TO THE EDITOR:
BCR-ABL+ acute myeloid leukemia (AML) has recently been listed in the 2016 revised World Health Organization (WHO) classification of myeloid malignancies as a provisional entity.1 BCR-ABL+ AML comprises a group of de novo AML in patients without evidence of an underlying chronic myeloid leukemia (CML) and without cooccurring aberrations such as CEBPA, NPM1, inv(16), and inv(3) that would lead to the classification as “AML with recurrent genetic aberrations.” Although there is some overlap between BCR-ABL+ AML, myeloid CML blast crisis, and BCR-ABL+ mixed phenotype acute leukemia, no definite criteria have yet been defined to distinguish among these entities. However, a loss of IKZF1 and CDKN2A as well as cryptic deletions in IGH and TRG genes have recently been reported in BCR-ABL+ AML and seem to be absent in myeloid blast crisis of CML. Therefore, these additional molecular markers may be helpful for differential diagnosis in clinically difficult situations.2
According to the recently updated European Leukemia Net (ELN)3 and current National Comprehensive Cancer Network guidelines,4 BCR-ABL+ AML is classified as high-risk disease. Remarkably, neither guidelines explicitly comment on BCR-ABL+ AML cases in which BCR-ABL cooccurs with otherwise favorable molecular/cytogenetic markers such as CEBPA, NPM1, and inv(16) (these would exclude a diagnosis as BCR-ABL+ AML sensu stricto according to WHO 2016) or without additional adverse molecular/cytogenetic features. Considering recent data on the prognosis of BCR-ABL+ AML, we believe that a general classification as high-risk AML according to ELN might not be appropriate. Given the paucity of clinical cases and the lack of robust data sets on survival and treatment responses, we have recently analyzed all published cases of BCR-ABL+ AML since 1975. To avoid inclusion of CML blast crisis as far as possible, we excluded cases that, because of the reported clinical features or insufficient information provided, might in fact represent CML blast crisis than de novo AML.5 Our retrospective study contains the largest cohort of BCR-ABL+ AML that has been published so far. Nevertheless, its results regarding the prognosis of BCR-ABL+ AML are also limited by its retrospective nature, which comprises a period that includes the pre–tyrosine-kinase inhibitor (TKI) era and the lack of data concerning recently reported molecular markers such as IKZF1, CDKN2A, and deletions in IGH or TRG2 that may help to distinguish between de novo BCR-ABL+ AML and myeloid blast crisis of CML.
Considering the results of our retrospective analysis in the different ELN risk groups, we wonder whether a general classification of BCR-ABL+ as high-risk feature in AML is really justified. Our reasons for challenging the current view are the following.
1.Previous analyses of survival in BCR-ABL+ AML have demonstrated an adverse prognosis.6 However, this study collected data over an extended period and might have included many cases of CML blast crisis; also, the start of data collection was in the pre-TKI era. Furthermore, the survival data in these studies might be confounded by other poor-risk molecular/cytogenetic features that have not always been reported.
The largest original data set on survival of BCR-ABL+ AML currently available is included in a retrospective cytogenetic risk group analysis of myelodysplasia-related change AML trials between 1988 and 2009.6 In this study with an impressively high number of BCR-ABL+ AML cases, an inferior 10-year overall survival of 11% was reported for 44 BCR-ABL+ AML patients; however, 18 of these patients also had a complex karyotype and monosomy 7 was present (6 patients). Thus, these patients already belonged to the high-risk group irrespective of BCR-ABL. Furthermore, the study contains patients with BCR-ABL+ AML who had a history of an antecedent myeloproliferative disorder; therefore, the inferior overall survival of BCR-ABL+ AML within this study cannot be as easily generalized.
In our recent analysis,5 a significant percentage of cases, irrespective of BCR-ABL itself, in fact belonged to the high-risk group according to ELN 2010 (mostly determined by a monosomal or a complex karyotype).7 However, there was also a significant number of cases belonging to the ELN favorable-risk group as determined by cooccurring aberrations (mainly inv(16) and some NPM1 mutations). Regarding the outcome of this favorable subgroup of patients with BCR-ABL+ AML, it is surprising that many of these patients were long-term survivors, although they had been treated with chemotherapy ± TKI alone (without allogeneic stem cell transplantation).5,8-10 These data clearly suggest that the presence of BCR-ABL did not alter the overall favorable outcome in these subgroups (Table 1). Although this point is reflected in the 2016 WHO classification by the fact that recurrent genetic aberrations such as CEBPA, NPM1, and inv(16) take precedence over a classification as “BCR-ABL+ AML,” both the ELN and National Comprehensive Cancer Network risk classifications are less clear-cut because BCR-ABL is listed as a high-risk feature.
