Key Points
The Lu/BCAM adhesion molecule is gradually activated during erythrocyte aging due to loss of sialic acid on glycophorin-C.
Upon activation, Lu/BCAM engages a sialic acid–dependent interaction with the extracellular matrix protein laminin-α5.
Abstract
Lutheran/basal cell adhesion molecule (Lu/BCAM) is a transmembrane adhesion molecule expressed by erythrocytes and endothelial cells that can interact with the extracellular matrix protein laminin-α5. In sickle cell disease, Lu/BCAM is thought to contribute to adhesion of sickle erythrocytes to the vascular wall, especially during vaso-occlusive crises. On healthy erythrocytes however, its function is unclear. Here we report that Lu/BCAM is activated during erythrocyte aging. We show that Lu/BCAM-mediated binding to laminin-α5 is restricted by interacting, in cis, with glycophorin-C–derived sialic acid residues. Following loss of sialic acid during erythrocyte aging, Lu/BCAM is released from glycophorin-C and allowed to interact with sialic acid residues on laminin-α5. Decreased glycophorin-C sialylation, as observed in individuals lacking exon 3 of glycophorin-C, the so-called Gerbich phenotype, was found to correlate with increased Lu/BCAM-dependent binding to laminin-α5. In addition, we identified the sialic acid–binding site within the third immunoglobulin-like domain within Lu/BCAM that accounts for the interaction with glycophorin-C and laminin-α5. Last, we present evidence that neuraminidase-expressing pathogens, such as Streptococcus pneumoniae, can similarly induce Lu/BCAM-mediated binding to laminin-α5, by cleaving terminal sialic acid residues from the erythrocyte membrane. These results shed new light on the mechanisms contributing to increased adhesiveness of erythrocytes at the end of their lifespan, possibly facilitating their clearance. Furthermore, this work may contribute to understanding the pathology induced by neuraminidase-positive bacteria, because they are especially harmful to patients suffering from sickle cell disease and are associated with the occurrence of vaso-occlusive crises.
Introduction
Lutheran/basal cell adhesion molecule (Lu/BCAM) is a transmembrane adhesion molecule found on erythrocytes, endothelial cells, and several types of cancer cells.1,2 It is expressed as a long (Lu) and a short [Lu(v13)] isoform that differ only in their cytoplasmic tail, which is 59- and 19-amino-acid residues long, respectively. Both isoforms are highly expressed during the early stages of erythropoiesis after which the expression levels strongly decline.3 Erythrocytes express 500 to 4000 Lu/BCAM molecules on their surface, a quantity that gradually decreases as erythrocytes shed membrane and become increasingly dense during their lifespan.4,5 Lu/BCAM can interact with the extracellular matrix (ECM) component laminin-α5, allowing the capturing of erythrocytes through this interaction even under flow conditions.6,7 As the ECM is normally not exposed to circulating erythrocytes, the function of Lu/BCAM on terminally differentiated erythrocytes is currently unclear. However, in sickle cell disease (SCD), the vascular endothelium can be substantially damaged, causing exposure of the ECM to the circulation,8,9 potentially allowing an interaction between erythrocytes and laminin-α5 to occur.7,10 Such Lu/BCAM-mediated adhesion of sickle erythrocytes to the vascular wall is believed to contribute to vaso-occlusive crises.11
Activation is required for Lu/BCAM to interact with laminin-α5. In SCD and polycythemia vera (PV), Lu/BCAM is constitutively activated on a large proportion of the erythrocytes through phosphorylation.12,13 In SCD, increased baseline levels of cyclic adenosine monophosphate lead to PKA activation and ultimately to Lu/BCAM phosphorylation.13 Epinephrine can further increase cyclic adenosine monophosphate levels, especially in the younger fraction of sickle erythrocytes.13 In PV, the JAK2V617F mutation causes activation of the RAP1/Akt signaling pathway that eventually leads to Lu/BCAM activation through phosphorylation.14,15 Upon phosphorylation, Lu/BCAM is believed to detach from the underlying spectrin network16,17 and thus to more easily cluster upon ligation of laminin-α5. Although both Lu and Lu(v13) contain the cytoplasmic motif that interacts with the underlying spectrin network,17 only the Lu cytoplasmic tail seems to contain phosphorylation sites that modulate this interaction.12 The notion that Lu/BCAM activation, at least to a certain extent, is dependent on its release from the spectrin network is corroborated by the finding that erythrocytes from patients suffering from hereditary spherocytosis adhere more frequently to laminin-α5.16 There exists some evidence that during the sickle erythrocyte lifespan Lu/BCAM activity is changing. The low-density sickle erythrocytes, containing young erythrocytes, were found to be more susceptible to epinephrine stimulation, whereas the old, highly dense erythrocytes, that contain fewer Lu/BCAM molecules, were found to be most adherent to laminin-α5.5
Here we report a novel mechanism that drives Lu/BCAM activation on aging erythrocytes, which can account for abnormal erythrocyte adhesion in SCD and other pathological states such as sepsis. We identified a domain within Lu/BCAM that bears homology to the SIGLEC family of sialic acid–binding receptors. This domain interacts, in cis, with sialic acid on erythroid Glycophorin-C (GpC), preventing Lu/BCAM from binding to laminin-α5 in trans. We show that this interaction is gradually decreasing during erythrocyte aging, ultimately leading to Lu/BCAM activation. We strengthen our findings with data from individuals that lack exon 3 of GpC, the so-called Gerbich phenotype, which are unable to restrict Lu/BCAM activity. Last, we provide data that indicate that encapsulated bacteria, to which sickle cell patients are highly susceptible,18 can similarly activate Lu/BCAM by removing erythrocyte sialic acid.
