Key Points
Th17 cells differentiate early after allo-SCT in both mouse and man and display a high degree of cytokine plasticity.
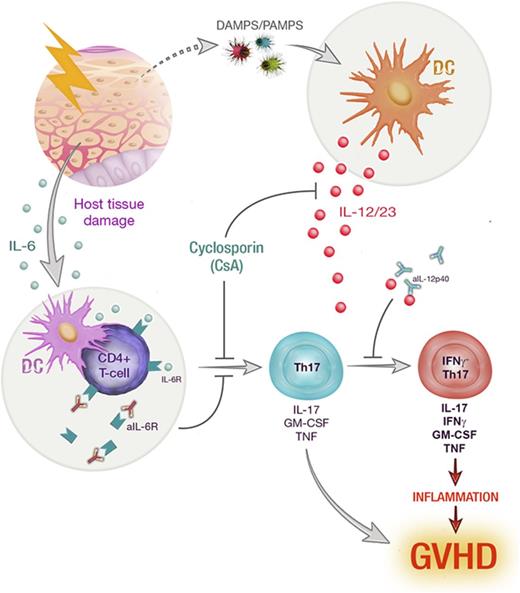
IL-12/IL-23 drives further differentiation of Th17 toward an inflammatory phenotype that is inhibited by cyclosporine treatment.
Abstract
T-helper 17 (Th17) cells have been widely implicated as drivers of autoimmune disease. In particular, Th17 cytokine plasticity and acquisition of an interleukin-17A+(IL-17A+)interferon γ(IFNγ)+ cytokine profile is associated with increased pathogenic capacity. Donor Th17 polarization is known to exacerbate graft-versus-host disease (GVHD) after allogeneic stem cell transplantation (allo-SCT); however, donor Th17 cytokine coexpression and plasticity have not been fully characterized. Using IL-17 “fate-mapping” mice, we identified IL-6-dependent Th17 cells early after allo-SCT, characterized by elevated expression of proinflammatory cytokines, IL-17A, IL-22, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor. This population did not maintain lineage fidelity, with a marked loss of IL-17A and IL-22 expression late posttransplant. Th17 cells were further segregated based on IFNγ coexpression, and IL-17A+IFNγ+ Th17 displayed an enhanced proinflammatory phenotype. Th17 cytokine plasticity and IFNγ production were critically dependent upon donor-derived IL-12p40, and cyclosporine (CsA) treatment regulated this differentiation pathway. This observation was highly concordant with clinical samples from allo-SCT recipients receiving CsA-based immune suppression where although the IFNγ-negative–Th17 subset predominated, IFNγ+-Th17 cells were also present. In sum, Th17 polarization and ensuing differentiation are mediated by sequential inflammatory signals, which are modulated by immunosuppressive therapy, leading to distinct phenotypes within this lineage.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is an important treatment for hematological malignancies, whose therapeutic efficacy resides in donor T-cell responses that drive antitumor immunity. However, donor T cells are also responsible for graft-versus-host disease (GVHD), a major complication that significantly impacts patient morbidity and mortality. Thus, understanding the factors that differentiate these outcomes is critical to improving allo-SCT. Due to the highly inflammatory environment induced following allo-SCT, T-cell polarization is a complex process culminating in highly plastic and heterogeneous cytokine expression. This effect is perhaps most prominent in interleukin-17 (IL-17)–producing donor T cells, which we and others have shown to be highly pathogenic in preclinical models of GVHD.1-6 Plasticity has been widely reported in CD4+IL-17A+ (T-helper 17 [Th17]) and CD8+IL-17A+ (Tc17) T cells in a range of infectious and autoimmune settings.6-12 We have recently described Tc17 cytokine plasticity in murine allo-SCT recipients,1 which is characteristic of a hyper-proinflammatory T-cell subset that contributes to GVHD but fails to mediate graft-versus-leukemia effects.1,3 Th17 cells have been reported to transdifferentiate toward highly functional distinct phenotypes, including the acquisition of regulatory and follicular B-cell helper function (Tfh).13-15 In addition, multiple studies of autoimmunity have described differential pathogenic capacity within Th17 subsets due to cytokine plasticity correlating with the acquisition of “Th1-like” phenotypes.6-8,16-18 These important features of Th17 development and differentiation are yet to be fully examined in GVHD, and we therefore characterized donor Th17 development and plasticity following both murine and clinical allo-SCT.
