Key Points
Rates of pediatric PE in hospitalized patients increased 184% from 2001 to 2014.
Mortality as a result of PE in children has decreased over time and is now comparable to that from VTE.
Abstract
Although rare in children, pulmonary embolism (PE) can cause significant morbidity and mortality. Overall rates of venous thromboembolism (VTE) are increasing in hospitalized children. By using the Pediatric Health Information System database, we evaluated incidence, treatment, and outcome of PE in children younger than age 18 years from 2001 to 2014. Demographic characteristics for those admitted with VTE alone and those admitted with PE were compared. Rates of PE by year were compared with the number of hospital and VTE admissions. Trends in medication use were analyzed. Over the period of the study, patients with PE made up 15.8% of VTE discharges. The overall rate of PE increased 200% (P < .001). Compared with all other age groups, adolescents (age 13-18 years) had the highest prevalence (55%; P < .001), the rate of which increased from 9.8 to 24.7 per 10 000 hospital discharges (152%; P < .001), and from 17.5 to 34.1 per 100 VTE discharges (95%; P < .001). Individuals with PE had a higher mortality (8.3% vs 6%; P < .001) and were less likely to have a complex chronic condition (58% vs 65%; P < .001) than those with VTE alone. However, PE mortality rates decreased over the time period studied. African American and Hispanic patients were more likely to experience recurrent PE than white patients (12% and 10.7% vs 8%; P = .002). During the study period, the use of unfractionated heparin decreased (P < .001), and the use of low molecular weight heparin increased (P < .001). Further research is required to determine what factors contribute to the higher rate of PE in adolescents and influence recurrence in African American and Hispanic patients.
Introduction
Pulmonary embolism (PE) is rare in children, but it can lead to significant morbidity and mortality. Evidence has shown that the incidence of venous thromboembolism (VTE) in pediatric tertiary care hospitals has increased over the past 10 years.1 However, the authors did not specifically evaluate the incidence of PE. Adult guidelines are often used to treat children, but the underlying causes of PE may be very different in pediatric patients when compared with adults, much as they are with VTE. Factors that have been shown to be predictive of PE in adults, such as age older than 65 years, tachycardia, a history of immobilization or surgery, previous PE, deep vein thrombosis (DVT), hemoptysis, and malignancy, may not be applicable in the pediatric population.2,3 Few data exist specifically regarding the epidemiology and outcomes of PE in children or to guide treatment.
Previous estimations of PE prevalence in children have been derived from discharge data and have often been reported in conjunction with other VTEs. The Canadian Registry reported a combined incidence of DVT and PE of 5.3 per 10 000 hospital admissions. Of the 137 children in that registry, 22 (16%) were identified as having PEs.4 Another study that used the US National Hospital Discharge Survey evaluated the rates of DVT and PE in children. That study found that from 1979 to 2001, PE was diagnosed in 13 000 infants and children, and DVT was found in 64 000, with an overall VTE cohort of 75 000 (17% PE). The rate of PE diagnosis in that cohort was 0.9 per 100 000 discharged children per year. That study also found that the rate of VTE (DVT and PE) was higher in infants and teenagers, in keeping with the classic bimodal distribution identified in other studies, and that the rate of VTE was higher in girls.5 Raffini et al1 reported an increase in VTE cases from 34 to 58 per 10 000 hospital admissions from 2001 to 2007. Of the total cohort (14 917), 1434 (9.6%) were identified as having PEs.
In this study, we used the Pediatric Health Information Systems (PHIS) database to evaluate the trend in the rate of PE in children from 2001 to 2014 and the changes in clinical management over that time period. We hypothesized that the incidence of PE had increased over the time period of the study.
Methods
The Children’s Hospital Association (Lenexa, KS) maintains the PHIS database, which comprises comprehensive inpatient data from 48 not-for-profit, tertiary care pediatric hospitals throughout the United States located in 27 states and the District of Columbia. Participating hospitals provide discharge data regarding patient demographics, diagnoses, and procedure and billing data. Data are de-identified and undergo a number of reliability and validity checks through the Children’s Hospital Association and participating hospitals.
This was a retrospective analysis of children up to age 18 years admitted to hospitals that contributed data to PHIS from 2001 to 2014 and who were diagnosed with a PE as identified through diagnosis codes 415.11, 415.12, and 415.19. There were no specific exclusion criteria. Data obtained included demographic characteristics such as age at admission, sex, race, ethnicity, and other complex chronic conditions (CCCs). This study was approved by the local institutional review board.