Methods
Blood samples, isolation of dense and light erythrocytes, and erythrocyte deformability
Heparinized venous blood was obtained and washed as previously described.19 GpC-ex3 erythrocytes were kindly provided by the Department of Serology, Sanquin (Amsterdam, The Netherlands). Dense and light erythrocytes were isolated using Percoll (GE Healthcare, Little Chalfont, UK) density centrifugation. Briefly, isotonic Percoll was prepared by adding 8.1 mL 10× phosphate-buffered saline (PBS) per 100 mL Percoll. Next, Percoll buffer (26.3 g/L bovine serum albumin, 132 mM NaCl, 4.6 mM KCl, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES]) was used to dilute isotonic Percoll to 1.096 (80%), 1.087 g/mL (71%), 1.080 g/mL (64%), and 1.060 g/mL (40%), respectively (supplemental Figure 1D). Deformability of young and old erythrocytes was measured using an automated rheoscope and cell analyzer at a shear stress of 30 dyne/cm2 (3 Pa).20
Flow cytometry, flow assays, FRAP, antibodies, and reagents
Flow cytometric analysis was performed on an LSRII + HTS (BD Biosciences, Franklin Lakes,NJ), and data were analyzed by FACSDiva software (BD Biosciences). Imaging and fluorescence recovery after photobleaching (FRAP) experiments were performed using the confocal laser scanner platform Leica TCS SP8. LASX software was used to set up and analyze the experiments (Leica Microsystems, Wetzlar, Germany). Anti-GpC/D antibodies, BRIC4, BRIC10, and BRIC100, were a kind gift from IBRGL (Bristol, United Kingdom). Anti-CD235a-PE (M1732) was purchased from Sanquin Reagents (Amsterdam, The Netherlands). Erythrocyte adhesion to laminin-α5 was assessed by coating laminin from human placenta (Sigma-Aldrich, St Louis, MO) or recombinant laminin-511 (BioLamina, Sundyberg, Sweden) through passive adsorption on an uncoated IBIDI u-slideVI0.4 flow chamber. Adhesion frequency was quantified by EVOS microscopy (Thermo Fisher, Waltham, MA). Briefly, 1 × 106 erythrocytes are pumped over a laminin-α5–coated chamber at 0.2 dyn/cm2. Adhesion frequency was assessed by quantifying 9 representative locations (2 mm2) on the IBIDI slide. These data were assumed to follow a normal distribution. The data were analyzed using Graphpad Prism 6 software and presented as mean ± standard deviation. Parametric Student t tests were used to determine P values. The Pearson correlation coefficient was used to express the correlation strength between Lu, GpC, and erythrocyte adhesion frequency (fold, compared with control) to laminin-α5.
Terminal sialic residues were removed from laminin-α5 and the erythrocyte membrane by 0.15 mU of neuraminidase from Vibrio cholerae at 37°C for 4 hours and 30 minutes, respectively (V cholerae filtrate and purified type II neuramindase; Sigma-Aldrich). Successful desialylation was confirmed using Arachis hypogea lectin (Sigma-Aldrich) that agglutinates erythrocytes upon desialylation.21 Streptococcus pneumoniae (Cultiloops ref #4609015; Thermo Fisher) were grown on chocolate agar (Anaerobe Systems, Morgan Hill, CA) resuspended to optical density = 1 in HEPES+ (132 mM NaCl, 20 mM HEPES, 6 mM KCl, 1 mM MgSO4, 1.2 mM K2HPO4, all from Sigma-Aldrich) and incubated with erythrocytes at 1:1000 and 1:50, respectively, for 3 hours at 37°C.
Lu/BCAM expression in K562 and HEK293T cells
The coding sequence of Lutheran (PubMed ID NM_005581) was ordered without stop codon at Genscript, with 5′ flanking sequence of GGATCCGCCACC and 3′ flanking sequence of GGATATC, and subsequently cloned into the BamHI and EcoRV sites of pENTR1A (Invitrogen, Carlsbad, CA). The resulting plasmid pENTR1A-Lutheran was recombined with pLenti6.3/V5 using LR Clonase II (Invitrogen), creating pLenti6.3–Lutheran-V5. Lentiviral particles were generated by transient cotransfection of 293T cells with pLenti6.3–Lutheran-V5, pMDL-gp, RSVrev, and pCMV-VSVg, using TransIT-LT1 (Mirus Bio, Madison, WI). Supernatant containing virus was harvested on days 2 and 3 after transfection and filtered through 0.45 μM, and 1 mL was used on 5 × 105 K562 cells on 2 successive days. The transduced K562 cells were selected with 10 μg/mL blasticidin (Invitrogen). Expression of Lutheran-V5 was determined by western blot and flow cytometry using goat anti-human Lu/BCAM (R&D Systems, Minneapolis, MN).
Production and purification of the extracellular domain of Lu/BCAM
Substitution of c.1012A>G and c.1013G>C (ENSEMBL ENST00000270233.10) in Lutheran, resulting in substitution of arginine residue 338 into an alanine (R338A), was performed using the QuickChange Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) using the primers 5′-GGGACCTATGGCTGCGCAGTGGAGGATTACGAC-3′ (nt. 997-1029) and 5′-GTCGTAATCCTCCACTGCGCAGCCATAGGTCCC-3′ on pIgPlus plasmid containing the Lutheran extracellular domain (ECD). Correct mutagenesis was verified by Sanger sequencing. Lu-ECD-IgG1-Fc and Lu-ECD-R338A-IgG1-Fc protein was generated by transient cotransfection of Freestyle HEK cells (Invitrogen) with pIgPlus–Lu-WT/R338A, pORF-p21, pORF27, and pSVLT, using 293Fectin (Invitrogen). Immunoglobulin G (IgG)1-Fc-tagged Lu-WT and Lu-R338A were purified from culture supernatant using protein G sepharose Fastflow (GE Healthcare), subsequently desalted by PD10 column buffer exchange (GE Healthcare), and concentrated by Amicon-15 Ultracel 10-kDa column filtration (EMD Millipore, Billerica, MA).
Immunoprecipitation, erythrocyte membrane isolation, and western blotting
For immunoprecipitation, 75 µL Protein G Dynabeads (Thermo Fisher) were washed twice in PBS and incubated with 20 µL of either BRIC4, BRIC10, or BRIC100 for GpC immunoprecipitation, 20 µL of anti–glycophorin-A-PE (Sanquin), and Mouse-IgG isotype control (Thermo Fisher) antibody for 1 hour in 300 µL PBS. Amounts of 2 × 108 erythrocytes and 5 × 106 K562 cells were lysed for 15 minutes at 4°C in lysis buffer (10 mM Tris, 30 mM NaCl, 0.5% NP40, 10% glycerol, 1:100 Halt protease inhibitor cocktail [Thermo Fisher] ) and incubated with the antibody-coated protein-G beads. Detergent-resistant and soluble membrane (DRM/DSM) from 100 × 106 erythrocytes were isolated using 1% Triton X-100 buffered in 50 mM Tris, 150 mM NaCl supplemented with 1:1000 protease/phosphatase inhibitor mix (HALT; Sigma). The DRM and DSM were separated by centrifugation at 20 000g for 30 minutes at 4°C.