Methods
Mice
Female C57Bl/6 (B6 [H-2Db]), Balb/c (H-2Dd), and B6D2F1 (H-2Db/d) mice were purchased from the Animal Resources Center, WA, Australia. IL-17Cre (B6, cre-recombinase H-2Db), Rosa26eYFP (B6, enhanced yellow fluorescent protein H-2Db), IL-12p40−/− (B6, H-2Db),19 IL-12p40YFP (B6, yellow fluorescent protein H-2Db),20 and TEa T-cell transgenic21-23 (B6, H-2Db) mice were bred and housed at QIMR. IL-17Cre and Rosa26eYFP mice were provided by Brigitta Stockinger (Francis Crick Institute, UK) and crossed to generate IL-17creRosa26eYFP heterozygous mice.7 Mice were housed in sterilized microisolator cages and received acidified autoclaved water (pH 2.5) after transplantation.
Experimental stem cell/bone marrow transplantation
Recipient mice received 900 (Balb/c), 1000 (B6), or 1100 (B6D2F1) cGy total-body irradiation (TBI) (137Cs source at 84.6 cGy/min) split over 2 doses (day −1 [d-1]). For granulocyte-colony stimulating factor (G-CSF) mobilized stem cell grafts, 10 × 106 splenocytes from G-CSF–treated donors were injected (d0). Otherwise, grafts consisted of 10 × 106 bone marrow (BM) cells from donor strains as indicated with 2 × 105 fluorescence-activated cell sorted (FACS)–purified wild-type.B6 (WT.B6) T cells. Recipients of alloantigen-specific transgenic T cells were injected intravenously on d12 with 2 × 106 TEa T cells. TEa (>98%; Vβ6+Vα2+) cells were purified by sorting (MoFlo [Beckman Coulter] or FACSAria [BD]). Cyclosporine A (CsA) -treated mice received 5 to 50 mg/kg intraperitoneally (IP) d0-7 posttransplant. Rapamycin-treated mice received 0.6 μg/kg IP d0-7 posttransplant. Recombinant human G-CSF was given subcutaneously to donors at 10 µg per dose per animal (4-6 days).3 Mice were monitored daily after transplant, and systemic GVHD was assessed weekly using a cumulative scoring system.24 Those with GVHD scores of ≥6 were culled, and the date of death recorded as the next day.
Patients
Patients were enrolled (n = 53) as part of an observational study tracking hematopoietic reconstitution after allo-SCT to treat hematological malignancies, including acute myeloid leukemia (36%), acute lymphoblastic leukemia (26%), myelodysplasia (13%), lymphoproliferative disorders (13%), and myeloproliferative disorders (11%). Recipients (60% male and 40% female), aged between 19 and 65 years, received either a myeloablative conditioning regimen consisting of cyclophosphamide (Cy) at 60 mg/kg per day for 2 days and 12 Gy of fractionated TBI given over 3 days or a reduced-intensity conditioning regimen consisting of fludarabine (Flu) at 25 mg/m2 day for 5 days (d-7 to d-3) and melphalan (Mel) at 120 mg/m2 on d-2 (Cy/TBI = 51%, Flu/Mel = 49%). All patients received a T-cell–replete G-CSF-mobilized peripheral blood stem cell graft from HLA-matched donors (51% related, 49% unrelated). GVHD prophylaxis consisted of CsA (5 mg/kg d-1 to d1 and thereafter 3 mg/kg daily with subsequent adjustment to target a therapeutic range of 140-250 ng/mL) and methotrexate on d1 (15 mg/m2), 3, 6, and 11 (10 mg/m2). Cross-correlation analysis of plasma cytokine data in this study is a secondary analysis of a control group previously reported.25 After allo-SCT, the incidence of acute GVHD grade II-IV = 41.5% (n = 53), overall chronic GVHD = 58.8% (n = 50), extensive chronic GVHD26 = 33.5% (n = 50), and relapse of primary disease = 35.1% (n = 53).
Monoclonal antibodies
Antibodies used in flow cytometry were purchased from Biolegend: anti-mouse CD3 (145-2C11), CD4 (GK1.5), CD8α (53-6.7), CD90.2 (30-H12), IL-10 (JES5-16E3), IL-17A (TC11-18H10), interferon γ (IFNγ) (XMG1.2), tumor necrosis factor (TNF) (MP6-XT22), IL-22 (poly5164), Tbet (4B10), anti-human CD3 (UCHT1), CD4 (RPA-T4), CD8α (RPA-T8), TNF (MAb11), IL-17A (BL168), IFNγ (45.B3), or eBioscience: anti-mouse granulocyte-macrophage colony-stimulating factor (GM-CSF; MP1-22E9), IL-13 (eBio13A), RORγt (BD2). Rat anti-mouse IL-6R monoclonal antibodies (mAb) (MR16-1; provided by Chugai Pharmaceutical Co) or rat immunoglobulin G (IgG; Sigma-Aldrich) was given IP at 500 μg per dose on d-1 and d3 post-SCT as described.27 Anti–IL-12p40 (C17.8) and matched control IgG (anti-AGP3) (4D2) were administered IP at 500 μg per dose on d-2, d0, d2, d4, and d6 pre-/posttransplant (Amgen Inc). Anti-IFNγ (XMG1.2) or rat IgG control mAbs were given at 500 μg per dose IP on d0 and d3 posttransplant.
