Key Points
Low-dose cytarabine treatment reduced mortality in symptomatic TMD patients compared with the historical control.
An MRD monitoring–based low-dose cytarabine treatment does not prevent progression from preleukemic TMD to ML-DS.
Abstract
Approximately 5% to 10% of children with Down syndrome (DS) are diagnosed with transient myeloproliferative disorder (TMD). Approximately 20% of these patients die within 6 months (early death), and another 20% to 30% progress to myeloid leukemia (ML-DS) within their first 4 years of life. The aim of the multicenter, nonrandomized, historically controlled TMD Prevention 2007 trial was to evaluate the impact of low-dose cytarabine treatment on survival and prevention of ML-DS in patients with TMD. Patients received cytarabine (1.5 mg/kg for 7 days) in case of TMD-related symptoms at diagnosis (high white blood cell count, ascites, liver dysfunction, hydrops fetalis) or detection of minimal residual disease (MRD) 8 weeks after diagnosis. The 5-year probability of event-free and overall survival of 102 enrolled TMD patients was 72 ± 5% and 91 ± 3%, respectively. In patients eligible for treatment because of symptoms (n = 43), we observed a significantly lower cumulative incidence (CI) of early death as compared with symptomatic patients in the historical control (n = 45) (12 ± 5% vs 33 ± 7%, PGray = .02). None of the asymptomatic patients in the current study suffered early death. However, the treatment of symptomatic or MRD-positive patients did not result in a significantly lower CI of ML-DS (25 ± 7% [treated] vs 14 ± 7% [untreated], PGray = .34 [per protocol analysis]; historical control: 22 ± 4%, PGray = .55). Thus, low-dose cytarabine treatment helped to reduce TMD-related mortality when compared with the historical control but was insufficient to prevent progression to ML-DS. This trial was registered at EudraCT as #2006-002962-20.
Introduction
Approximately 5% to 10% of neonates and infants with Down syndrome (DS) present with a transient clonal proliferation of myeloid blasts with megakaryoblastic or erythroblastic features, called transient abnormal myelopoiesis, transient leukemia, or transient myeloproliferative disorder (TMD).1-3 Most of these children are asymptomatic and achieve a spontaneous remission without therapeutic intervention. Nevertheless, ∼20% of the patients die within 6 months (referred to as early death),4-7 which is frequently caused by liver infiltration of blasts and subsequent hepatic failure.4,6,8 Previous studies suggested the benefit of a therapeutic intervention with cytarabine to reduce blast burden in children presenting with symptoms associated with early death, such as high white blood cell (WBC) count, ascites, bleeding diatheses, and preterm delivery.5,8 However, the treatment indications and dosing are under debate and have yet to be defined.4
Of the children diagnosed with TMD, another 16% to 23% progress to myeloid leukemia (ML-DS) within the first 4 years of life.4-6 For both, TMD and ML-DS, mutations in the hematopoietic transcription factor GATA1 are detected, leading to the exclusive expression of a truncated GATA1 protein (GATA1s).9-11 In several cases, the same patient-specific GATA1-mutation was present in TMD and ML-DS,12 implying a clonal evolution with the acquisition of additional mutations.13 These observations argue that elimination of the preleukemic TMD clone would prevent the development of ML-DS.
The aim of the TMD Prevention 2007 (TMD07) trial reported here was to reduce the incidence of ML-DS in children diagnosed with TMD from >22% to 7% by applying a low-dose cytarabine treatment (1.5 mg/kg per day for 7 days) in combination with minimal residual disease (MRD) monitoring to eradicate the GATA1-mutated clone. All patients with TMD-related symptoms (high WBC count, ascites, liver dysfunction, hydrops fetalis) at diagnosis or that were MRD positive 8 weeks after diagnosis were eligible for treatment. Children that did not respond adequately to treatment were eligible for up to 3 courses of cytarabine. This is the first trial that prospectively evaluated an MRD monitoring–based treatment approach in order to prevent the progression from a preleukemic condition (TMD) to leukemia (ML-DS). The prospectively defined treatment indications allowed us to evaluate the impact of low-dose cytarabine on the survival of the patients and the prevention of ML-DS.
Patients and methods
Patients
The multicenter, nonrandomized, historically controlled TMD07 study (EudraCT 2006-002962-20) was opened on 1 April 2007 and closed on 8 February 2015. All children with trisomy 21 in Germany and the Netherlands that showed >5% myeloid blasts or with detection of a GATA1-mutation (exon 1, 2, or 3) in the peripheral blood and/or bone marrow within the first 3 months of life were eligible for inclusion in the study. Informed consent was obtained for all children from the parents/custodians. The final protocol was approved by the ethic committee of the Hannover Medical School (Hannover, Germany) (no. 4378M) and the Erasmus University Medical Center (Rotterdam, The Netherlands) according to national laws and regulations for all participating hospitals. The historical control is a previously reported cohort of patients that were reported to the Acute Myeloid Leukemia Berlin-Frankfurt-Münster (AML-BFM) study group between 1 January 1993 and 31 December 2006 using similar criteria for in- and exclusion (see supplemental Methods).5 Analyses of the morphology and immunophenotype and confirmation of diagnosis were performed centrally at the AML-BFM reference laboratory (Hannover/Essen, Germany) and the Dutch Childhood Oncology Group (Den Haag, The Netherlands). Immunophenotyping was performed by flow cytometry as described previously.14 In the follow-up analysis, the specific immunophenotype of the respective patient was monitored. Data for the immunophenotype were available for 85 patients (83%).