CCCs in PHIS were defined by using a previously published classification scheme that used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes6 that included the following disorders: neuromuscular, cardiovascular, respiratory, renal, gastrointestinal, hematology/immunodeficiency, metabolic, other congenital/genetic defect, and malignancy. Identification of patients with VTE was carried out as previously described.1 Clinical variables evaluated included mortality, PE recurrence, type of initial treatment, and length of hospital stay. The primary outcome measure was the trend in incidence of pediatric PE over the course of the study. The secondary outcomes evaluated were rates of recurrence, treatment patterns, and trends for PE.
Statistical analysis
Categorical variables were described using frequencies and values. Comparisons of demographic information or health service use between PE discharges and non-PE discharges and patients with a single PE discharge vs patients with a recurrent PE were made by using χ2 tests for categorical variables and the Wilcoxon rank sum test for comparison of medians in continuous variables.
PEs per 100 VTEs and per 10 000 discharges were modeled over time by using generalized linear mixed models (GLMMs), assuming an underlying Poisson distribution and a natural log link function. Variables included in the model were year of study, age group, and the 2-way interaction. To account for any effect of the clustering of patients within hospitals by age group over time, we fit a random hospital effect, a random hospital-by-age-group effect, and a random hospital-by-year-of-study effect in our GLMM. The assumption of any additional variance/covariance components, such as a first-order autoregressive covariance structure, did not improve the model fit and were not included in the final GLMM.
Logistic regression was used to assess trends in the use of medication. We fit a generalized estimating equation assuming a first-order autoregressive variance/covariance structure to account for potential correlation in prescribing patterns across years within the same hospital. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). P < .05 was considered statistically significant.
Results
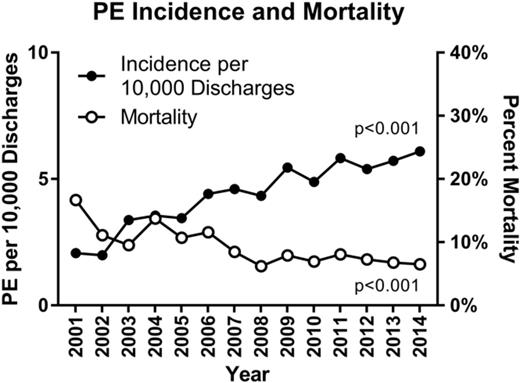
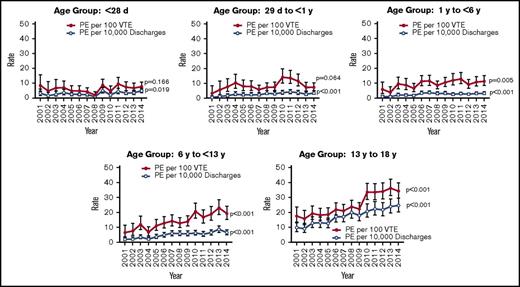
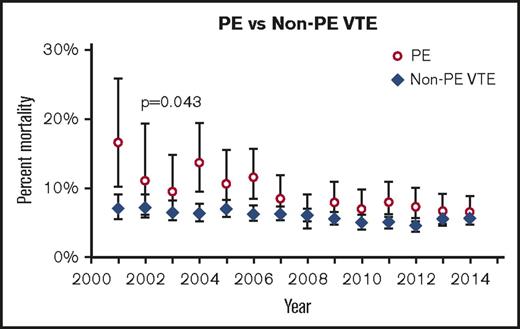
Over the period of the study, patients discharged with a diagnosis of PE (n = 4644) made up 15.8% of all pediatric discharges with VTE (n = 29 380). The mean overall rate of PE increased by 200% from 2001 to 2014 (P < .001), from 2 to 6 per 10 000 hospital discharges, and from 7 to 13 per 100 VTE discharges. Compared with all other age groups, adolescents (age 13-18 years) were the most likely to present with PEs (55%; P < .001) (Table 1). In addition, the mean rate of PE in this age group increased from 9.8 to 24.7 per 10 000 hospital discharges, a 152% increase (P < .001), and from 17.5 to 34.1 per 100 VTE discharges, a 95% increase (P < .001) (Figure 1) The bimodal age distribution seen in most VTEs was not apparent in the population with PEs, which showed a continued increase with advancing age (Table 1). Those with PEs had a higher mortality than those with VTEs alone (8.3% vs 6%; P < .001). However, the overall mortality from PEs decreased over the time period and by 2014 was comparable to that of VTEs (Figure 2). Rates of pulmonary imaging per VTE diagnosis increased from 4.7% in 2007 to 13.1% in 2014 (P < .001; years for which data were available).