Cell preparation, culture, and cytokine analysis
Murine samples were isolated by mechanical disruption and treated with lysis buffer to remove contaminating erythrocytes. Leukocytes were further enriched from liver tissue via Percoll density gradients and lung tissue by digestion using Collagenase type 3 (1 mg/mL; Worthington) and DNase I (100 μg/mL; Worthington). Human samples were collected from patients on d14, d120, and d240 after allo-SCT, and peripheral blood mononuclear cells were purified from whole blood using Ficoll-paque centrifugation and stored in liquid nitrogen for subsequent analysis. For intracellular cytokine staining, cells were cultured with phorbol 12-myristate 13-acetate (5 μg/mL) and Ionomycin (50 μg/mL) for 4 hours with Brefeldin A (BioLegend) included in the final 3 hours of incubation. Cells were surface labeled and processed for intracellular staining; cytokines were assessed via cytofix/cytoperm kit (BD Biosciences), and intranuclear staining was performed via fixation and permeabilization (EBioscience). All samples were acquired on a BD LSR Fortessa (BD Biosciences) using BD FACSDiva (v7.0) and analyzed with FlowJo (v9.7).
Gene expression analysis
T cells were isolated from liver, spleen, and lymph nodes (LNs) on either d7 or d21 after allo-SCT. Target populations were sorted (>95% purity) from 6 to 10 mice per experiment and pooled prior to storage in RLT buffer (Qiagen). Each sample analyzed was therefore derived from an independent experiment consisting of either CD4+YFP+ or CD4+YFP– T cells isolated from multiple mice. Total RNA was extracted with RNeasy Micro kits (Qiagen) and cDNA prepared with Maxima H Minus First Strand cDNA synthesis kits (Thermo Fisher). Quantitative polymerase chain reaction (qPCR) was performed using Taqman gene expression assays (Applied Biosystems), and all measurements were run in parallel with, and normalized to, the housekeeping gene Hprt.
BMDC differentiation and stimulation
BM cells were cultured in complete RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (Iscove modified Dulbecco medium) in the presence of 200 ng/mL FLT3-L (Peprotech) for 9 days at 37°C. On day 9, cells were stained and quantified for CD11c+MHC (major histocompatibility complex) II+ (I-A/I-E+) bone marrow–derived dendritic cell (BMDC) proportions using flow cytometry. BMDCs were cultured in triplicate at 1 × 105 cells/100 μL overnight in the presence of 10 ng/mL recombinant mouse GM-CSF (BioLegend), 10 ng/mL recombinant mouse IFNγ (BD), in the presence or absence of 10 μM CpG 1585 (InvivoGen). CsA was included in increasing concentrations of 0 to 10 μg/mL. Supernatants were collected for the detection of analytes (IL-6, IL-12p70, IL-12p40, IL-23, and TNFα) using Cytometric Bead Array detection kits (BD). Cytokine concentrations were quantified using FCAP Array v3.0 software (BD). Data were normalized by calculating the fold change in cytokine concentration between treated and untreated wells.
Confocal imaging
Lymph node explants were imaged on the LSM780 upright confocal microscope (Carl Zeiss). Z-stacks were acquired up to 80 μm deep, with slices every 3 μm. Samples were excited using a MaiTai multiphoton laser at 890 nm, and emission was detected on the spectral detectors in λ mode. Cyan fluorescent protein (CFP), YFP, and collagen spectra were unmixed using Zen software, and references for each were obtained from single color controls. Images were analyzed on Imaris v8.2.0 (Bitplane) to create surfaces and determine the volume of YFP cells within each z-stack.
Study approval
All animal experiments were approved by and performed in accordance with the QIMR Berghofer Animal Ethics Committee. Ethics approval to undertake clinical studies was obtained from both the QIMR Berghofer Medical Research Institute and the Royal Brisbane and Women’s Hospital Human Ethics Committees with written informed consent obtained from participating patients.