Cytogenetic and molecular analysis
The constitutional trisomy 21 was confirmed by the participating hospitals. The screening for mutations in exon 1, 2, or 3 of the transcription factor GATA1 in the patients’ blasts was conducted in the AML-BFM reference laboratory (Hannover/Essen, Germany) and the Department of Pediatric Oncology at the Erasmus University Medical Center (Rotterdam, The Netherlands) as previously described.9 Comprehensive cytogenetic data from 90 children (88%) were available.
MRD monitoring
Monitoring of MRD was done by flow cytometry2 or based on molecular genetics and was measured in peripheral blood samples. A detailed description and correlation of the methods can be found in the supplemental Methods and supplemental Figure 1. Patients were considered MRD positive, if ≥0.1% of the specific TMD immunophenotype was detectable or if the patient-specific GATA1s mutation could be detected by quantitative polymerase chain reaction with a sensitivity of ≥0.01% (depending on the patient-specific primers).
Treatment plan
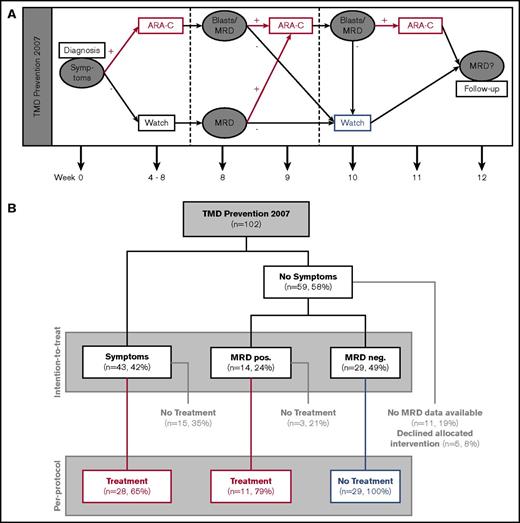
The patients were monitored at 10 different time points: week 0, 4 to 8, 8, and 12, as well as month 6, 9, 12, 18, 24, and 36 after diagnosis. At these time points, routine laboratory testing, evaluation of morphology, and MRD monitoring were performed. The patients included in the study were eligible for low-dose (1.5 mg/kg IV/subcutaneously) cytarabine treatment of 7 days if they met the following criteria: (1) presentation with TMD-related clinical symptoms at diagnosis (high WBC count [>100 × 109/L], ascites, liver dysfunction [hepatomegaly and elevated liver enzymes and/or cholestasis], hydrops fetalis); (2) MRD positive in week 8; and (3) no adequate response to cytarabine treatment (because of 1 or 2) as evaluated by detectable blasts and/or MRD positivity after treatment. Patients could receive a maximum of 3 courses with an interval of at least 1 week. A Karnofsky index of at least 60% was required prior to treatment (Figure 1A). Patients were excluded from intervention if they presented with conditions that precluded treatment. Also, children with severe anemia (hemoglobin <9 g/dL), thrombocytopenia (<100 × 109/L), and/or neutropenia (<0.5 × 109/L) that were not caused by the TMD were not eligible for treatment (eg, immune thrombocytopenia, congenital neutropenia, or hemolytic anemia). Toxicity was evaluated after each course of low-dose cytarabine and documented using modified National Cancer Institute Common Terminology Criteria for Adverse Events v3.0. Toxicity-related data were available for 38 treated patients (86%). If patients developed ML-DS, they were treated according to the protocol of the ML-DS 2006 trial.14 In the historical control, treatment and treatment criteria were not applied systematically.5 A treatment (0.5-1.5 mg/kg cytarabine for 3-12 days) was considered for patients with clinical impairment (WBC >50 × 109/L, platelets <100 × 109/L, cholestasis, liver dysfunction).5
Overview of the TMD Prevention 2007 study. (A) Treatment plan of the TMD07 study. (B) Flowchart of patients enrolled in the TMD07 study.
Overview of the TMD Prevention 2007 study. (A) Treatment plan of the TMD07 study. (B) Flowchart of patients enrolled in the TMD07 study.
Statistical analysis and definitions
The main objective of the nonrandomized, historically controlled trial was to reduce the rate of children with TMD that subsequently develop ML-DS from 22% in the historical control to 7%. In order to detect this difference with a power of 80% and an α of 5%, it was estimated that 100 patients were needed for each group. The study could not be performed as a randomized trial because of the low incidence rate and expected randomization problems (imbalance in the benefit-risk ratio of the options).
The primary end point of the study was the cumulative incidence (CI) of ML-DS (constructed by the method of Kalbfleisch and Prentice15 ) 3 years after diagnosis of TMD. The null hypothesis (no difference from the historical control or higher rate in the study cohort) was tested using the 1-sided 95% confidence interval of the difference. In addition, Gray’s test was used for comparisons.16 The secondary end point of the study was the MRD level (independent of prior treatment) at week 12. Cases of death without previous ML-DS and the development of other malignant tumors were considered as competing events. Events were defined as either death from any cause or the development of ML-DS. Event-free survival (EFS) was defined as the time from TMD diagnosis to the date of the first event. Survival was defined as the time from diagnosis to the date of death from any cause. Early death was defined as death from any cause within the first 6 months of life. The Kaplan-Meier method was used to estimate survival rates.17 The 2-sided log-rank test was used to compare differences between different groups.18 Standard errors were obtained using Greenwoods formula. Differences in the distribution of individual parameters among patient subsets were analyzed using Fisher’s exact test for categorized variables and the Mann-Whitney U test for continuous variables.