Results
Lu/BCAM binding to laminin-α5 increases during erythrocyte aging as a consequence of membrane sialic acid loss
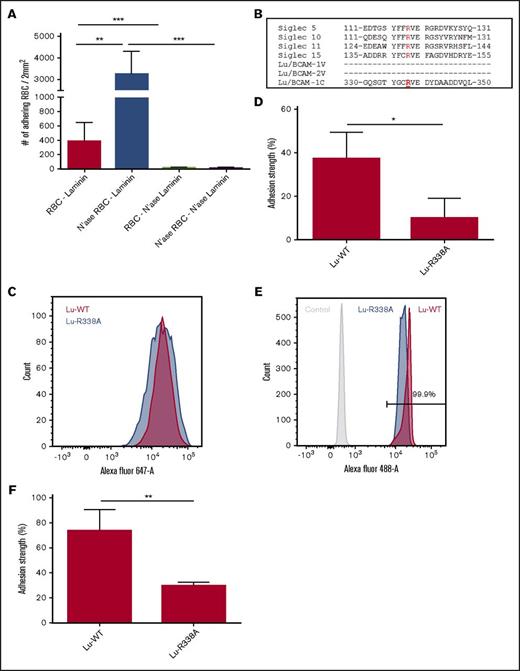
In freshly isolated erythrocytes from healthy donors, there is a fraction of erythrocytes that display Lu/BCAM-mediated binding to laminin-α5. This fraction is much smaller than that found in erythrocytes isolated from SCD patients (Figure 1A; supplemental Figure 1A). Notably, within the population of sickle erythrocytes, the dense erythrocytes adhere to laminin-α5 more frequently, despite a marked reduction in Lu/BCAM expression,5,22 thus suggesting that Lu/BCAM expression levels are not the only determinant of activity. To assess if the same phenomenon is also occurring in erythrocytes from healthy donors, we isolated young and old erythrocytes based on density. Also, in healthy donors, we found that mainly the dense, non-deformable erythrocytes adhered most frequently to laminin-α5 (Figure 1B; supplemental Figure 1B). We aimed to determine how Lu/BCAM is activated on these cells. During erythrocyte lifespan, sialic acid content is gradually lost.23-25 Indeed, the dense erythrocytes we isolated that adhered better to laminin-α5 contained significantly less α2,3-linked sialic acid (Figure 1C). We tested whether red cell membrane sialic acid loss would induce Lu/BCAM activation. We treated erythrocytes from healthy donors with neuraminidase, thereby removing membrane sialic acid, and found that these erythrocytes adhered to laminin-α5 at least as frequently as sickle erythrocytes, which was shown to be Lu/BCAM dependent using a blocking antibody (Figure 1D). Because aged sickle erythrocytes adhere more to laminin-α5, we hypothesized that loss of sialic acid from the erythrocyte membrane might cause Lu/BCAM activation, also in SCD. We therefore treated sickle erythrocytes with neuraminidase and found these cells to adhere even more frequently to laminin-α5 upon desialylation (Figure 1E).
Loss of sialic acids on the erythrocyte membrane activates Lu/BCAM. (A) Representative micrograph of adhesion of healthy erythrocytes (top panel; original magnification ×10) and sickle erythrocytes (lower panel) to a laminin-α5–coated ibidi chamber at 0.2 dyn/cm. (B) Quantification of adhesion frequency of old and young erythrocytes to laminin-α5 at 0.2 dyn/cm2 (n = 3). Percoll dilutions were stacked in a 15-mL tube; erythrocytes isolated from the fraction denser than 1.096 g/mL Percoll were defined as dense and old erythrocytes (roughly 3% of total red blood cell [RBC]), whereas erythrocytes lighter than 1.060 g/mL Percoll are here defined as light and young erythrocytes (roughly 0.75% of total RBC). (C) Biotinylated Maackia amurensis lectin type II (MA) was used to quantify α2,3-linked sialic acid (SIA) by flow cytometry (Data shown as mean fluorescence intensity [MFI] and normalized [Norm.] to control erythrocytes [aaRBC].). (D) Membrane sialic acid was removed from control erythrocytes by V cholerae neuraminidase (N'ase RBC), and adhesion frequency was assessed at 0.2 dyn/cm2 (n = 5). Specificity was addressed using an Lu/BCAM blocking polyclonal antibody. (E) Sickle erythrocyte (ssRBC) adhesion frequency to laminin-α5 at 0.2 dyn/cm2, either treated or not treated with neuraminidase for 30 minutes at 37°C (n = 5). *P < .05; ***P < .001.
Loss of sialic acids on the erythrocyte membrane activates Lu/BCAM. (A) Representative micrograph of adhesion of healthy erythrocytes (top panel; original magnification ×10) and sickle erythrocytes (lower panel) to a laminin-α5–coated ibidi chamber at 0.2 dyn/cm. (B) Quantification of adhesion frequency of old and young erythrocytes to laminin-α5 at 0.2 dyn/cm2 (n = 3). Percoll dilutions were stacked in a 15-mL tube; erythrocytes isolated from the fraction denser than 1.096 g/mL Percoll were defined as dense and old erythrocytes (roughly 3% of total red blood cell [RBC]), whereas erythrocytes lighter than 1.060 g/mL Percoll are here defined as light and young erythrocytes (roughly 0.75% of total RBC). (C) Biotinylated Maackia amurensis lectin type II (MA) was used to quantify α2,3-linked sialic acid (SIA) by flow cytometry (Data shown as mean fluorescence intensity [MFI] and normalized [Norm.] to control erythrocytes [aaRBC].). (D) Membrane sialic acid was removed from control erythrocytes by V cholerae neuraminidase (N'ase RBC), and adhesion frequency was assessed at 0.2 dyn/cm2 (n = 5). Specificity was addressed using an Lu/BCAM blocking polyclonal antibody. (E) Sickle erythrocyte (ssRBC) adhesion frequency to laminin-α5 at 0.2 dyn/cm2, either treated or not treated with neuraminidase for 30 minutes at 37°C (n = 5). *P < .05; ***P < .001.