Statistical analysis
Unpaired Student t tests were predominantly used to evaluate differences in cytokine expression, and cell frequencies, paired Student t tests, or analysis of variance was used as indicated when comparisons were made between 3 or more groups. Unpaired 2-tailed Mann-Whitney tests were used to evaluate differences in gene expression. Data are mean ± standard error of the mean (SEM) unless otherwise stated, and P < .05 was considered significant. Cytokine concentrations in naive patient sera were evaluated by Pearson correlation. To test for correlations across time between pairs of plasma cytokine datasets, we estimated the cross-correlation of each cytokine at lag 0. This method differs from applying traditional Pearson and Spearman correlation to longitudinal data; there are well-known biases with these statistics on time series, going back nearly 100 years.28 The cross-correlation statistic is the extension of the autocorrelation to 2 separate time series. In our scenario, we only estimated lag 0 cross-correlations, which did not consider whether a given cytokine was leading or lagging a different cytokine. We tested for significance in the cross-correlation using the large-sample normal approximation vs a null hypothesis of a cross-correlation of 0.
Results
Th17 development after allo-SCT is an early and transient phenomenon
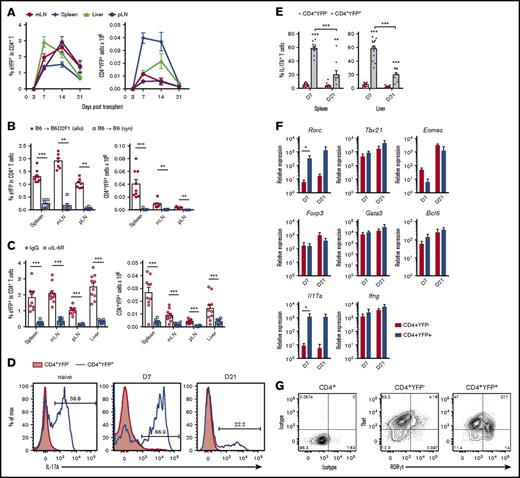
Using IL-17A “fate-mapping” reporter grafts (B6.IL-17CreRosa26eYFP), we followed donor Th17 differentiation after allo-SCT. CD4+YFP+ Th17 cells were present in all murine lymphoid tissues and GVHD target organs examined, with particularly rapid expansion between d3 and d7 (Figure 1A). CD4+YFP+ numbers peaked between 1 and 2 weeks posttransplant and then contracted significantly, with up to 30-fold fewer CD4+YFP+ cells at d21 compared with d7. Th17 differentiation was dependent upon the presence of alloantigen, because CD4+YFP+ frequencies remained low in syngeneic transplant recipients (Figure 1B). Using an allo-SCT–induced model of lung injury, we have previously demonstrated the importance of IL-6 in driving Th17 development.2 Here, we also observed Th17 differentiation to be highly dependent upon IL-6R signaling in all tissues examined (Figure 1C). IL-17A expression was transient such that the proportion of CD4+YFP+ T cells expressing IL-17A declined significantly over time (Figure 1D-E). This plasticity in Th17 cytokine expression was observed in both central lymphoid and GVHD target tissue, demonstrating the requirement for IL-17 “fate-mapping” reporter grafts to fully characterize Th17 development in this model. Prior studies of Th17 in autoimmune disease have demonstrated significant functional plasticity in Th17 populations, including the acquisition of Tfh and regulatory T-cell function.14,15 We therefore followed transcription factor gene expression over time by performing qPCR analysis of sort-purified CD4+YFP+ T cells early and late posttransplant. The Th17-defining transcription factor Rorc and IL17a expression were significantly increased in CD4+YFP+ T cells at d7 (Figure 1F), which remained high at d21 despite reduced IL-17A protein expression at this time point. Taken together, these data suggest that posttranscriptional regulation may play a role in the decline in IL-17A expression late posttransplant. No evidence was found in CD4+YFP+ T cells for enhanced expression of transcription factor genes Tbx21, Eomes, Bcl6, Gata3, or Foxp3, normally associated with cytotoxicity, Tfh, Th2, and regulatory T-cellfunction, respectively. Because Tbx21/Tbet expression by Th17 has been reported to be necessary to induce autoimmune pathology in murine models of experimental autoimmune encephalomyelitis,18,29,30 we confirmed RORγt and Tbet coexpression in CD4+YFP+ T cells by intranuclear staining and flow cytometry. Interestingly, both Tbet+ and Tbet– populations were apparent in the RORγt+Th17 compartment at d7 (Figure 1G), suggesting that after allo-SCT, donor Th17 subsets emerge that may have differential capacity to drive inflammation.