Results
Patient characteristics
In total, 102 patients (median age: 4 days; range: 0-88 days) were enrolled to the TMD07 trial. Patient characteristics are summarized in Table 1 in comparison with the historical control. Several patients included in the study presented with comorbidities: 69 (69%) were diagnosed with cardiac defects, and 14 (15%) showed other malformations. The median WBC count was 24.0 × 109/L (range: 3.2 × 109/L to 306 × 109/L). Overall, 43 patients (42%) presented with defined TMD-related clinical symptoms (high WBC count [>100 × 109/L], n = 17; ascites, n = 17; liver dysfunction [hepatomegaly and elevated liver enzymes and/or cholestasis], n = 30; hydrops fetalis, n = 7) and were therefore eligible for treatment at the time of diagnosis. In comparison with the historical control, significantly fewer patients presented with pleural effusions and hepatomegaly, and the percentage of blasts in the peripheral blood was significantly lower. The rate of ascites and cardiac defects, however, was higher in the current study and more children received intensive care.
Patient characteristics
| . | TMD07 . | ML-DS . | . | Historical control . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | N (of) . | % . | N (of) . | % . | P . | N (of) . | % . | P . |
| Total | 102 | 100 | 17 | 100 | 146 | 100 | ||
| Elevated WBC | 17 (102) | 17 | 4 (17) | 24 | .476 | 21 (128) | 16 | .148 |
| Elevated aspartate transaminase | 17 (87) | 20 | 2 (17) | 12 | .506 | 27 (96) | 28 | .225 |
| Elevated alanine transaminase | 26 (90) | 29 | 4 (17) | 24 | .769 | 23 (96) | 24 | .507 |
| Pathologic coagulation | 20 (54) | 37 | 3 (9) | 33 | 1.000 | 23 (104) | 22 | .059 |
| Hydrops fetalis | 7 (96) | 7 | 1 (17) | 6 | 1.000 | 7 (146) | 5 | .416 |
| Pleural effusion | 6 (94) | 6 | 0 (17) | 0 | .588 | 24 (146) | 16 | .027 |
| Pericardial effusion | 19 (95) | 20 | 2 (17) | 12 | .510 | 17 (146) | 12 | .096 |
| Ascites | 17 (98) | 17 | 3 (17) | 18 | 1.000 | 12 (146) | 8 | .042 |
| Splenomegaly | 28 (97) | 29 | 5 (17) | 29 | 1.000 | 44 (104) | 42 | .056 |
| Hepatomegaly | 43 (97) | 44 | 9 (17) | 53 | .592 | 62 (104) | 60 | .035 |
| Cholestasis | 21 (91) | 23 | 2 (16) | 13 | .344 | 16 (104) | 15 | .202 |
| Liver dysfunction* | 30 (97) | 31 | 5 (17) | 29 | 1.000 | 21 (104) | 20 | .105 |
| Intensive care | 41 (75) | 55 | 7 (11) | 64 | .745 | 37 (146) | 25 | <.001 |
| Ventilation required | 30 (76) | 39 | 4 (11) | 36 | 1.000 | 30 (104) | 29 | .152 |
| Cardiac defects | 69 (100) | 69 | 13 (17) | 76 | .573 | 68 (146) | 47 | <.001 |
| . | TMD07 . | ML-DS . | . | Historical control . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | N (of) . | % . | N (of) . | % . | P . | N (of) . | % . | P . |
| Total | 102 | 100 | 17 | 100 | 146 | 100 | ||
| Elevated WBC | 17 (102) | 17 | 4 (17) | 24 | .476 | 21 (128) | 16 | .148 |
| Elevated aspartate transaminase | 17 (87) | 20 | 2 (17) | 12 | .506 | 27 (96) | 28 | .225 |
| Elevated alanine transaminase | 26 (90) | 29 | 4 (17) | 24 | .769 | 23 (96) | 24 | .507 |
| Pathologic coagulation | 20 (54) | 37 | 3 (9) | 33 | 1.000 | 23 (104) | 22 | .059 |
