment of effective stem-cell mobilization and stem-cell product quality, given that EMCN is a more specific marker of functional human HSCs than CD34, which is not expressed by lineage-committed HPCs. In this context, EMCN also represents a potential marker for simplified HSC purification strategies, including 2-color flow cytometry–based or 2-step immunomagnetic cell sorting protocols. Significantly, such EMCN-based sorting could easily be adapted in automated cell separation protocols already applied in the clinical setting, allowing for production of highly pure stem-cell products devoid of T cells or CD34–expressing malignant cells, and thus have a profound impact on patients undergoing allogeneic or autologous SCT, respectively.18
In summary, our study identifies EMCN as a novel HSC marker, which holds great potential to advance future studies on human HSC biology and improve HSC-based therapies.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all bone marrow donors. The authors would like to acknowledge Bente Langelund Kristensen for professional animal care and Anna Fossum for expert flow cytometric analysis and cell sorting.
This work was supported by grants from Enid Ingemann Fonden (K.R.), Barncancerfonden Sverige (PDU13/004) (K.R.), Børnecancerfonden (2013-37) (K.R.), and Dagmar Marshalls Fond (K.R. and K.T.-M.). Work in the Porse Laboratory was supported through a center grant from the Novo Nordisk Foundation (Novo Nordisk Foundation Center for Stem Cell Biology, DanStem; NNF15CC0027852, NNF17CC0027852). K.T.-M. is supported by a clinical research fellowship and a center grant from the Novo Nordisk Foundation (100191; Novo Nordisk Foundation Center for Stem Cell Biology, DanStem; NNF17CC0027852) and by grants from the Danish Council for Strategic Research (133100153), the Danish Cancer Society (R72-A4572-13-S2), the Novo Nordisk Foundation (34220), Børnecancerfonden (2016-0255), Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis Foundation, and Tømrermester Jørgen Holm og Hustru Elisa F. Hansen’s Mindelegat.
Authorship
Contribution: K.R. and K.T.-M. conceived and designed the study; N.B., F.A., K.G., and K.T.-M. provided bone marrow and cord blood samples; D.V. provided essential monoclonal antibodies; K.R., H.K., A.S.H., M.E.A., A.G.D., N.R., and K.T.-M. collected and assembled the data; K.R. and K.T.-M. analyzed and interpreted the data; K.R. and K.T.-M. drafted and wrote the manuscript; and D.V., A.G., B.P., and C.N. interpreted the data and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Niels Borregaard died on 10 January 2017.
Correspondence: Kim Theilgaard-Mönch, Department of Hematology & Finsen Laboratory, National University Hospital and Biotech Research and Innovation Centre, University of Copenhagen, Ole Maaloes Vej 5, 2200 Copenhagen, Denmark; e-mail: kim.theilgaard@finsenlab.dk.

![Figure 1. EMCN is highly expressed by HSCs in human adult BM. (A) Microarray GEPs of EMCN in highly purified human hematopoietic BM cell populations including immunophenotypic HSCs (n = 5), MPPs (n = 3), CMPs (n = 7), GMPs (n = 9), MEPs (n = 6), mature neutrophils (polymorphonuclear neutrophils [PMN]; n = 3), and monocytes (Mono; n = 4). One-way analysis of variance (ANOVA) of EMCN expression in HSCs vs MPPs, CMPs, GMPs, MEPs, PMNs, and Monos: P ≤ .0001. (B) Representative gating strategy of viable (7AAD−) human BM populations including all lineage-committed HPCs (L−34+38+; black), HSCs (L−34+38−45RA−90+; green), MPPs (L−34+38−45RA−90−; red), and LMPPs (L−34+38−45RA+90−; blue). (C) Average frequencies (± standard deviation; n = 7) of EMCN+ cells among HSCs (65.1% ± 6.8%), MPPs (26.8% ± 9.5%), LMPPs (11.1% ± 8.1%), HPCs (0.7% ± 0.5%). One-way ANOVA of EMCN+ cells in HSCs vs MPPs, LMPPs, and HPCs: P ≤ .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/13/10.1182_bloodadvances.2018015743/3/m_advances015743f1.jpeg?Expires=1765962478&Signature=oT28wU-Tax-rjD7ywDFY6md~g100xubKaYrV24aDnvUDtI-bHNU8eLyZPVrMwx6pYyjeBC6RTnCz4uErk0227eMAo4L~r50KA7AMJbl6IdHL2fm1QTj0X1VrB3Ie2h6nO6H-5EZsB-5K1x1vVPpazL-1zpOZFEFDWG8bv41JaobF5zMzC8YQMzPv2WV9HkwSMyjUedRut81k53CgdUkQmU8s7OI4leuc9FdthztA8qTO48PF4WELRhzjniUR0GolxQRFlJiJImD7OW-K9e5AxRIMWdJ8K1cjoYM-~y5gBiVzIv5vxvwVkzoR21Ys0xKxaaLatE4vyYuk5sPWYwv5zA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)