Demographic characteristics
| Characteristic . | All VTE discharges (N = 29 380) . | Discharges without PE (n = 24 736) . | Discharges with PE (n = 4644) . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Age | <.001 | ||||||
| <28 d | 4 192 | 14.3 | 3 921 | 15.9 | 271 | 5.8 | |
| 29 d to <1 y | 4 323 | 14.7 | 3 953 | 16 | 370 | 8.0 | |
| 1 to <6 y | 5 692 | 19.4 | 5 119 | 20.7 | 573 | 12.3 | |
| 6 to <13 y | 5 560 | 18.9 | 4 719 | 19.1 | 841 | 18.1 | |
| 13-18 y | 9 613 | 32.7 | 7 024 | 28.4 | 2589 | 55.7 | |
| Male sex | 15 855 | 54 | 13 447 | 54.4 | 2408 | 51.9 | .0016 |
| Payer | <.001 | ||||||
| Government | 14 405 | 49.0 | 12 231 | 49.4 | 2174 | 46.8 | |
| Commercial | 11 157 | 38.0 | 9 259 | 37.4 | 1898 | 40.9 | |
| Other | 3 818 | 13.0 | 3 246 | 13.1 | 572 | 12.3 | |
| Characteristic . | All VTE discharges (N = 29 380) . | Discharges without PE (n = 24 736) . | Discharges with PE (n = 4644) . | P . | |||
|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | ||
| Age | <.001 | ||||||
| <28 d | 4 192 | 14.3 | 3 921 | 15.9 | 271 | 5.8 | |
| 29 d to <1 y | 4 323 | 14.7 | 3 953 | 16 | 370 | 8.0 | |
| 1 to <6 y | 5 692 | 19.4 | 5 119 | 20.7 | 573 | 12.3 | |
| 6 to <13 y | 5 560 | 18.9 | 4 719 | 19.1 | 841 | 18.1 | |
| 13-18 y | 9 613 | 32.7 | 7 024 | 28.4 | 2589 | 55.7 | |
| Male sex | 15 855 | 54 | 13 447 | 54.4 | 2408 | 51.9 | .0016 |
| Payer | <.001 | ||||||
| Government | 14 405 | 49.0 | 12 231 | 49.4 | 2174 | 46.8 | |
| Commercial | 11 157 | 38.0 | 9 259 | 37.4 | 1898 | 40.9 | |
| Other | 3 818 | 13.0 | 3 246 | 13.1 | 572 | 12.3 | |
Rate of PEs per 10 000 discharges and 100 VTE admissions. Error bars indicate standard deviation.
Rate of PEs per 10 000 discharges and 100 VTE admissions. Error bars indicate standard deviation.
Mortality over time of PE vs VTE. Error bars indicate standard deviation.
Males were noted to have a higher prevalence of PEs within the whole cohort (54% vs 46%; P = .0016). However, there was a higher prevalence in females within the 13- to 18-year age group when analyzed separately (51.6% vs 48.4%; P < .001). African American and Hispanic patients were more likely to experience a recurrent PE than white patients (12% and 10.7% vs 8%; P < .001). Payer source was evaluated and was not found to be associated with an increased risk of recurrence. However, PEs were associated with a higher percentage of government-paid hospitalizations (P < .001), and patients with recurrent PEs had significantly shorter length of stay and lower costs on their initial hospital stay (19 vs 25 days; P = .0006).
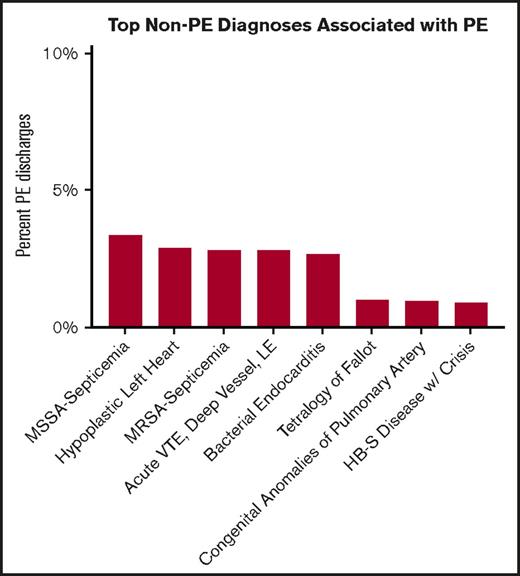
Individuals with PEs were less likely to have a CCC than those with VTE alone (58% PE vs 65% VTE; P < .001). The most common diagnoses associated with PEs were analyzed (Figure 3). The majority of those had infectious etiology, with methicillin-susceptible Staphylococcus aureus septicemia being the most common. Congenital heart disease, specifically hypoplastic left heart syndrome and tetralogy of Fallot, were also among the diagnoses associated with PEs. In the 13- to 18-year-old age group, the top associated diagnoses were DVT of the proximal lower extremity or another site, bacterial endocarditis, and S aureus sepsis (methicillin sensitive and methicillin resistant).