| Hydrops fetalis | 7 (96) | 7 | 1 (17) | 6 | 1.000 | 7 (146) | 5 | .416 |
| Pleural effusion | 6 (94) | 6 | 0 (17) | 0 | .588 | 24 (146) | 16 | .027 |
| Pericardial effusion | 19 (95) | 20 | 2 (17) | 12 | .510 | 17 (146) | 12 | .096 |
| Ascites | 17 (98) | 17 | 3 (17) | 18 | 1.000 | 12 (146) | 8 | .042 |
| Splenomegaly | 28 (97) | 29 | 5 (17) | 29 | 1.000 | 44 (104) | 42 | .056 |
| Hepatomegaly | 43 (97) | 44 | 9 (17) | 53 | .592 | 62 (104) | 60 | .035 |
| Cholestasis | 21 (91) | 23 | 2 (16) | 13 | .344 | 16 (104) | 15 | .202 |
| Liver dysfunction* | 30 (97) | 31 | 5 (17) | 29 | 1.000 | 21 (104) | 20 | .105 |
| Intensive care | 41 (75) | 55 | 7 (11) | 64 | .745 | 37 (146) | 25 | <.001 |
| Ventilation required | 30 (76) | 39 | 4 (11) | 36 | 1.000 | 30 (104) | 29 | .152 |
| Cardiac defects | 69 (100) | 69 | 13 (17) | 76 | .573 | 68 (146) | 47 | <.001 |
| . | Median (range) . | Median (range) . | P . | Median (range) . | P . | |||
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, d | 4 (0-88) | 7 (0-88) | .278 | 3 (0-65) | .216 | |||
| Birth weight, kg | 2.9 (1.1-4.3) | 3.2 (1.6-3.7) | .098 | 2.9 (1.3-5.6) | .874 | |||
| Gestational age, wk | 37 (28-43) | 37 (30-39) | .965 | 37 (30-41) | .928 | |||
| WBC count, × 109/L | 24.0 (3.2-306) | 23.0 (3.2-149.1) | .447 | 40.3 (3.9-556) | .177 | |||
| Platelets, × 109/L | 102 (13-901) | 94 (15-505) | .803 | 119 (4-1047) | .609 | |||
| Hemoglobin, g/L | 15 (6.7-24.2) | 13.1 (7-20.2) | .408 | 14.5 (4.8-25.7) | .259 | |||
| Blasts peripheral blood, % | 27.5 (0-91) | 41 (0-89) | .368 | 39 (2-95) | .042 | |||
| Blasts bone marrow, % | 15.0 (6-60) | 8.0 | 32 (5-93) | .465 | ||||
| . | Median (range) . | Median (range) . | P . | Median (range) . | P . | |||
|---|---|---|---|---|---|---|---|---|
| Age at diagnosis, d | 4 (0-88) | 7 (0-88) | .278 | 3 (0-65) | .216 | |||
| Birth weight, kg | 2.9 (1.1-4.3) | 3.2 (1.6-3.7) | .098 | 2.9 (1.3-5.6) | .874 | |||
| Gestational age, wk | 37 (28-43) | 37 (30-39) | .965 | 37 (30-41) | .928 | |||
| WBC count, × 109/L | 24.0 (3.2-306) | 23.0 (3.2-149.1) | .447 | 40.3 (3.9-556) | .177 | |||
| Platelets, × 109/L | 102 (13-901) | 94 (15-505) | .803 | 119 (4-1047) | .609 | |||
| Hemoglobin, g/L | 15 (6.7-24.2) | 13.1 (7-20.2) | .408 | 14.5 (4.8-25.7) | .259 | |||
| Blasts peripheral blood, % | 27.5 (0-91) | 41 (0-89) | .368 | 39 (2-95) | .042 | |||
| Blasts bone marrow, % | 15.0 (6-60) | 8.0 | 32 (5-93) | .465 | ||||
“N (of)” refers to the number of patients for whom the data for the respective category/parameter were available. As not all parameters were available for all patients, this number is lower than the total number of patients included in the study. Bolded numbers indicate P < .05.
Hepatomegaly and elevated liver enzymes and/or cholestasis.
