Background
The process of managing chronic myeloid leukemia (CML) at the National Hospital Abuja was frustrating to both the clinicians and patients because the hospital did not have facilities for molecular diagnosis, which is a prerequisite to access imatinib mesylate through the Glivec International Patient Assistance Program (GIPAP; free access to imatinib) program. The testing was outsourced, and in addition to being very expensive, the results usually take months to get back to the hospital. In 2014, the International Chronic Myeloid Leukemia Foundation offered clinical CML preceptorship training to C.U. at the Hammersmith Hospital, London, United Kingdom, (Figure 1) under the mentorship of Jane Apperley. In 2016, the International Chronic Myeloid Leukemia Foundation extended the collaboration by providing a grant of $10 000 US dollars for the purchase of a GeneXpert polymerase chain reaction (PCR) machine for the diagnosis and monitoring of CML patients at the National Hospital Abuja. The Max Foundation provided a supplementary grant and donated 50 test kits to set up on-site PCR (GeneXpert) testing for molecular diagnosis and monitoring of CML patients (Figure 2).
The program has trained 4 hematology residents and fellows through the clinical preceptorship program off-shore and 17 staff on-site through the Cepheid education and training unit.
Introduction
CML is a triphasic clonal proliferation characterized by the presence of the Philadelphia chromosome (Ph+) in >95% of cases.1,2 The identified underlying nonrandom balanced chromosomal translocation between chromosomes 9q34 and 22q213 gives rise to the chimeric BCR-ABL1 gene on chromosome 22,4 and this has been found to be responsible for the oncogenesis of CML. This gives rise to the uncontrolled proliferation primarily of the granulocytic and megakaryocytic lineages, resulting in the accumulation of immature granulocytic precursors in the bone marrow, blood, and other organs.5,6
The discovery of the molecular basis of CML and other subsequent studies and discoveries have impacted significantly the understanding of the biology, diagnosis, treatment, and monitoring of the disease. The discovery of imatinib mesylate, a tyrosine kinase inhibitor (TKI), and later other second- and third-generation TKIs has led to a paradigm shift in the management of the disease.
The World Health Organization has defined 3 distinct phases of CML: chronic, accelerated, and blastic.6 The presence of the BCR-ABL1 mutation is specific for the diagnosis, and this can be from peripheral blood or bone marrow aspirates using reverse transcriptase PCR. The Ph1 chromosome can also be demonstrated by G-banded metaphase cytogenetic or fluorescent in situ hybridization. Monitoring treatment outcome is essential in ascertaining remission or disease progression.
Objectives
To provide access to diagnosis for patients suspected of having CML and monitor treatment outcomes in patients on TKIs.
To provide training to clinical and laboratory staff on molecular diagnosis and monitoring of CML.
Methods
All patients with clinical suspicion of CML are clerked; signs and symptoms with biodata are collected after clinical examination.
A complete blood count is done with examination of peripheral blood smears.
Patients with a provisional diagnosis of CML are counseled and sent to the molecular laboratory for PCR (BCR-ABL) analysis of the peripheral blood.
PCR (GeneXpert) is run with a fresh blood sample after payment of the prescribed fees, and the results are sent back to the clinic within 48 hours.
Patients with positive results are referred to the GIPAP treatment center to be registered and obtain imatinib mesylate for free. They are subsequently monitored in the clinic initially on a monthly basis and later every 3 months.
Patients identified as having failed imatinib treatment or having a suboptimal response are counseled for TKD mutation analysis at the Hammersmith molecular pathology laboratory if they can afford the test.
The on-site training was conducted online for 5 consecutive days by the Cepheid education and training unit, and certificates were issued.
Results
Twenty-one clinical and laboratory staff members trained through a 5-day online training program.
Twenty-eight PCR tests done so far.
Fifteen new diagnoses.
Seven negative tests.
Six follow-up monitoring tests.
Four cases of hypersensitivity to imatinib mesylate (severe neutropenia and thrombocytopenia).
Five TKD mutation analyses done; 4 positive results (2 E255K, 1 F359V, and 1 F359I).
Turnaround time reduced from an average of 9 weeks to 3 weeks.
Discussion
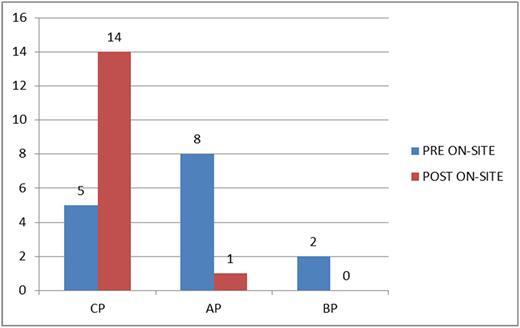
The onset of on-site PCR testing at the National Hospital Abuja has led to a significant reduction of the stress patients go through to access a diagnosis of CML, although patients still need to travel to the GIPAP center in Ife (∼600 km) for the TKIs. We still have some patients presenting late to the hospital, but the time between presentation and diagnosis has been reduced significantly (Table 1), thereby reducing disease progression before commencement of TKI therapy (Figure 3). It is also correct to infer that the cost to patients is also reduced because less time is spent outside patients' localities at the GIPAP center.
Turnaround time for diagnosis before and after on-site testing
| Turnaround time, wk . | Before on-site testing (N = 15) . | After on-site testing (N = 15) . |
|---|---|---|
| Mean | 9.20 | 2.67 |
| Standard deviation | ±6.7 | ±3.8 |
| Range | 1-22 | 1-16 |
| Turnaround time, wk . | Before on-site testing (N = 15) . | After on-site testing (N = 15) . |
|---|---|---|
| Mean | 9.20 | 2.67 |
| Standard deviation | ±6.7 | ±3.8 |
| Range | 1-22 | 1-16 |
P = .006.
Comparison of phase at commencement of TKI therapy before and after on-site testing.
Comparison of phase at commencement of TKI therapy before and after on-site testing.
Take-home messages
The provision of high-technology molecular diagnosis and monitoring of CML patients is feasible in low-income countries with adequate staff training.
On-site PCR testing for BCR-ABL in the management of CML significantly reduces the turnaround time for diagnostic workup and reduces disease progression to advanced stages.
On-site PCR testing improves treatment outcome.
Effective collaboration between high- and low-/middle-income countries will invariably improve the health indices of low-/middle-income countries.
Acknowledgment: The authors thank the International CML Foundation, the Max Foundation, management and staff of the Department of Haematology, National Hospital Abuja, and management of the Federal Medical Centre, Keffi.
Conflict-of-interest disclosure: J.A.: Bristol-Myers Squibb (research funding, consultancy, and honoraria); Ariad (research funding, honoraria, consultancy, and membership on board of directors or advisory committees); Incyte (honoraria); and Novartis (speakers’ bureau, honoraria, and consultancy). The remaining authors declare no competing financial interests.
Correspondence: Chi-kadibia Ukoma, Department of Clinical Haematology, Federal Medical Centre, Keffi, Nasarawa, Nigeria; e-mail: komasaint@yahoo.com, uckadibia@nationalhospitalabuja.net.