Background
Immunophenotypic and genetic analyses have become routine in the diagnosis and monitoring of hematological malignancies in the developed world. However, these advances have not been translated to developing countries. The technology required is expensive and has previously not been available. Peshawar, the capital of Khyber Pakhtunkhwa province of Pakistan, caters to the health needs of ∼30 million people in the province. Despite huge numbers of patients, most hospitals lack an advanced laboratory for genetic and immunophenotypic diagnosis and monitoring of blood diseases, and diagnoses are made on morphology alone. We sought to establish a diagnostic facility using flow cytometry and genetic analysis that is tailored to the diseases that we encounter, but that is also affordable. Khyber Medical University and Rehman Medical Institute (RMI) in Peshawar have been working with the University of Glasgow and affiliated hospitals to establish such a laboratory in Peshawar.
Redundant polymerase chain reaction and general laboratory equipment from Queen Elizabeth University Hospital donated to Peshawar.
Redundant polymerase chain reaction and general laboratory equipment from Queen Elizabeth University Hospital donated to Peshawar.
Training on polymerase chain reaction was provided at the Institute of Infection, Immunity and Inflammation, University of Glasgow.
Training on polymerase chain reaction was provided at the Institute of Infection, Immunity and Inflammation, University of Glasgow.
Validated protocols shared by the Department of Molecular Pathology, Queen Elizabeth University Hospital, Glasgow.
Validated protocols shared by the Department of Molecular Pathology, Queen Elizabeth University Hospital, Glasgow.
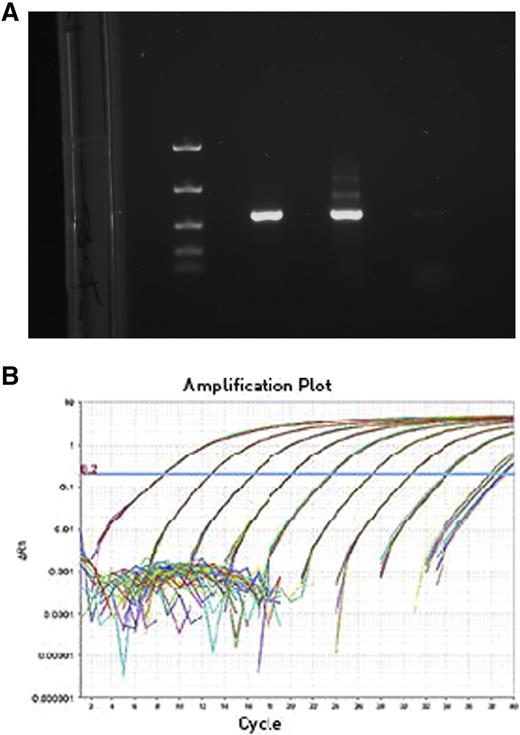
Low-cost polymerase chain reaction assays for JAK2-V617F and BCR-ABL mutations were optimized, validated, and made available at RMI, Peshawar. Two technicians were further trained on polymerase chain reaction procedures and competence-based training manuals were designed.
Low-cost polymerase chain reaction assays for JAK2-V617F and BCR-ABL mutations were optimized, validated, and made available at RMI, Peshawar. Two technicians were further trained on polymerase chain reaction procedures and competence-based training manuals were designed.
Based on the experience of flow cytometry experts in Glasgow, procurement of a low-cost flow cytometer is under way.
Based on the experience of flow cytometry experts in Glasgow, procurement of a low-cost flow cytometer is under way.
Y.Y. undertook intensive training in morphological assessment of blood and bone marrow, which is essential in identifying target cell populations and the interpretation of flow cytometry data.
Y.Y. undertook intensive training in morphological assessment of blood and bone marrow, which is essential in identifying target cell populations and the interpretation of flow cytometry data.
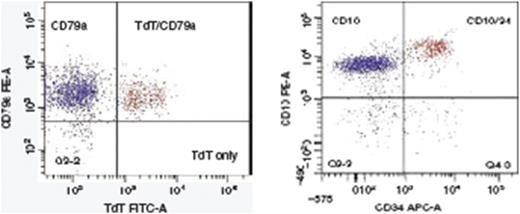
We have designed specific antibody panels for use in Peshawar, relevant to the diagnosis of leukemias.
We have designed specific antibody panels for use in Peshawar, relevant to the diagnosis of leukemias.
The acquired experience and expertise are to be implemented at RMI, Peshawar. Competency-based training manuals from Glasgow have been adapted for implementation in technician training.
The acquired experience and expertise are to be implemented at RMI, Peshawar. Competency-based training manuals from Glasgow have been adapted for implementation in technician training.
Molecular diagnostics for therapeutically targetable genetic mutations in leukemia
The World Health Organization classification uses genetic mutations for the diagnosis and classification of a number of acute and chronic malignancies. The identification of these mutations is critical for patient management. The following were therefore prioritized:
JAK2 mutation
BCR-ABL mutation
PML-RARα mutation
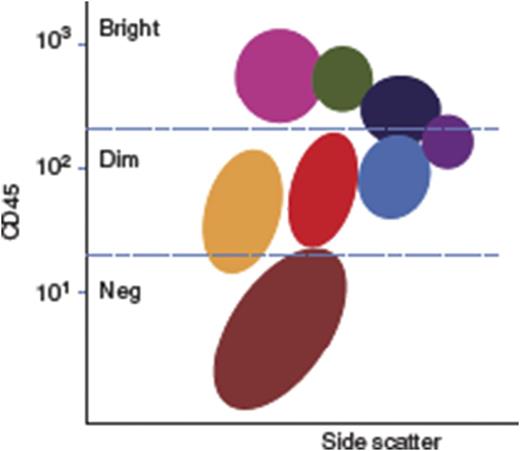
Flow cytometry for immunophenotypic diagnosis and monitoring of leukemia
The establishment of a flow cytometry laboratory requires careful selection of a flow cytometer, technical and clinical training on flow-based diagnosis, and the design of affordable antibody panels of high diagnostic yield. Under the American Society of Hematology Visitor Training Program, Y.Y. undertook a 12-week attachment to the diagnostic flow cytometry laboratory at Gartnavel Hospital, Glasgow. Y.Y. was trained in flow cytometry technology using a platform relevant to that planned for use at RMI.
Conclusions
We have successfully initiated a molecular diagnostic hematology laboratory in Peshawar. Ongoing training of technical staff will ensure sustainability of this service. We are now in a position to introduce a dedicated flow cytometry service in Peshawar essential to our needs. The low test costs will ensure that such technology is accessible and routinely available to a larger segment of our population. This demonstrates that mentoring and guidance from laboratories in the developed world can be highly effective in helping establish an affordable and accessible diagnostic hematology service in low-/middle-income countries.
Conflict-of-interest disclosure: M.C.: Incyte (research funding, honoraria, speakers’ bureau, membership on board of directors or advisory committees); Bristol-Myers Squibb (research funding, honoraria, speakers’ bureau, membership on board of directors or advisory committees). C.H.: Jazz Pharmaceuticals (consultancy, other). The remaining authors declare no competing financial interests.
Correspondence: Yasar Mehmood Yousafzai, Institute of Basic Medical Sciences, Khyber Medical University, Peshawar, Pakistan; e-mail: yasaryousafzai@live.com.