Lu/BCAM is a sialic acid–binding protein and bears functional and sequence homology to SIGLECs
Based on the finding that loss of sialic acid causes activation of Lu/BCAM, we hypothesized that on healthy young erythrocytes Lu/BCAM activity is restricted by interacting with erythrocyte membrane sialic acid. Therefore, we reasoned that after erythrocyte desialylation Lu/BCAM is able to interact, in trans, with sialic acids on laminin-α5. To test this, laminin-α5 was treated with neuraminidase after which the interaction with neuraminidase-treated erythrocytes was assessed (Figure 2A). Removal of sialic acid from laminin-α5 abolished the interaction with untreated and neuraminidase-treated erythrocytes. This shows that sialic acid residues on laminin-α5 are important to establish an interaction with Lu/BCAM on erythrocytes. A well-known group of receptors interacting with sialic acid in a similar manner are sialic acid–binding immunoglobulin-type lectins (SIGLECs).26 They interact with sialic acid either in cis, masking the SIGLEC, or in trans, for example, after neuraminidase treatment or cellular activation.26 Although there are exceptions, in most currently known SIGLECs, the first V-type domain harbors the arginine residue that is critical for sialic acid interaction.26 Alignment of a subset of SIGLECs reveals a conserved stretch of amino acids around the arginine residue that is critical for sialic acid binding. In Lu/BCAM, a homologous region was found. Of note, this homologous region was not present in the first V-type immunoglobulin-like domain, but rather in the third domain, which bears more resemblance to a C-type immunoglobulin-like domain (Figure 2B). We mutated Lu/BCAM, substituting the arginine in Lu/BCAM that is homologous to the sialic acid–binding arginine in SIGLECs into an alanine (R338→A) and transfected wild-type (WT) and mutant Lu/BCAM in K562 cells. Transfected K562 cells expressed similar levels of Lu-WT and Lu-R338A, as was assessed by flow cytometry (Figure 2C). The adhesion strength with which the Lu-R338A transfected K562 cells adhered to laminin-α5 was significantly reduced (Figure 2D). In addition, we produced and purified the ECD of Lu-WT-Fc and Lu-R338A-Fc, which we coated on Protein-G Dynabeads. Equal coating density on the Dynabeads for Lu-WT-Fc and Lu-R338A-Fc protein was confirmed by flow cytometry (Figure 2E). Similar to K562 transfectants, Lu-R338A-Fc–coated protein-G beads adhered to laminin-α5 with significantly decreased strength compared with control (Figure 2F). These results suggest that arginine residue 338 within Lu/BCAM interacts with sialic acid in a manner similar to SIGLECs.
Lu/BCAM amino acid residue R338 is critical for laminin-α5 binding. (A) Laminin-α5–coated ibidi chambers and erythrocytes were treated with neuraminidase and compared with nontreated erythrocytes (n = 4), and adhesion frequency to laminin-α5 was assessed at 0.2 dyn/cm2. (B) Various Lu/BCAM domains were aligned to SIGLEC sequences that are centered around the arginine residue critical for interaction with sialic acid residues. (C) Flow cytometry histogram comparing expression of Lu-WT (red) and Lu-R338A (blue) in transfected K562 cells. (D) Adhesion of K562 cells transfected with Lu-WT and Lu-R338A to laminin-α5–coated ibidi chambers (n = 3). K562 cell adhesion strength was measured to correct for variation between controls. Adhesion strength was defined as the percentage of cells that remain attached after gradually increasing flow shear from a static to 0.2 dyn/cm2 up to a maximum of 2.5 dyn/cm2. (E) Flow cytometric comparison of control (light gray), Lu-WT-Fc (red), and Lu-R338A-Fc (blue) coated protein-G beads. (F) Adhesion strength of Lu-WT-Fc and Lu-R338A-Fc–coated protein-G beads (n = 3). *P < .05; **P < .01; ***P < .001.
Lu/BCAM amino acid residue R338 is critical for laminin-α5 binding. (A) Laminin-α5–coated ibidi chambers and erythrocytes were treated with neuraminidase and compared with nontreated erythrocytes (n = 4), and adhesion frequency to laminin-α5 was assessed at 0.2 dyn/cm2. (B) Various Lu/BCAM domains were aligned to SIGLEC sequences that are centered around the arginine residue critical for interaction with sialic acid residues. (C) Flow cytometry histogram comparing expression of Lu-WT (red) and Lu-R338A (blue) in transfected K562 cells. (D) Adhesion of K562 cells transfected with Lu-WT and Lu-R338A to laminin-α5–coated ibidi chambers (n = 3). K562 cell adhesion strength was measured to correct for variation between controls. Adhesion strength was defined as the percentage of cells that remain attached after gradually increasing flow shear from a static to 0.2 dyn/cm2 up to a maximum of 2.5 dyn/cm2. (E) Flow cytometric comparison of control (light gray), Lu-WT-Fc (red), and Lu-R338A-Fc (blue) coated protein-G beads. (F) Adhesion strength of Lu-WT-Fc and Lu-R338A-Fc–coated protein-G beads (n = 3). *P < .05; **P < .01; ***P < .001.
