Key Points
Single-agent vemurafenib leads to a rapid and sustained clinical response in severe multisystem LCH but does not eradicate the disease.
Longitudinal assessment of BRAF V600E during treatment shows that clinical remission can occur despite significant amounts of mutated BRAF.
Introduction
Langerhans cell histiocytosis (LCH) is a systemic histiocytic disorder.1 The clinical manifestations range from a benign single-system disease to severe multisystem disease (MS-LCH). MS-LCH that fails to respond to initial standard therapy has a poor prognosis,2,3 and optimal salvage treatment of this subgroup still needs to be established. A combination of high-dose cytarabine with cladribine4 or reduced intensity conditioning hematopoietic stem cell transplantation (HSCT)5 are the 2 most effective treatment options to date. However, both are associated with toxicity and require extensive supportive care. For some of these patients, targeted treatment with a RAF inhibitor might be an option because activation of the MAPK pathway is found in all LCH cells6 and ∼50% to 60% of LCH patients harbor the activating mutation of the B-RAF kinase (BRAF V600E).6-8 So far, successful treatment of 4 adult patients with Erdheim Chester disease with concomitant LCH9,10 and of an infant with refractory LCH11 with vemurafenib has been published, and a larger case series is in preparation (Donadieu et al12 ). Furthermore, a multicenter pediatric phase 1/2a trial of dabrafenib (#NCT01677741), which included LCH patients, has recently been completed. In view of these promising results, international trials with RAF inhibitors in LCH are needed and currently being planned.12 However, important aspects in the design of future trials are not clear yet. For instance, dosing regimen, optimal treatment duration, and whether monotherapy suffices is a matter of debate. In this report, we describe the effect of vemurafenib treatment in a child with life-threatening refractory LCH. We provide evidence that treatment with the inhibitor alone is possibly not sufficient to cure the disease. Assessment of the percentage of the BRAF V600E allele in peripheral blood was used to monitor disease activity and suggests that clinical remission is compatible with considerable amounts of residual mutated BRAF.
Case description
In this report we describe a patient with refractory multisystem LCH who achieved clinical remission upon treatment with the RAF inhibitor vemurafenib.
Methods
Histopathology
Immunohistochemistry for BRAF V600E using a mutation-specific antibody (clone VE1) and CD1a was performed as described previously.13,14
Molecular analysis
DNA was isolated from whole blood using the QIAamp DNA blood mini kit (Qiagen, Germany). Allele-specific quantitative polymerase chain reaction was performed based on a method described in Thierry et al15 with modifications (see supplemental Data).
This study was conducted in accordance with the Declaration of Helsinki after approval by the local institutional review board and written and informed consent of the parents.
Results and discussion
A 15-month-old child presented with MS-LCH with involvement of skin, gingiva, skeleton, and bone marrow. Therapy according to LCH-III protocol was initiated (supplemental Figure 1).16 After a 6-week initial therapy course, remarkable improvement was documented and maintenance therapy with mercaptopurine and vinblastine/prednisolone was started. After 2 months of therapy, LCH relapsed. Second-line treatment with 2-chlorodeoxyadenosine cycles according to LCH-S-98 protocol17 was initiated. Just before the fifth cycle, the clinical condition of the patient deteriorated, and the patient developed fever, hepatosplenomegaly, and progression of the skin lesions. Laboratory workup showed marked pancytopenia and hypoalbuminemia. HSCT was considered,5 but because of the critical clinical condition, this seemed too hazardous. Because we detected the BRAF V600E mutation in the peripheral blood (Figure 1A) and because of the rapid deterioration of the patient, we started therapy with the RAF inhibitor vemurafenib at a dose of 2 × 10 mg/kg per day, based on the report by Héritier et al.11 The BRAF mutation was later confirmed in the original biopsy (Figure 1B). Three days after treatment was initiated, the patient improved clinically, fever decreased, and laboratory values started to stabilize (Figure 1C-D). Dosage was increased to 2 × 15 mg/kg per day on day 6. Vemurafenib was tolerated well by the patient. He had no overt signs of toxicity during treatment with the exception of a maculopapular exanthema, which the boy developed on day 28 and which improved within days after dose reduction to 2 × 10 mg/kg per day. After 3 months, the dose was decreased to 10 mg/kg per day because there were no signs of active disease. We stopped the inhibitor after a total of 8 months. At that time, the patient was well without any clinically apparent signs of disease. However, 1 week later, the boy developed high fever and laboratory values showed decreasing platelets, hemoglobin, and albumin, presumably reflecting rapid reactivation of LCH. Consequently, vemurafenib was restarted, which led to rapid resolution of the fever and normalization of his laboratory values (Figure 1C-D), similar to a previous observation by Héritier et al.11 Because there is only limited experience on long-term effects of RAF inhibition,18,19 and because previous chemotherapy was inefficient, we decided to perform an allogeneic reduced intensity conditioning HSCT.5 At 455 days after starting treatment with vemurafenib, the boy was transplanted from a matched sibling donor (supplemental Figure 1). HSCT was well tolerated, and 1 month after transplant, the patient was clinically well and showed no signs of disease.