As described previously,2 the blasts of patients with TMD showed a particular immunophenotype (CD33+, CD13+/−, CD117+, CD34+, CD7+, CD56+/−, CD36+, CD42b+, CD41+/−; supplemental Figure 1) that could be used for MRD monitoring via flow cytometry. Eighty children (89%) showed a free constitutional trisomy 21, 5 (6%) presented with trisomy 21 mosaicism, and 5 (6%) showed translocations of chromosome 21. Of the patients that were molecularly analyzed (n = 81), 78 (96%) showed a mutation in exon 1, 2, or 3 of GATA1. In 3 patients (4%), no GATA1 mutation could be detected, and they were diagnosed with TMD at the age of 1, 4, and 0 days based on the presence of 49%, 12%, and 9% blasts with typical TMD morphology (FAB M6/M7 morphology) in the peripheral blood, respectively. The patient-specific GATA1 mutations were used for MRD monitoring via quantitative polymerase chain reaction.
Outcome of all patients
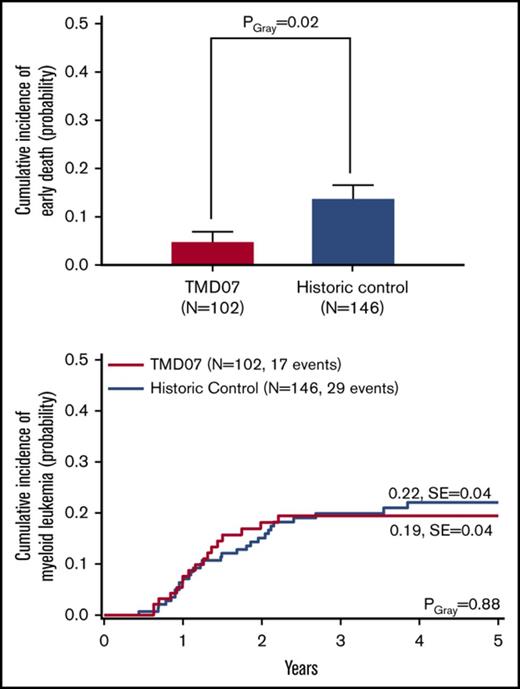
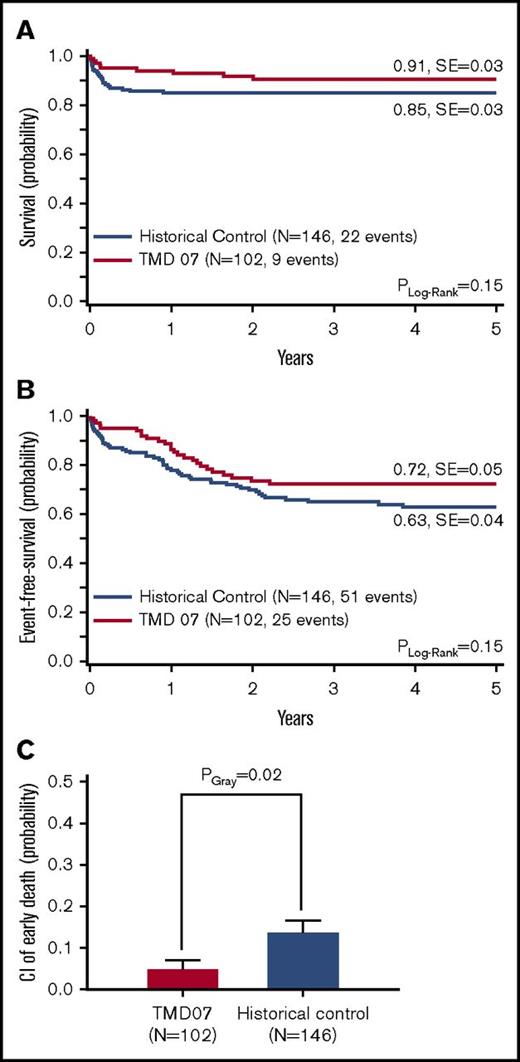
Nine patients (9%) died, resulting in a 5-year probability of overall survival (5y-pOS) of 91 ± 3%. Causes of death were sepsis (n = 2), cardiac events (n = 2), liver failure (n = 2), and asphyxia (n = 1). One child died secondary to ML-DS, and for 1 patient the cause of death is unknown. Hence, 5 patients died because of complications that can be directly (n = 2) or possibly (n = 3) attributed to TMD (supplemental Table 1). The 5-year probability of event-free survival (5y-pEFS) was 72 ± 5%. Compared with the historical control, there were no significant differences in the 5y-pEFS (63 ± 4%, PLog-Rank = .15) or 5y-pOS (85 ± 3%, PLog-Rank = .15) (Figure 2A-B). Interestingly, the CI of early death is significantly lower in the current study (5 ± 2% vs 14 ± 3%, PGray = .02) (Figure 2C).
Outcome of TMD07 patients compared with the historical control. (A) OS. (B) EFS. (C) CI of early death. Five-year probabilities are given in panels A and B.
Outcome of TMD07 patients compared with the historical control. (A) OS. (B) EFS. (C) CI of early death. Five-year probabilities are given in panels A and B.
Outcome of symptomatic and asymptomatic patients
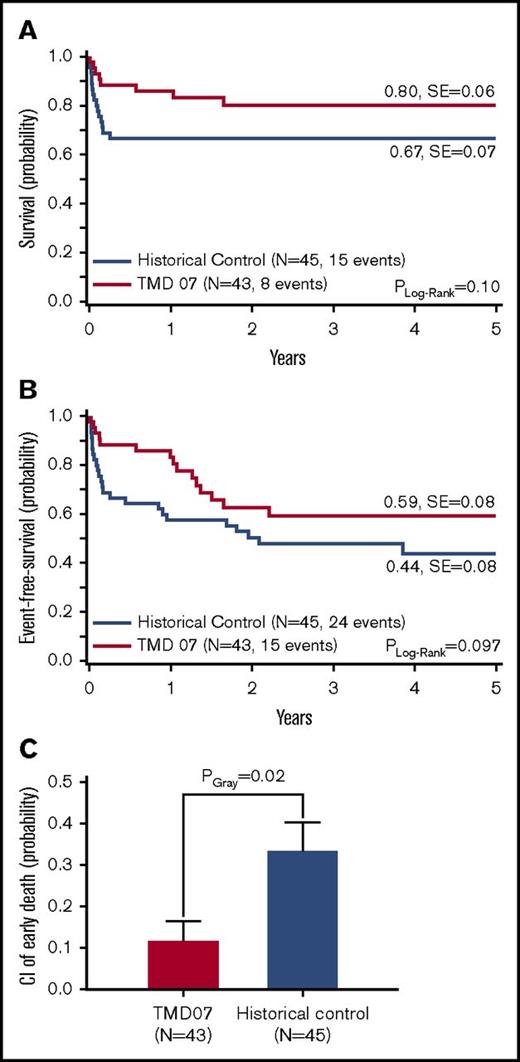
The lower CI of early death suggests that awareness of the risk, followed by intensive care and early intervention based on clear treatment indications might provide a survival benefit to the patients. Forty-three patients that presented with TMD-related clinical symptoms (high WBC count, ascites, liver dysfunction [hepatomegaly and elevated liver enzymes and/or cholestasis], hydrops fetalis) at diagnosis were eligible for the treatment with low-dose dose cytarabine (1.5 mg/kg per day) for 7 days (intention-to-treat group at diagnosis). In comparison with the historical control, where 45 patients showed these symptoms (16 [27%] received treatment of any kind5 ), the CI of early death decreased significantly (12 ± 5% vs 33 ± 7%, PGray = .02), which resulted in a trend for better 5y-pEFS (59 ± 8% vs 44 ± 8%, PLog-Rank = .097) and 5y-pOS (80 ± 6% vs 67 ± 7%, PLog-Rank = .10) (Figure 3A-C).
Outcome of symptomatic TMD07 patients (intention-to-treat at diagnosis) compared with symptomatic patients in the historical control. (A) OS. (B) EFS. (C) CI of early death. Five-year probabilities are given in panels A and B.
Outcome of symptomatic TMD07 patients (intention-to-treat at diagnosis) compared with symptomatic patients in the historical control. (A) OS. (B) EFS. (C) CI of early death. Five-year probabilities are given in panels A and B.