GpC restricts Lu/BCAM binding to laminin-α5
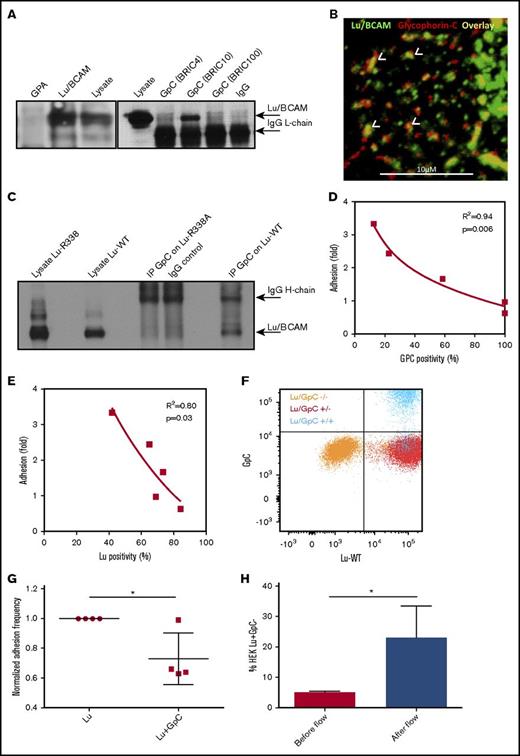
We wanted to test whether specific sialylated proteins within the erythrocyte membrane restrict Lu/BCAM binding to laminin-α5. The most abundant group of erythrocyte membrane proteins that are known for their high degree of glycosylation and thus high sialic acid content are glycophorins.27 We hypothesized that sialic acid on these glycoproteins interact with arginine residue 338 within Lu/BCAM and thereby restrict Lu/BCAM from interacting with laminin-α5. We performed immunoprecipitation of Glycophorin-A and GpC in erythrocyte lysates and found by western blot that Lu/BCAM coimmunoprecipitates with GpC (Figure 3A). Confocal microscopy demonstrated clusters of Lu/BCAM and GpC that partially colocalized (Figure 3B). To test if arginine residue 338 within Lu is responsible for interacting with GpC, we immunoprecipitated GpC from K562 cells that were cotransfected with either Lu-WT or Lu-R338A. Western blot analysis showed that Lu-R338A does not coimmunoprecipitate with GpC, as opposed to Lu-WT (Figure 3C). To demonstrate that Lu/BCAM is kept inactive by GpC-derived sialic acid, we assessed Lu/BCAM activation in erythrocytes from donors lacking GpC exon 3 (GpC-ex3), the so-called Gerbich blood group. We first assessed GpC-ex3 sialic acid content as GpC-ex3 glycosylation has previously been reported to differ from WT GpC.28 We used the monoclonal antibody BRIC4 directed against a sialic acid–containing epitope on GpC. We found by flow cytometry that this sialylated epitope in GpC-ex3 erythrocytes is reduced by up to 75% compared with control, indicating a loss of sialic acid due to the lack of expression of exon 3 (Figure 3D). The reduction GpC-ex3 sialylation was found to strongly correlate with increased binding of these erythrocytes to laminin-α5 (Figure 3D). We verified that the increase in binding was not due to increased expression of Lu/BCAM (Figure 3E). Finally, to show the inhibitory effect of GpC on Lu/BCAM-mediated adhesion to laminin-α5, we transduced HEK293T cells with Lu-WT, with and without GpC. The expression levels of Lu/BCAM in both transfectants were checked by flow cytometry and found to be identical (Figure 3F). Simultaneous introduction of GpC resulted in a significantly reduced binding to laminin-α5 compared with HEK293T cells expressing Lu-WT alone (Figure 3G). In line with this, 5.2% of HEK293T cells that were transduced with both Lu-WT and GpC failed to express GpC (Figure 3F). This Lu+GpC− fraction of HEK293T cells was enriched approximately fivefold on laminin-α5 substrate compared with the Lu+GpC+ cells (Figure 3H), further illustrating the inhibitory effect of GpC on laminin-α5 binding. Last, we aimed to identify the epitope on GpC that is interacting with Lu/BCAM, thereby restricting it from interacting with laminin-α5. In an attempt to interfere with the sialic acid–dependent interaction between Lu/BCAM and GpC, we treated erythrocytes with BRIC4, which recognizes a sialylated epitope on GpC, and assessed erythrocyte adhesion frequency. However, we did not detect an effect on adhesion frequency (supplemental Figure 1C).
GpC restricts Lu/BCAM activity. (A) BRIC4, 10, and 100 were used to immunoprecipitate (IP) GpC from an erythrocyte lysate. Only BRIC10 was able to co-IP Lu/BCAM, suggesting that Lu/BCAM may mask the sialylated GpC epitope recognized by BRIC4 and possibly BRIC100 as well. Anti-CD235 was used to IP glycophorin-A (GpA). Western blotting for Lu/BCAM shows that BRIC10 co-IPs Lu/BCAM (top arrow). The anti-goat horseradish peroxidase–linked antibody directed against primary Lu/BCAM antibody was found to cross-react with the mouse light chain of the BRICS used to IP GpC (lower arrow). (B) Confocal micrograph showing Lu/BCAM clusters (fluorescein isothiocyanate, green; original magnification ×40) partially colocalizing (yellow, indicated with white arrowheads) with GpC (BRIC10, PE, red). (C) Western blot of GpC immunoprecipitation from Lu-WT and Lu-R338A transfected cells. GpC was found to co-IP only with Lu-WT. (D) GpC-ex3 (Gerbich phenotype GPC) sialylation was quantified by flow cytometry using BRIC4 and is expressed as a percentage compared with control erythrocytes (x-axis). GpC sialylation was then plotted against adhesion frequency to laminin-α5 (n = 5), which was also normalized to control (y-axis). The squared correlation coefficient (R2) is indicated. (E) Gerbich phenotype erythrocyte adhesion negatively correlates with Lu/BCAM expression. (F) Flow cytometric comparison, using BRIC4 and goat anti-human Lu/BCAM (R&D Systems), of Lu−GpC− (orange), Lu+GpC− (red), and Lu+GpC+ (blue) transfected HEK293T cells. (G) Adhesion frequency of Lu+GpC− (normalized to 1) and Lu+GpC+ HEK293T cells to laminin-α5. (H) Of the HEK293T transfected with both Lu and GpC (blue population), 5.2% failed to express GpC but did express Lu (blue population in red gate, right lower quadrant), as was assessed by flow cytometry. Upon flowing the total HEK293T cell population over laminin-coated ibidi chambers, the 5.2% GpC negative fraction was significantly enriched for on laminin-α5–coated ibidi chambers as determined by fluorescence microscopy (n = 4). *P < .05.