Top non-PE associated diagnoses associated with PEs. HB-S, hemoglobin S; LE, lower extremity; MRSA, methicillin-resistant S aureus; MSSA, methicillin-sensitive S aureus.
Top non-PE associated diagnoses associated with PEs. HB-S, hemoglobin S; LE, lower extremity; MRSA, methicillin-resistant S aureus; MSSA, methicillin-sensitive S aureus.
Over the period of the study, the use of unfractionated heparin decreased (P < .001) and the use of low molecular weight heparin (LMWH) increased (P < .001) (Figure 4). The use of recombinant tissue plasminogen activator remained steady over the course of the study (P = .055). Patients with recurrent PEs were more likely to have been prescribed warfarin (P = .0041) or LMWH (P < .0001) for the treatment of their first PE compared with thrombolysis or unfractionated heparin. The presence of a central line was similar between those with VTEs and those with PEs (51% vs 50%; P = .200).
Initial use of anticoagulants in patients with PEs.P values represent change in anticoagulant use over time. Error bars indicate standard deviation. tPA, tissue plasminogen activator.
Initial use of anticoagulants in patients with PEs.P values represent change in anticoagulant use over time. Error bars indicate standard deviation. tPA, tissue plasminogen activator.
Discussion
Our study shows a similar percentage of PE diagnoses when compared with all VTE diagnoses, as in previously published studies.1,4,5 Much like the overall incidence of VTEs in the hospitalized pediatric population, our study demonstrates that PE rates have increased from 2001 to 2014. However, when compared with the 70% increase identified by Raffini et al,1 PE has experienced a more rapid rise in incidence. Increased use of medications such as hormonal contraceptives or more prevalent obesity and other lifestyle choices may have contributed to this apparent rise. Alternatively, this may be a result of more sensitive radiologic techniques to identify less clinically significant PEs or to a higher index of suspicion in pediatric providers. Evaluation of trends in the use of computed tomographyfor diagnosing PE in adults has shown a dramatic increase in the number of tests performed.7 Other studies have indicated that the difficulty of interpreting radiographic studies may result in overdiagnosis of PEs by pulmonary computed tomography angiography.8 Our data also demonstrate an increase in pulmonary imaging in children with VTEs. With these data, it is not possible to determine whether these tests were obtained in response to significant clinical symptoms, but it is possible that the increase in diagnosis of PEs is related, at least in part, to the increased use of imaging.
There are many risk-prediction scores for PEs for adults.9-11 However, these have not been validated in children, and when these have been applied to a pediatric population, they do not perform as well, and those typically used clinical findings such as the D-dimer have been nondiscriminatory.12,13 A case-control study investigating risk factors and clinical features that distinguished children with PE from those without identified current or recent use of the oral contraceptive pill as the most significant risk factor. Although the patients with PEs tended to more often have tachycardia, clinical signs were not effective in identifying those with PEs.14 Risk prediction scores developed for children thus far have focused on the risk of the development of hospital-acquired VTEs.13-15 It is possible that the development of a more accurate prediction tool for pediatric PEs could decrease the risk of overdiagnosis and allow for more appropriate imaging in high-risk patients.
In most studies regarding pediatric VTEs, the highest risk is seen in infants younger than 1 year of age and in adolescents, which creates a bimodal distribution.1,4,5 It is striking that the age distribution of PEs we found varies from that of VTEs, with a steady increase in prevalence from infancy with the highest risk in the adolescent population. If this distribution is true, it suggests that risk factors for PE and VTE differ, particularly after puberty. It may be that the pathophysiology of PEs in adolescents is more akin to that of adults and that strategies used to mitigate risk in the adult population may be applicable in this pediatric subset.