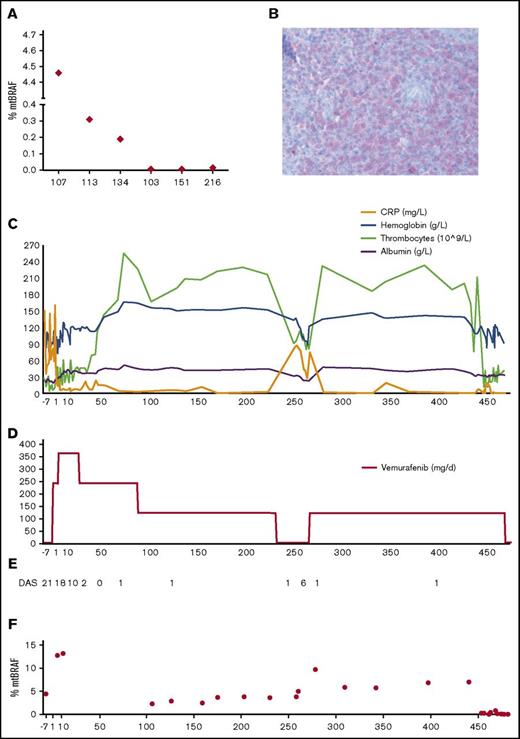
Effect of vemurafenib on disease and BRAF V600E levels. (A) Percentage of the BRAF V600E allele in the peripheral blood of patients at time of relapse (patient 107) or diagnosis (5 other patients). Patient 107 is the patient described in this case report; patients 113 and 134 had MS-LCH, patients 103 and 216 had multifocal bone LCH, and peripheral blood of patient 151 was used as a negative control (no BRAF V600E mutation in lesional cells). (B) Immunohistochemistry of a biopsy (gingiva) of patient 107 with an antibody against V600E. Original magnification ×40. (C) Monitoring of laboratory parameters under vemurafenib. (D) Vemurafenib dosage (milligrams per day) administered to the patient. (E) Disease activity score (DAS) during treatment with vemurafenib until HSCT (see also supplemental Table 1). (F) Percentage of circulating BRAF V600E alleles in the peripheral blood (whole blood) during therapy.
Effect of vemurafenib on disease and BRAF V600E levels. (A) Percentage of the BRAF V600E allele in the peripheral blood of patients at time of relapse (patient 107) or diagnosis (5 other patients). Patient 107 is the patient described in this case report; patients 113 and 134 had MS-LCH, patients 103 and 216 had multifocal bone LCH, and peripheral blood of patient 151 was used as a negative control (no BRAF V600E mutation in lesional cells). (B) Immunohistochemistry of a biopsy (gingiva) of patient 107 with an antibody against V600E. Original magnification ×40. (C) Monitoring of laboratory parameters under vemurafenib. (D) Vemurafenib dosage (milligrams per day) administered to the patient. (E) Disease activity score (DAS) during treatment with vemurafenib until HSCT (see also supplemental Table 1). (F) Percentage of circulating BRAF V600E alleles in the peripheral blood (whole blood) during therapy.