GpC restricts Lu/BCAM activity. (A) BRIC4, 10, and 100 were used to immunoprecipitate (IP) GpC from an erythrocyte lysate. Only BRIC10 was able to co-IP Lu/BCAM, suggesting that Lu/BCAM may mask the sialylated GpC epitope recognized by BRIC4 and possibly BRIC100 as well. Anti-CD235 was used to IP glycophorin-A (GpA). Western blotting for Lu/BCAM shows that BRIC10 co-IPs Lu/BCAM (top arrow). The anti-goat horseradish peroxidase–linked antibody directed against primary Lu/BCAM antibody was found to cross-react with the mouse light chain of the BRICS used to IP GpC (lower arrow). (B) Confocal micrograph showing Lu/BCAM clusters (fluorescein isothiocyanate, green; original magnification ×40) partially colocalizing (yellow, indicated with white arrowheads) with GpC (BRIC10, PE, red). (C) Western blot of GpC immunoprecipitation from Lu-WT and Lu-R338A transfected cells. GpC was found to co-IP only with Lu-WT. (D) GpC-ex3 (Gerbich phenotype GPC) sialylation was quantified by flow cytometry using BRIC4 and is expressed as a percentage compared with control erythrocytes (x-axis). GpC sialylation was then plotted against adhesion frequency to laminin-α5 (n = 5), which was also normalized to control (y-axis). The squared correlation coefficient (R2) is indicated. (E) Gerbich phenotype erythrocyte adhesion negatively correlates with Lu/BCAM expression. (F) Flow cytometric comparison, using BRIC4 and goat anti-human Lu/BCAM (R&D Systems), of Lu−GpC− (orange), Lu+GpC− (red), and Lu+GpC+ (blue) transfected HEK293T cells. (G) Adhesion frequency of Lu+GpC− (normalized to 1) and Lu+GpC+ HEK293T cells to laminin-α5. (H) Of the HEK293T transfected with both Lu and GpC (blue population), 5.2% failed to express GpC but did express Lu (blue population in red gate, right lower quadrant), as was assessed by flow cytometry. Upon flowing the total HEK293T cell population over laminin-coated ibidi chambers, the 5.2% GpC negative fraction was significantly enriched for on laminin-α5–coated ibidi chambers as determined by fluorescence microscopy (n = 4). *P < .05.
Loss of GpC binding does not increase Lu/BCAM mobility in the erythrocyte membrane
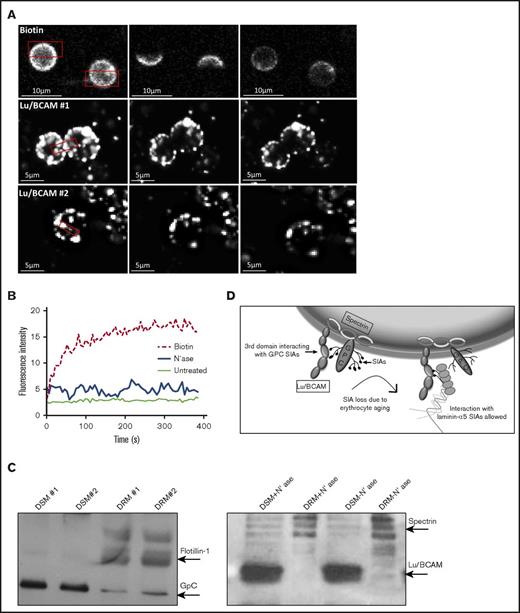
GpC, and Lu/BCAM to a certain extent, is anchored in the erythrocyte spectrin network.16,17,29,30 In sickle cell erythrocytes, release of Lu/BCAM from the spectrin network as a consequence of Lu/BCAM phosphorylation is hypothesized to increase Lu/BCAM clustering after ligand binding and therefore enhance erythrocyte adhesion to laminin-α5.16,30 We wanted to verify whether the mobility of Lu/BCAM within the erythrocyte membrane is impacted by neuraminidase treatment. To test this, we performed FRAP experiments with control and neuraminidase-treated erythrocytes and used biotinylated erythrocytes as control. We observed that Lu/BCAM is fixed within the erythrocyte membrane, which is unchanged after neuraminidase treatment (Figure 4A-B). The anchorage of Lu/BCAM within lipid rafts would explain such observation. Using detergent extraction, we aimed to pinpoint the localization of Lu/BCAM with respect to detergent-soluble and -insoluble membranes (ie, lipid rafts). We found that Lu/BCAM is almost exclusively localized to the DSM, which is unchanged upon neuraminidase treatment (Figure 4C). Taken together, this indicates that GpC does not function to restrict Lu/BCAM within erythrocyte lipid rafts and that the increased interaction of Lu/BCAM with laminin-α5 after neuraminidase treatment is probably not driven by changes in the linkage between Lu/BCAM and the spectrin network (Figure 4D).
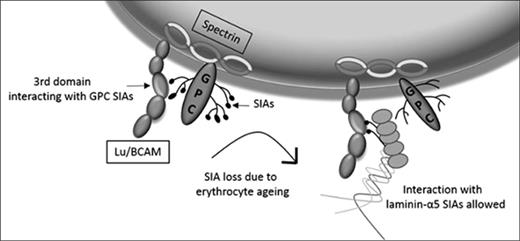
Lu/BCAM membrane localization. (A) FRAP of biotin-labeled erythrocytes (top row; original magnification ×40) and Lu/BCAM-labeled, neuraminidase-treated, erythrocytes (bottom row). Alexa Fluor 488 was bleached at 70% output power for 3 iterations for a duration of 1.29 seconds. (B) FRAP quantification of biotin-labeled erythrocytes (dotted line), untreated Lu/BCAM-labeled erythrocytes (thin line), and neuraminidase-treated Lu/BCAM-labeled erythrocytes (thick line). (C) Lu/BCAM western blot of DSM and DRM either treated with neuraminidase (+) or not treated (−). Membrane was separated using 1% Triton X-100. Flotillin was used as a positive control to show successful isolation of lipid rafts (DRM). (D) Depicted is how GpC-derived sialic acid residues interact with arginine 338 on the third domain of Lu/BCAM, inhibiting the interaction with laminin-α5. Upon loss of sialic acid residues, through either erythrocyte aging or removal of sialic acid by neuraminidase, this interaction is lost, leading exposure of the sialic acid binding domain of Lu/BCAM, facilitating an interaction with laminin-α5–derived sialic acid residues.
