Introduction
Before 2015, only 2 doctors in Malawi were able to care for people with hemophilia. However, there was no laboratory facility able to provide complete diagnosis for the patients. As a result, only 7 patients were identified with symptoms across the country. With a projected population of 17 million inhabitants, it was expected that at least 1700 patients with hemophilia would be present in Malawi (based on an estimated frequency of 1 in 10 000 births).1
Illustrative cases on lack of hemophilia care
The cases of 2 boys who presented to Queen Elizabeth Central Hospital (QECH) and Kamuzu Central Hospital (KCH) illustrate the extent of the problems patients with hemophilia used to face in Malawi. KCH and QECH are the 2 teaching hospitals in Malawi; KCH serves the Central Region of Malawi and QECH serves a large part of the Southern Region of Malawi.
First case
In 2008, a 10-year-old boy was referred to QECH in Blantyre from Zomba Central Hospital with a history of recurrent hemoptysis for a period of ∼1 year. He had undergone above-the-knee amputation of his left leg in the past for recurrent joint swelling and pain. The working diagnosis for his left joint problem had been tuberculosis (TB) of the left knee with a differential diagnosis of cancer. For his current presentation, the differentials on referral included lung metastases, TB, and bronchiectasis. Chest radiograph and chest computed tomography scan showed normal lungs. During a routine ward round at QECH, a hematologist (Y.B.M.) suspected hemophilia on the basis of the shape of the boy’s right knee (Figure 1). He obtained the boy’s family history from his mother, who said that her son had had 6 siblings: 3 girls who were all alive and well, and 3 brothers, the eldest of whom (first born in the family) died at 3 months of age, and she did not know the cause of death. A second boy died at the age of 7 from excessive bleeding, and the third brother was alive and well. The hematologist performed PT and aPTT tests for the patient using a water bath, and the values were 17 seconds (normal, 10-12 seconds) and 81 seconds (normal, 30-40 seconds), respectively. No mixing studies were done, so this could be moderate to mild prothrombin, fibrinogen, and factor V, X, or combined factor deficiencies.
Undiagnosed patient with hemophilia, first case. The patient had his left leg amputated when hemarthropathy was misdiagnosed as joint TB or malignancy. Figure 2. Undiagnosed patient with hemophilia, second case.
Undiagnosed patient with hemophilia, first case. The patient had his left leg amputated when hemarthropathy was misdiagnosed as joint TB or malignancy. Figure 2. Undiagnosed patient with hemophilia, second case.
Second case
A 13-year-old boy presented to KCH in April 2013 with bleeding from the right knee. The history of the presenting complaint for the current case was that the boy developed knee pain and swelling while playing football at school. He was brought to the local health facility where a clinician suspected septic arthritis and placed a needle into the right knee joint to aspirate reactive fluid or pus. Instead, he aspirated frank red blood, and a few minutes later, the patient developed continuous bleeding from the puncture site on the right knee. Unable to control the bleeding at the local health facility, the patient was referred to KCH. At KCH, the patient continued to experience severe pain and bleeding from the right knee. He could not place his right leg on the hospital bed for fear of soiling the beddings with blood, so his right leg was kept dangling on the floor (Figure 2A). His left knee showed features of hemophilic arthropathy (Figure 2B). At KCH, before a hematologist (Y.B.M.) saw the patient, the management plan included whole blood transfusion and orthopedic consultation. No coagulation tests, including screening tests, were available at KCH.
An international expert demonstrating a procedure on the new coagulation equipment under the NNHF project.
An international expert demonstrating a procedure on the new coagulation equipment under the NNHF project.
The problems of rare disease in a low-income country
In late 2014, the Society of Hemophilia and Allied Bleeding Disorders (SHAD) was formed in Malawi, bringing together a few patients with only clinical diagnoses, their relatives, and health care professionals. SHAD faced an uphill task to achieve its vision of “diagnosis and relief of all people suffering from inherited bleeding disorders.”
As a low-income country, ranked 151 out of 189 countries by gross domestic product by the World Bank in 2016,2 Malawi has very limited resources with many competing health care needs. Hemophilia as a rare disease faces common problems, such as: (1) lack of access to correct diagnosis, (2) delay in diagnosis, (3) lack of quality information on the disease, (4) lack of scientific knowledge of the disease, (5) heavy social consequences for patients, (6) lack of appropriate quality health care, and (7) inequities and difficulties in access to treatment and care.3 ,4 In Malawi, as SHAD painfully found out, these problems are seemingly insurmountable.
The value of collaboration
SHAD was introduced to World Haemophilia Federation (WFH) then Haemophilia Scotland and Novo Nordisk Haemophilia Foundation (NNHF). In a collaborative effort, 2 projects were implemented in 2016. A hemophilia awareness campaign was implemented by Haemophilia Scotland, and an NNHF-funded project was implemented by SHAD. Under the NNHF project, a coagulation unit (Figure 3) together with a hemophilia clinic unit (Figure 4) were established at KCH, representing the first-ever such center in Malawi. In addition, an awareness campaign was executed strategically to identify undiagnosed hemophilia patients.
An international clinical expert under the NNHF project discussing a patient with a nurse in the new hemophilia clinic.
An international clinical expert under the NNHF project discussing a patient with a nurse in the new hemophilia clinic.
Outcome
So far, 48 patients were newly diagnosed with hemophilia and 12 with other bleeding disorders. The NNHF project included a patient camp (Figure 5) where the diagnosed patients received training about their condition and how best to deal with it.
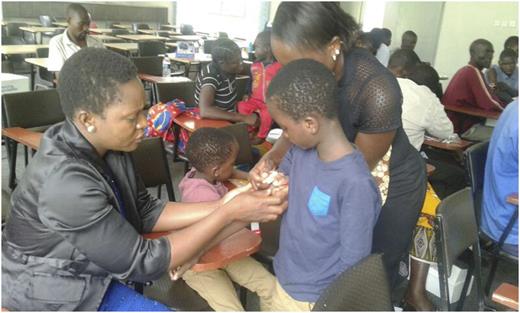
A parent placing a butterfly needle on her son to infuse factor VIII.
With confirmed cases of hemophilia A and hemophilia B, the WFH Humanitarian Aid Program commenced donations of factor VIII and factor IX concentrates. Among those diagnosed with severe hemophilia A was the first illustrative case above, the boy from Zomba. This young man would not have had an amputation of his leg if he had been able to access what he has now been able to access in Malawi. Subsequent to the availability of a proper diagnosis and proper treatment, NNHF supported a patient camp (Figure 6) during World Haemophilia Day 2017 to train patients on home treatment and self-infusion therapy. To date, all the diagnosed hemophilia patients in Malawi are receiving the standard of care. Our future plans include efforts to identify more hemophilia patients in the country and expand the diagnostic and clinical services to at least all the 3 major regions of Malawi.
Acknowledgment
This work was supported by the Novo Nordisk Foundation.
Authorship
Conflict of interest disclosure: S.S.: Novo Nordisk Haemophilia Foundation (employment). Other authors: No competing financial interests declared.
Correspondence: Christine Chilinga, Medicine Department, Kamuzu Central Hospital, P.O. Box 149, Lilongwe, Malawi. E-mail: christinechilinga@gmail.com.