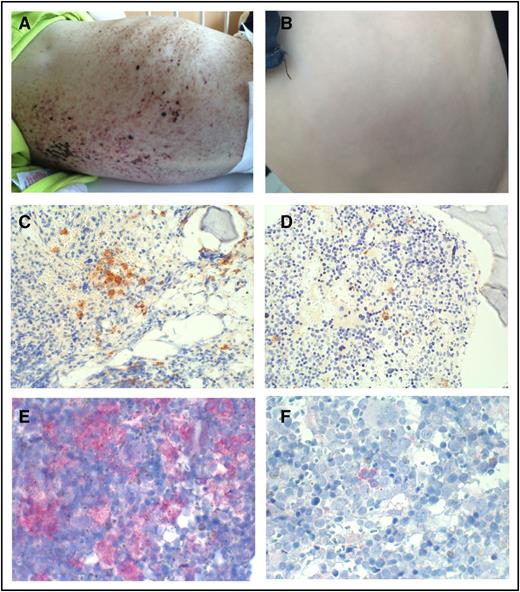
Disease activity score, a quantitative scoring system for assessment of LCH disease activity,20 was used to systematically evaluate disease status in the patient. Figure 1E and supplemental Table 1 show the rapid and sustained clinical efficiency of vemurafenib in this patient. BRAF V600E–positive cells and cell-free DNA can be detected in the peripheral blood of patients with LCH who have this mutation.21-24 To assess the percentage of the BRAF V600E allele in the peripheral blood, we have developed an allele-specific quantitative polymerase chain reaction based on a method described by Thierry et al.15 This enabled us to monitor total BRAF allele levels at a sensitivity of 0.01% (supplemental Figure 2). In our patient, although the percentage of mutated BRAF decreased markedly during therapy, it never became negative (Figure 1E). Therefore, even though BRAF V600E inhibition brought the patient into clinical remission (Figures 1C and 2B), it did not eradicate all the cells harboring the mutation. Consequently, immediately after cessation of the inhibitor, levels of mutated BRAF in the peripheral blood rose again, and the clinical condition of the patient worsened. In line with this finding, immunohistochemistry showed high numbers of BRAF mutated cells in the bone marrow before treatment with vemurafenib, which, although declining considerably during treatment, were nevertheless still detectable (Figure 2C-F). This implies that clinical remission can occur despite significant amounts of mutated BRAF.
Effect of vemurafenib on skin lesions and bone marrow. Skin lesions at start of therapy with vemurafenib (A) and after 10 months of treatment with vemurafenib (B). Bone marrow biopsy stained with an antibody against CD1a before (C) and after 9 months (D) of treatment with vemurafenib. Expression of the mutated BRAF protein in the bone marrow before (E) and after 9 months (F) of treatment with vemurafenib. Original magnifications ×20 (C-D) and ×40 (E-F).
Effect of vemurafenib on skin lesions and bone marrow. Skin lesions at start of therapy with vemurafenib (A) and after 10 months of treatment with vemurafenib (B). Bone marrow biopsy stained with an antibody against CD1a before (C) and after 9 months (D) of treatment with vemurafenib. Expression of the mutated BRAF protein in the bone marrow before (E) and after 9 months (F) of treatment with vemurafenib. Original magnifications ×20 (C-D) and ×40 (E-F).
Taken together, we report the rapid response to vemurafenib in a child in critical condition with life-threatening MS-LCH, in line with the previous report by Héritier et al.11 Even though this study focuses on a single patient,25 this case elucidates important aspects that should be taken into account in the design of a prospective clinical trial for LCH involving RAF inhibitors: We advocate to test the usefulness of systematic monitoring of the BRAF V600E mutation in the peripheral blood using a sensitive method as described here as a complement to conventional clinical staging, because a persistence of the mutated allele could indicate a higher probability of relapse after the cessation of a therapy. If proven, these measurements could be a gauge for treatment efficacy and help in treatment decisions. Furthermore, if a patient fails to get into molecular remission with vemurafenib, early therapy intensification such as combination with an extracellular signal-regulated kinase (ERK) inhibitor or chemotherapy should be considered. Innovative studies addressing these approaches are necessary. The inhibitor as single agent, although being an excellent bridge to a more intensive salvage therapy, might not suffice to cure LCH, and unnecessary treatment with RAF inhibitors should be avoided in view of the unknown long-term effects of these drugs in children.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients and their families for participating in this project to make this research possible, and Jozef Ban and Andrea Cechvalova for support in this project.
C.H. received a clinical investigator–driven grant from the St. Anna Kinderkrebsforschung. Additional financial support was provided by the Deutsche Histiozytosehilfe e.V.
Authorship
Contribution: A.K., J.H., I.B., and L.G. were involved in the clinical part of the project, were responsible for patient care, and provided clinical data; C.H. devised the project; M.M. and W.H. provided senior clinical and conceptual advice; R.S., G.J., and C.H. designed experiments and interpreted data; R.S. and G.J. performed the sample processing and BRAF analyses; C.K., I.S.-K., and L.P. performed histopathology analyses; and R.S., T.S., and A.K. provided figures; and A.K. and C.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caroline Hutter, St. Anna Kinderspital, Kinderspitalgasse 6, 1090 Vienna, Austria; e-mail: caroline.hutter@stanna.at.