Lu/BCAM membrane localization. (A) FRAP of biotin-labeled erythrocytes (top row; original magnification ×40) and Lu/BCAM-labeled, neuraminidase-treated, erythrocytes (bottom row). Alexa Fluor 488 was bleached at 70% output power for 3 iterations for a duration of 1.29 seconds. (B) FRAP quantification of biotin-labeled erythrocytes (dotted line), untreated Lu/BCAM-labeled erythrocytes (thin line), and neuraminidase-treated Lu/BCAM-labeled erythrocytes (thick line). (C) Lu/BCAM western blot of DSM and DRM either treated with neuraminidase (+) or not treated (−). Membrane was separated using 1% Triton X-100. Flotillin was used as a positive control to show successful isolation of lipid rafts (DRM). (D) Depicted is how GpC-derived sialic acid residues interact with arginine 338 on the third domain of Lu/BCAM, inhibiting the interaction with laminin-α5. Upon loss of sialic acid residues, through either erythrocyte aging or removal of sialic acid by neuraminidase, this interaction is lost, leading exposure of the sialic acid binding domain of Lu/BCAM, facilitating an interaction with laminin-α5–derived sialic acid residues.
Lu/BCAM binding to laminin-α5 is induced by S pneumoniae
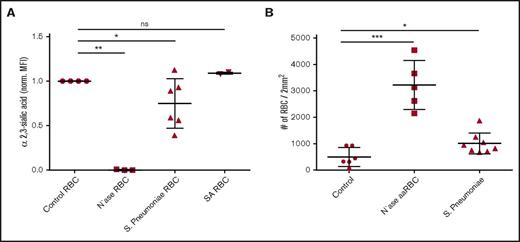
In SCD, recurring vaso-occlusive crises cause the spleen to be damaged, ultimately resulting hyposplenism or even functional asplenia.31 This renders patients suffering from SCD to be susceptible to infection by encapsulated bacteria, which is associated with mortality. Pathogens that are a major threat to SCD patients include S pneumoniae, which expresses a neuraminidase,31,32 that scavenges sialic acid from host cells in order to decorate their bacterial surface and evade the immune system.33,34 S pneumoniae has previously been shown to be able to desialylate platelets.35 This prompted us to assess whether this pathogen can also decrease erythrocyte sialic acid content and thereby induce Lu/BCAM activation. This is especially relevant in the context of SCD and vaso-occlusive crises, as in this disease laminin-α5 has been found to be exposed and leak into the circulation due to damaged vascular endothelium.8,9 We thus incubated erythrocytes with S pneumoniae and used Staphylococcus aureus as a negative control. As expected, S aureus did not affect erythrocyte sialic acid content because this bacterium does not express a neuraminidase (Figure 5A). In contrast, we observed varying degrees of erythrocyte α2.3-sialic acid loss upon incubation with S pneumoniae. Even partial α2.3-sialic loss was found to cause a twofold increase in erythrocyte adhesion to laminin-α5 (Figure 5B).
S pneumoniae activates Lu/BCAM through desialylation of the erythrocyte membrane. (A) Effect of S pneumoniae, S aureus, and purified neuraminidase from V cholerae on α2,3-linked sialic acid content of erythrocytes using M amurensis lectin and flow cytometry (n = 3-6). (B) Effect of erythrocyte incubation with S pneumoniae, S aureus, and purified neuraminidase from V cholerae on Lu/BCAM-mediated erythrocyte adhesion frequency to laminin-α5 (n = 5-7). *P < .05; **P < .01; ***P < .001. ns, not significant.
S pneumoniae activates Lu/BCAM through desialylation of the erythrocyte membrane. (A) Effect of S pneumoniae, S aureus, and purified neuraminidase from V cholerae on α2,3-linked sialic acid content of erythrocytes using M amurensis lectin and flow cytometry (n = 3-6). (B) Effect of erythrocyte incubation with S pneumoniae, S aureus, and purified neuraminidase from V cholerae on Lu/BCAM-mediated erythrocyte adhesion frequency to laminin-α5 (n = 5-7). *P < .05; **P < .01; ***P < .001. ns, not significant.
Discussion
Here we reveal a novel mechanism by which Lu/BCAM binding to laminin-α5 is regulated. The interaction of Lu/BCAM with laminin-α5 was found to be inhibited by interacting, in cis, with GpC-derived sialic acid residues on the erythrocyte. Upon loss of this interaction, as occurs during erythrocyte aging or after incubation with a neuraminidase, Lu/BCAM can interact, in trans, with sialic acid present on laminin-α5 (Figure 4D).
Several groups have previously reported regions within Lu/BCAM and laminin-α5 that are important to establish an interaction.36-40 An initial study by Zen et al, using murine erythroleukemia cells expressing various Lu/BCAM domains, determined that the membrane proximal domain, domain 5, contributes to adhesion of these cells to laminin-α5.38 Later, this finding was seemingly contradicted by 2 groups that used recombinant Lu/BCAM protein and BIACORE technology to address the same question.37,41 Also, negatively charged residues within domains 2 and 3 as well as the linker region between these domains were determined to be important in establishing an interaction with laminin-α5.39 Together these studies show that the 3-dimensional orientation of Lu/BCAM toward the substrate, the nature of the interaction, thus under static or flow conditions, as well as electrostatic interactions are important in establishing an interaction with laminin-α5. In line with these reports, we found the positively charged R338 residue within domain 3 of Lu/BCAM to be important for interacting, in a sialic acid–dependent fashion, with laminin-α5. Although substitution of this arginine residue in several models causes a strong decrease in adhesion strength, the interaction was not totally abolished (Figure 2C-D). We thus propose that on erythrocytes, which harbor relatively few Lu/BCAM molecules, the sialic acid binding property contributes significantly in interacting with laminin-α5. However, as Lu/BCAM expression levels increase, such as is seen in the Lu-transfected K562 and HEK293T cells used here, or in the Lu-coated protein-G microbead adhesion assays we employed, Lu/BCAM electrostatic and conformation-dependent interactions37,39,41 with laminin-α5 may become more prominent.