Overall, 28 (65%) of the symptomatic patients received treatment. This compliance rate is comparable to previous studies.4 The reasons not to comply with the treatment recommendation were death before start of treatment (n = 2; 5%) or at the discretion of the local physician (n = 13; 30%). However, comparing symptomatic patients that received treatment (n = 28; per protocol group) to symptomatic patients in the historical control (n = 45) showed the same result (CI of early death: 11 ± 6% vs 33 ± 7%, PGray = .03; supplemental Figure 2).
For asymptomatic patients (TMD07: n = 59; historical control: n = 101) no significant differences were seen regarding 5y-pEFS (81 ± 5% vs 71 ± 5%, PLog-Rank = .27) and 5y-pOS (98% vs 93 ± 3%, PLog-Rank = .16) (supplemental Figure 3). None of the asymptomatic patients in the current study suffered from early death.
Prevention of ML-DS progression by therapeutic intervention
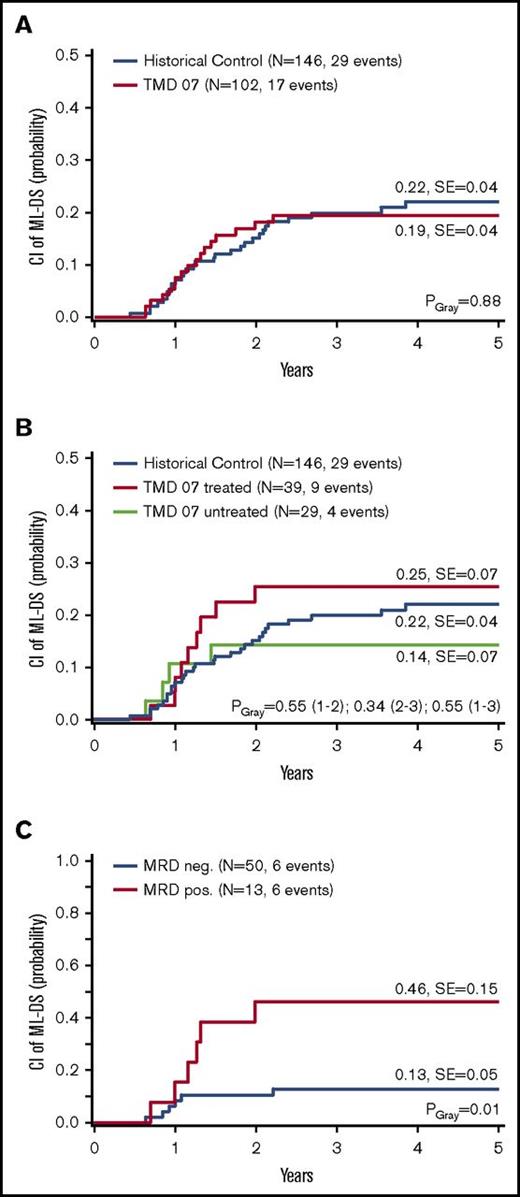
The primary objective of the TMD07 trial was to analyze whether low-dose cytarabine treatment could prevent the progression from TMD to ML-DS. In total, 17 children (5-year cumulative incidence of ML-DS: 19 ± 4%) developed ML-DS in the follow-up period (supplemental Table 1). The median follow-up was 36.1 months. These patients were on average 15.3 months old when ML-DS was diagnosed. No clinical or laboratory parameters present at diagnosis were associated with the development of ML-DS (Table 1). In the historical control, the 5y-CI of ML-DS was with 22 ± 4% similar compared with the results of the TMD07 study (PGray = .88) (Figure 4A). After 3 years, the difference of the CI of ML-DS was 1% (TMD07, 19% vs historical control, 20%) with a lower limit of the 1-sided 95% confidence interval of −9% (primary end point).
Progression to ML-DS. CI of ML-DS: (A) TMD07 patients compared with the historical control. (B) Per protocol analysis: Treated TMD07 patients compared with asymptomatic, MRD-negative, untreated TMD07 patients and to the historical control. (C) MRD-positive TMD07 patients compared with MRD-negative TMD07 patients in week 12. Five-year probabilities are given in panels A through C.
Progression to ML-DS. CI of ML-DS: (A) TMD07 patients compared with the historical control. (B) Per protocol analysis: Treated TMD07 patients compared with asymptomatic, MRD-negative, untreated TMD07 patients and to the historical control. (C) MRD-positive TMD07 patients compared with MRD-negative TMD07 patients in week 12. Five-year probabilities are given in panels A through C.
Even when considering subgroups, no differences were observed. The 5y-CI of ML-DS for the symptomatic patients (intention-to-treat group at diagnosis) was with 21 ± 7%, similar to the historical control (23 ± 7%, PGray = .91) (supplemental Figure 4). The same holds true when only including the symptomatic patients that received treatment (per protocol analysis; n = 28, 20 ± 8%, PGray = .90) (supplemental Figure 4).
Fourteen patients (24%) that were asymptomatic at diagnosis but were MRD positive in week 8 were eligible for treatment at this time point (intention-to-treat week 8), of which 11 (79%) received low-dose cytarabine (per protocol week 8). The 5y-CI of ML-DS did not significantly differ between asymptomatic patients that were either MRD positive or MRD negative in week 8 (intention-to-treat analysis; 31 ± 13% vs 14 ± 7%, PGray = .25) (supplemental Figure 4). The MRD-based treatment did not prevent the progression from TMD to ML-DS.