Our study found that the mortality associated with PEs decreased over the time period of the study until it approximated that of the overall VTE cohort (6.52% for PEs vs 5.69% for VTEs). This may be a result of improved treatment of the underlying VTE diagnosis, more prompt treatment, or from improved supportive care. Alternatively, it may be related to overdiagnosis resulting from more sensitive imaging techniques or provider concern, as described above. This raises a question regarding the need for treatment in small subsegmental or incidentally identified PEs. The risk of treatment with anticoagulation in these patients may be greater than the potential morbidity from PEs. Information regarding mortality in children specifically attributable to PEs is not readily found in the literature, which limits our ability to compare our results to historical data. Our study was unable to assess complications from treatment. Further prospective studies are required.
From our data, it seems that the population of hospitalized pediatric patients with PEs is distinct from the population that presents with VTEs alone. Importantly, the patients with PEs have a decreased rate of CCCs, as defined by PHIS. Infection, particularly that with S aureus, was associated with PEs in this population. We have previously demonstrated that S aureus sepsis was associated with VTEs and increased mortality in a retrospective, single-center cohort.16 Other investigators have also shown an association between infection with S aureus, often osteomyelitis, and VTE.17-19 Although central venous lines have been identified as a significant risk factor for pediatric VTEs, this association was not identified in this study regarding PEs.15,20-22
Treatment of PEs uses a variety of anticoagulants, including unfractionated heparin, LMWH, warfarin, and alternative anticoagulants. Our study demonstrates a trend in treatment away from unfractionated heparin and warfarin and toward LMWH. Interestingly, although there was an increase in PE over the time period of the study, the use of thrombolysis did not increase. In relation to the concern for overdiagnosis, this may be an indication that patients were identified before severe symptom development and cardiovascular collapse, or that some patients may not have required anticoagulation at all. Alternatively, the relatively stable use of thrombolysis in the face of increased LMWH usage may merely reflect changing practice patterns.
In our study, African American and Hispanic children with PEs were more likely to experience a recurrence. This association remained true when payer source was accounted for. However, it was noted that patients with recurrence had lower costs and shorter length of stay on their index hospitalization, raising the concern that these patients initially may have been undertreated. Further research is required to determine whether this increased risk of recurrence is accurate. If it is, possible contributory variables need to be elucidated. The previous study using National Hospital Discharge Survey data notes an increased rate of PEs in African American children of 1.6 per 100 000 per year compared with the baseline rate of 0.7 per 100 000 per year in white children.5 We did not see a similar higher rate in our data.
Limitations of this study include our inability to identify prothrombotic risk factors before patients are admitted to the hospital, such as use of oral contraceptives or other medications, or lifestyle choices such as smoking tobacco. We were also unable to identify inherited thrombophilia. The PHIS database did not capture whether a patient had a procedure at another hospital that put them at higher risk of a PE. We are also limited by the fact that this is a retrospective study, and the PHIS database includes only inpatient data. As previously noted, we could not ascertain complications of treatment. The PHIS database relies upon charge coding, and therefore some patients may have been incorrectly identified as having PEs when they did not, or vice versa. We were unable to confirm diagnoses with data from imaging studies, given the nature of the database.
From these data, it seems that the rate of PEs in hospitalized pediatric patients is increasing out of proportion to the rate of VTE increase, which has previously been described. The prevalence is highest in the adolescent age group and is increasing more rapidly in this group as well. Patients with PEs have a higher mortality rate, although mortality has decreased over the last 10 years and now approximates that for VTE. There seems to be a higher risk of recurrence in African American and Hispanic patients when compared with white patients, regardless of payer source. More research is required to confirm these trends and to evaluate causes and interventions.
Acknowledgment
The authors thank Tina Khaleghi who helped develop the study and presented an early version of the data at the 2014 Annual Meeting of the American Society of Hematology.
Authorship
Contribution: S.L.C. conceived the study, helped analyze the data, and wrote the manuscript; and M.H. and T.R. helped develop the study, performed the data and statistical analysis, and critically edited the manuscript.
Conflict-of-interest disclosure: S.L.C. received honoraria from Bayer, CSL Behring, HEMA Biologics, and Novo Nordisk for consulting services and serves on a study steering committee for Genentech and on an advisory board for Kedrion. The remaining authors declare no competing financial interests.
Correspondence: Shannon L. Carpenter, Children’s Mercy Hospital, Department of Pediatrics, 2401 Gillham Rd, Kansas City, MO 64108; e-mail: slcarpenter@cmh.edu.