We show that it is the sialic acid binding capacity of Lu/BCAM that allows GpC to perturb an Lu/BCAM-dependent interaction of erythrocytes with laminin-α5. On the erythrocyte, ∼2 × 105 GpC copies per cell are present that carry the Gerbich blood group antigens.42 The Gerbich phenotype is associated with GpC lacking exon 3, which translates into the deletion of amino acid residues 36 to 63. Although only a single O-glycosylation site has so far been described to localize to this region, the GpC glycosylation on erythrocytes expressing the Gerbich phenotype is markedly altered compared with WT GpC.28 We found that a reduction in Gerbich GpC sialic acid content strongly correlates with an increase in adhesion to laminin-α5. Interestingly BRIC10 and not BRIC4, which recognizes a sialylated epitope, was able to coimmunoprecipitate Lu/BCAM, suggesting that Lu/BCAM may mask the sialylated GpC epitope recognized by BRIC4. In line with the data we present here, elliptocytic erythrocytes, which because of loss of protein 4.1R also lack GpC,43 have also been described to adhere more frequently to laminin-α5.44 However, to make matters more complex, Lu/BCAM linkage to the spectrin network has also been shown to modulate Lu/BCAM activity.16 Because GpC links the spectrin network to the membrane, it is unclear whether in elliptocytosis Lu/BCAM is activated because of loss of the interaction with spectrin, with GpC, or with both. Similarly, it would be of great interest to further dissect how phosphorylation of Lu/BCAM, that has been described to be involved in Lu/BCAM activation in SCD and PV,12,13,15 exactly affects the interaction between GpC, Lu/BCAM, and the spectrin network.
In SCD, Lu/BCAM is thought to contribute to vaso-occlusive crises because of the increase in interactions between sickle erythrocytes, ECM proteins, sickle reticulocytes, and endothelium.9,11,36,45,46 Patients suffering from SCD are more prone to infection by encapsulated bacteria such as S pneumoniae, Haemophilus influenzae, Neisseria meningitides, and Salmonella spp, which can cause impairment of splenic function and exacerbation of vaso-occlusive crisis.31 We found that S pneumoniae, which is a hazardous pathogen for patients suffering from sickle cell anemia, can cleave terminal sialic acid leading to Lu/BCAM binding to laminin-α5. Lu/BCAM is not the only receptor on erythrocytes that interacts with ECM proteins, which are activated upon sialic acid loss. A study by Kerfoot et al showed that erythrocytes can tether to the ECM protein hyaluronic acid through the erythrocyte adhesion molecule CD44.47 Although the function of Lu/BCAM on healthy erythrocytes is not clear, the fact that these adhesion molecules are activated on aged erythrocytes tempts us to speculate that Lu/BCAM, similar to CD44, might contribute to clearance of aged, senescent, erythrocytes.
In summary, our study reveals a novel mechanism that activates the adhesion function of Lu/BCAM on normal and sickle erythrocytes. Unlike the previously reported mechanisms (ie, phosphorylation and dissociation from the spectrin skeleton), this new mechanism targets the extracellular side of the protein and can thus affect both Lu/BCAM isoforms. Investigating the role of this mechanism in activating other erythroid adhesion proteins both during erythrocyte aging and under pathological conditions would be of great interest, especially in the context of erythrocyte sequestration by splenic red pulp macrophages in the human spleen.
The full-text version of this article contains a data supplement.
Acknowledgment
This work was supported by a grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR1412) (R.v.B.).
Authorship
Contribution: T.R.L.K. performed experiments, wrote the article, and designed the figures; D.Z.d.B., P.J.J.H.V., P.J.A., B.M.B., M.V., J.B., and E.C. performed experiments; P.C.L., T.K.v.d.B., W.E.N., and R.v.Z. supervised; and R.v.B. designed experimental setup and supervised and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. van Bruggen, Sanquin Research and Laboratory Services, Department of Blood Cell Research, Plesmanlaan 125, 1066 CX, Amsterdam, The Netherlands; e-mail: r.vanbruggen@sanquin.nl.

![Figure 1. Loss of sialic acids on the erythrocyte membrane activates Lu/BCAM. (A) Representative micrograph of adhesion of healthy erythrocytes (top panel; original magnification ×10) and sickle erythrocytes (lower panel) to a laminin-α5–coated ibidi chamber at 0.2 dyn/cm. (B) Quantification of adhesion frequency of old and young erythrocytes to laminin-α5 at 0.2 dyn/cm2 (n = 3). Percoll dilutions were stacked in a 15-mL tube; erythrocytes isolated from the fraction denser than 1.096 g/mL Percoll were defined as dense and old erythrocytes (roughly 3% of total red blood cell [RBC]), whereas erythrocytes lighter than 1.060 g/mL Percoll are here defined as light and young erythrocytes (roughly 0.75% of total RBC). (C) Biotinylated Maackia amurensis lectin type II (MA) was used to quantify α2,3-linked sialic acid (SIA) by flow cytometry (Data shown as mean fluorescence intensity [MFI] and normalized [Norm.] to control erythrocytes [aaRBC].). (D) Membrane sialic acid was removed from control erythrocytes by V cholerae neuraminidase (N'ase RBC), and adhesion frequency was assessed at 0.2 dyn/cm2 (n = 5). Specificity was addressed using an Lu/BCAM blocking polyclonal antibody. (E) Sickle erythrocyte (ssRBC) adhesion frequency to laminin-α5 at 0.2 dyn/cm2, either treated or not treated with neuraminidase for 30 minutes at 37°C (n = 5). *P < .05; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/2/1/10.1182_bloodadvances.2017013094/3/m_advances013094f1.jpeg?Expires=1765887246&Signature=lx7OoxUgi~VIt8hwd3jEc9H4xCDC0NUrNRTMfRP0n~FauDHfnjlTtrfr7FAjWwRl9SqQ2ON7LR6cJxJP~dt8ex8FoG8ompWYFuN3jI7DRVUJbF7s881pCEwtNBLS2CC6RumK4Ci9TzFGtwdUsfJmWHCmuGrtYRrMZXT-UNncUoXMqiSO2L~BplmDS0C082V1UPTsEQ5AAEys3xxm1A2d-IdSdtHmuqpI4mu719qFxOLriOhujuqOVbp0UdzcQwWpnCWE2eaJt0iJWuRry~eINu4Wkmch~gyWaRIL9VqCBFo6s9qn7IGgpJz8NJipMVtnzAWmsDVPAzMzhWsqvspHwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)