In total, 28 (72%) patients received treatment because of symptoms at diagnosis and 11 (28%) because of detection of MRD (per protocol group; n = 39). Of the children treated at diagnosis, 12 (43%) received 1 course, 7 (25%) received 2 courses, and 6 (21%) received 3 courses, and of the patients treated because of detection of MRD, 9 (82%) received 1 course and 2 (18%) received 2 courses of low-dose cytarabine. The treatment did not decrease the 5y-CI of ML-DS (25 ± 7%) compared with MRD-negative, untreated patients (n = 29; 14 ± 7%, PGray = .34) or the historical control (22 ± 4%, PGray = .55) (Figure 4B). The treatment was well tolerated as evaluated by analyzing treatment-related adverse events after each course (toxicity grade 3 or higher; supplemental Figure 5). The most common causes were nausea (n = 6; 19%) and fatigue (n = 13; 36%). Severe neutropenia (<0.5 × 109/L) was observed in 12 (32%) and severe thrombocytopenia (<20 × 109/L) was seen in 10 (26%) patients.
The secondary end point of the study was the MRD level at week 12 irrespective of prior intervention. These data were available for 63 patients (63%), of which 50 (79%) were MRD negative and 13 (21%) MRD positive. As expected, the 5y-CI of ML-DS for patients that were MRD-positive in week 12 was significantly higher than for patients that achieved MRD-negativity at this time point (46 ± 15% vs 13 ± 5%, PGray = .01) (Figure 4C).
Discussion
The TMD07 trial demonstrates that the progression to ML-DS cannot be prevented by low-dose cytarabine. Even an MRD monitoring–based treatment approach was not able to lower the occurrence of ML-DS. However, the prospective setup of the trial helped us to reveal the benefit of this well-tolerated treatment for symptomatic children. The CI of death in the first 6 months was significantly reduced in these patients when compared with symptomatic patients in the historical control.
Our treatment recommendations at diagnosis were based on our previously identified clinical symptoms associated with early death: high WBC count (>100 × 109/L), hydrops fetalis, ascites, liver dysfunction/failure (including coagulopathy), and preterm delivery/low birth weight.5 Except for prematurity, where the association with early death is less clear, these symptoms are in accordance with other studies.2,4,6,7 Especially liver dysfunction, with elevated AST/ALT6 or direct bilirubin levels,7 seems to be directly linked to TMD blasts infiltrating the liver, leading to liver failure and TMD-related mortality.6,8 However, Gamis et al concluded that hepatomegaly alone is not sufficient as an intervention criterion.4 Consequently, we recommended treatment of children presenting with high WBC count, ascites, liver dysfunction, or hydrops fetalis at diagnosis. As clinical manifestation of TMD is highly variable, the final decision was left to the treating physician, which might explain the compliance rate of 65% that was also seen by others.4 Treating physicians likely decided not to apply therapy if they considered the symptoms to be only mild or symptoms resolved before the treatment recommendation was made. Interestingly, in this study, none of the patients considered as asymptomatic at diagnosis suffered from early death, and only 1 asymptomatic child died in the follow-up period because of progression to ML-DS (supplemental Table 1). Hence, our definition of symptoms reliably identifies the patients at risk of early death.
It has been shown that TMD blasts are highly susceptible to cytarabine.19 Therefore, it is commonly used for the treatment of TMD patients. In our previous study (historical control), no systematic treatment of children diagnosed with TMD has been applied, and no prospective treatment recommendation was given.5 A treatment with 0.5 to 1.5 mg/kg cytarabine for 3 to 12 days was considered in a few cases by the treating physician.5 However, many of the children with severe symptoms did not receive treatment and died.5 Gamis et al applied a 3.33 mg/kg per 24 hours continuous infusion cytarabine regimen for 5 days to patients with life-threatening symptoms.4 Treated patients had a 3y-pEFS of 33% and showed severe hematologic toxicity.4 In the TMD07 trial, we systematically applied a lower dose of cytarabine (1.5 mg/kg for 7 days) subcutaneously or IV. As described, for some patients the typical side effects of cytarabine such as fatigue, nausea, and hematologic toxicity were observed. Nevertheless, in general, the treatment was well tolerated, and compared with the historical control, the early death rate of symptomatic patients in our study is significantly reduced. This was observed when comparing symptomatic patients with an intention to treat at diagnosis (Figure 3C) as well as symptomatic patients that actually received treatment (per protocol at diagnosis, supplemental Figure 2) with symptomatic patients in the historical control (the same criteria were retrospectively applied). Still, awareness of this risk for children with TMD has increased in recent years, and supportive care has improved, which might have contributed to the better outcome. This is reflected by the significantly higher number of children that received intensive care as compared to the historical control (Table 1). However, a major factor contributing to early death in the historical control was liver fibrosis (n = 7 of 104; 7%), whereas in the current study this condition was not diagnosed in any of the 102 patients (PFisher = .01). This cannot be sufficiently explained by better supportive care but is rather because of early treatment intervention. Another factor causing early death in the historical control was bleeding diatheses caused by hyperleukocytosis. This condition is difficult to handle without reduction of blast burden. Both observations suggest that the reduction of early death was achieved by the early cytarabine treatment augmented by improved supportive care. Although the comparison against a historical control is a limitation of our study, we conclude that patients with severe TMD-related symptoms profit from low-dose cytarabine treatment (1.5 mg/kg for 7 days). As previous studies suggest that late therapy is not able to revert severe liver dysfunction,5 and as 2 patients presenting with TMD-related symptoms died before receiving treatment, we suggest considering treatment as early as possible.