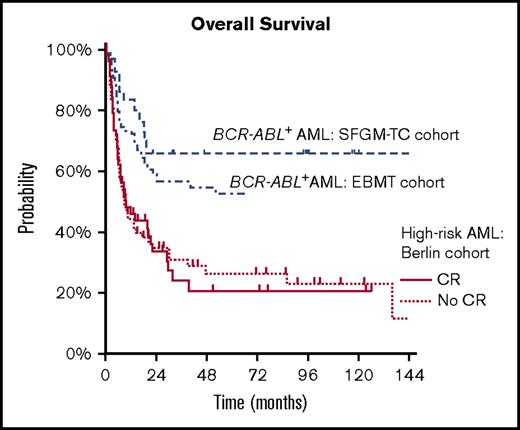
2.The outcome of BCR-ABL+ AML after hematopoietic stem cell transplantation (HSCT) appears to be better than in other high-risk AML cohorts. A retrospective study of the French Society of Bone Marrow Transplantation11 reported a 2-year overall survival of 68% in 19 patients with BCR-ABL+ AML who had undergone HSCT (90% of these patients had achieved a complete remission [CR] before HSCT). Comparable results were obtained by a survey of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation12 demonstrating a 5-year overall survival of 54% and a CR rate of 84% for 57 patients with BCR-ABL+ AML. Although the number of patients in the first analysis is certainly limited and both studies may be influenced by selection bias, both CR rate and overall survival are surprisingly high in contrast to other high-risk AML patient cohorts, whose outcome is still considered as unsatisfying. This is illustrated by survival data of 131 ELN high-risk AML patients who have received HSCT at Charité University Medical Center in Berlin between 1997 and 2015. In this patient cohort, which was in part recently published,13 a 2-year overall survival of 34% was observed. Interestingly, the survival did not substantially differ between patients achieving a CR before HSCT and patients who did not (34.7% vs 33.5%; Figure 1). These data are in line with other high-risk cohorts.14,15
Survival of BCR-ABL+ inv(16)+ AML
| Outcome . | Allo-HSCT . | No allo-HSCT . | Total . |
|---|---|---|---|
| Alive, n (%) | 6 (67) | 10 (71) | 16 (70) |
| Dead, n (%) | 3 (33) | 4 (29)* | 7 (30) |
| Total | 9 | 14 | 23 |
| Outcome . | Allo-HSCT . | No allo-HSCT . | Total . |
|---|---|---|---|
| Alive, n (%) | 6 (67) | 10 (71) | 16 (70) |
| Dead, n (%) | 3 (33) | 4 (29)* | 7 (30) |
| Total | 9 | 14 | 23 |
Long-term survival of patients with BCR-ABL+ AML with concurrent inv(16) from the literature. The table shows a substantial proportion of long-term survivors, even without allogeneic HSCT. The numbers represent absolute patient cases.
1 early death.
Comparison between overall survival in a high-risk AML cohort and BCR-ABL+AML. Overall survival in a high-risk AML cohort after allogeneic HSCT at Charité University Medical Center between 1997 and 2015. In comparison, the BCR-ABL+ AML cohorts from the European Society for Blood and Marrow Transplantation and French Society of Bone Marrow Transplantation (SFGM-TC) are included (data from Chantepie et al11 and Lazarevic et al12 ).
Comparison between overall survival in a high-risk AML cohort and BCR-ABL+AML. Overall survival in a high-risk AML cohort after allogeneic HSCT at Charité University Medical Center between 1997 and 2015. In comparison, the BCR-ABL+ AML cohorts from the European Society for Blood and Marrow Transplantation and French Society of Bone Marrow Transplantation (SFGM-TC) are included (data from Chantepie et al11 and Lazarevic et al12 ).
In our opinion, all of these issues support the view that the clinical course of BCR-ABL+ AML is not determined by BCR-ABL alone. It appears that the prognosis of BCR-ABL+ AML is largely determined by the specific genetic background (cooccurring mutations) rather than by BCR-ABL itself. Furthermore, it might make an important biological difference as to whether BCR-ABL is a primary driver mutation or an acquired secondary change occurring late during leukemia evolution. The situation might be comparable with FLT3-ITD in different molecular contexts (ie, NPM1 mutation vs no known cooccurring key driver).
In summary, the classification of BCR-ABL+ AML as a high-risk disease (with all of its therapeutic consequences) is justified in most cases. However, this is most likely not because of BCR-ABL itself but rather from other high-risk cytogenetic/molecular features that are present in the vast majority of cases. We suggest that the indication for allogeneic HSCT in first CR should be a matter of discussion, particularly in the presence of favorable genetic aberrations and/or the absence of high-risk features.
Authorship
Contribution: P.H., R.A., and J.I. collected and analyzed the transplantation data from Charité University Hospital; N.R.N. and J.W. wrote the paper; and B.D. and C.M.-T. supervised the study.
Conflict-of-interest disclosure: J.W. received research support and/or honoraria from Novartis, BMS, and Celgene. The remaining authors declare no competing financial interests.
Correspondence: Nina Rosa Neuendorff, Department of Hematology, Oncology and Rheumatology, Heidelberg University Hospital, Im Neuenheimer Feld 410, D-69120 Heidelberg, Germany; e-mail: ninarosa.neuendorff@med.uni-heidelberg.de.