Evaluating the effect of low-dose cytarabine treatment on the occurrence of ML-DS, our analysis indicates no prevention. The primary study goal was not achieved, and the CI of ML-DS for patients that received therapy per protocol is 25%, which is similar to the historical control (22%) and other studies (Table 2). Also, evaluating the outcome of asymptomatic patients, who were MRD positive in week 8, suggests that the evaluated MRD monitoring–based low-dose cytarabine intervention was not beneficial. Consequently, a general preventive chemotherapeutic treatment of children diagnosed with TMD cannot be recommended. The failure to prevent the development of ML-DS suggests that it is not possible to entirely eliminate the preleukemic GATA1-mutated clone via the applied intervention. Therefore, a more targeted therapy that is specifically directed to the preleukemic cells seems to be required to prevent the progression to ML-DS. Wee1 kinase inhibitors or histone deacetylase inhibitors might be valuable alternatives.20,21
Comparison of TMD trials reported in the literature
| Study . | Years . | N . | EFS, % . | OS, % . | Early death, % . | Death, % . | ML-DS, % . |
|---|---|---|---|---|---|---|---|
| TMD07 | 2007-2015 | 102 | 72 | 91 | 5 | 9 | 17 |
| Gamis et al4 | 1999-2004 | 135 | 57 | 77 | — | 21 | 16 |
| Klusmann et al5 | 1993-2006 | 146 | 63 | 85 | 14 | 15 | 20 |
| Muramatsu et al7 | 1985-2006 | 70 | — | — | 23 | 26 | 17 |
| Massey et al6 | 1996-1999 | 48 | — | — | 17* | — | 19 |
| Study . | Years . | N . | EFS, % . | OS, % . | Early death, % . | Death, % . | ML-DS, % . |
|---|---|---|---|---|---|---|---|
| TMD07 | 2007-2015 | 102 | 72 | 91 | 5 | 9 | 17 |
| Gamis et al4 | 1999-2004 | 135 | 57 | 77 | — | 21 | 16 |
| Klusmann et al5 | 1993-2006 | 146 | 63 | 85 | 14 | 15 | 20 |
| Muramatsu et al7 | 1985-2006 | 70 | — | — | 23 | 26 | 17 |
| Massey et al6 | 1996-1999 | 48 | — | — | 17* | — | 19 |
Dash indicates not reported.
Within first 9 months of life.
In the historical control, patients that presented with pleural effusions had a significantly higher risk of progression to ML-DS.5 However, this was not observed in the TMD07 study, where none of the patients developing ML-DS presented with pleural effusions at diagnosis (supplemental Table 1). Also, no other clinical or laboratory parameters present at diagnosis were associated with the progression to ML-DS (Table 1). An alternative option for risk stratification is MRD monitoring, which allows us to identify patients that fail to resolve the TMD with a high sensitivity. As expected, in our study, patients that fail to achieve MRD negativity 12 weeks after diagnosis face a significantly increased risk of developing ML-DS. Latest next-generation sequencing–based MRD approaches might even be superior in detecting subclones within the dominant GATA1s-mutated clone and monitor the clonal evolution over time.22,23 This higher sensitivity in detecting the TMD clones could also, in combination with the previously mentioned more targeted therapy, allow for eliminating the respective clone and thereby reduce progression to ML-DS, which needs to be evaluated in future trials.
In conclusion, our study establishes clear recommendations for the management of children with TMD: Patients that present with TMD-related clinical symptoms benefit from low-dose cytarabine treatment to prevent early death. Asymptomatic patients instead should not receive treatment as they are not at risk of TMD-related early death, and no effect of the treatment on the future risk of development of ML-DS was seen.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all colleagues, data managers, and technicians of the participating hospitals for their valuable cooperation. A complete listing of the participating institutions and investigators appears in the supplemental Data.
This work was supported by grants from the German Research Foundation (DFG RE 2580/1-1; 1-2). J.-H.K. receives funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant 714226). In The Netherlands, the study was supported by the KiKa Foundation (#15).
Authorship
Contribution: U.C., C.M.Z., J.-H.K., and D.R. were responsible for conception and design; D.R., J.-H.K., and C.M.Z. were responsible for financial support, provided administrative support, and provided study materials or patients; M.F., K.S., C.v.N., F.V., V.H.J.v.d.V., C.M.Z., V.d.H., K.R., J.-H.K., and D.R. performed collection and assembly of data; M.F., K.S., C.v.N., M.Z., and J.-H.K. performed data analysis and interpretation; M.F. and J.-H.K. wrote the manuscript; and all authors had final approval of the manuscript.
Conflict-of-interest disclosure: D.R. has consulting or advisory roles for Celgene, Pfizer, MSD, Astellas Pharma, BlueBirdBio, Amgen, Novartis, and Boehringer and receives research funding from Celgene. C.M.Z. has consulting or advisory roles for Pfizer, Daiichi Sankyo, Celgene, Novartis, Bristol-Myers Squibb, and Gilead Sciences and receives research funding from Karyopharm Therapeutics, Pfizer, Bristol-Myers Squibb, and GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Jan-Henning Klusmann, Pediatric Hematology and Oncology, Martin Luther University Halle-Wittenberg, Ernst-Grube-Str 40, 06108 Halle (Saale), Germany; e-mail: jan-henning.klusmann@uk-halle.de.
References
Author notes
M.F. and K.S. contributed equally to this study.
D.R. and J.-H.K. contributed equally to this study.