Donor-derived IL-17+and RORγt+Th17 cells develop early after allo-SCT in an IL-6-dependent manner. (A-G) CD4+YFP+ development was assessed in spleen, mesenteric LN (mLN), peripheral LN (pLN) and liver from lethally irradiated allogeneic (B6.IL-17CreRosa26eYFP → B6D2F1) or (B) syngeneic (B6.IL-17CreRosa26eYFP → B6) mice transplanted with G-CSF mobilized grafts. (A) Time course analysis of CD4+YFP+ frequencies and absolute numbers within the CD4+ T-cell compartment d3, d7, d14, and d21 posttransplant (mean ± SEM, n = 4-21 mice per group pooled from 2-4 independent experiments). (B) CD4+YFP+ frequencies and absolute numbers 7 days after syngeneic or allogeneic SCT (mean ± SEM, n = 7-9 mice per group pooled from 2 independent experiments, **P < .01, ***P < .001). (C) CD4+YFP+ frequencies and absolute numbers 7 days after allogeneic SCT in the presence of either IL-6R blocking mAbs or control IgG (mean ± SEM, n = 10 mice per group pooled from 2 independent experiments, ***P < .001). (D) IL-17A expression in naive and donor CD4+ T cells d7 and d21 after transplant in (D-E) spleen and (E) liver (mean ± SEM, n = 10-15 mice per group pooled from 2-3 independent experiments, **P < .01, ***P < .001). (F) YFP+ and YFP– CD4+ T cells isolated from spleen, LNs, and liver by FACS on d7 and d21 followed by qPCR (mean ± SEM, n = 3-4 independent experiments each composed of 10 pooled mice per group, *P < .05). (G) RORγt and Tbet expression by CD4+ T-cell populations isolated from spleen, mLNs, and liver d7 posttransplant.
Donor-derived IL-17+and RORγt+Th17 cells develop early after allo-SCT in an IL-6-dependent manner. (A-G) CD4+YFP+ development was assessed in spleen, mesenteric LN (mLN), peripheral LN (pLN) and liver from lethally irradiated allogeneic (B6.IL-17CreRosa26eYFP → B6D2F1) or (B) syngeneic (B6.IL-17CreRosa26eYFP → B6) mice transplanted with G-CSF mobilized grafts. (A) Time course analysis of CD4+YFP+ frequencies and absolute numbers within the CD4+ T-cell compartment d3, d7, d14, and d21 posttransplant (mean ± SEM, n = 4-21 mice per group pooled from 2-4 independent experiments). (B) CD4+YFP+ frequencies and absolute numbers 7 days after syngeneic or allogeneic SCT (mean ± SEM, n = 7-9 mice per group pooled from 2 independent experiments, **P < .01, ***P < .001). (C) CD4+YFP+ frequencies and absolute numbers 7 days after allogeneic SCT in the presence of either IL-6R blocking mAbs or control IgG (mean ± SEM, n = 10 mice per group pooled from 2 independent experiments, ***P < .001). (D) IL-17A expression in naive and donor CD4+ T cells d7 and d21 after transplant in (D-E) spleen and (E) liver (mean ± SEM, n = 10-15 mice per group pooled from 2-3 independent experiments, **P < .01, ***P < .001). (F) YFP+ and YFP– CD4+ T cells isolated from spleen, LNs, and liver by FACS on d7 and d21 followed by qPCR (mean ± SEM, n = 3-4 independent experiments each composed of 10 pooled mice per group, *P < .05). (G) RORγt and Tbet expression by CD4+ T-cell populations isolated from spleen, mLNs, and liver d7 posttransplant.
Donor Th17 are highly inflammatory and plastic in their cytokine profile
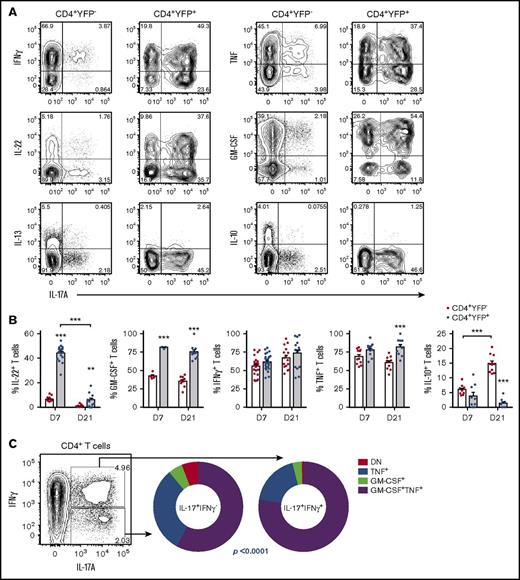
To explore this concept further, we assessed cytokine production in Th17 cells. At d7, CD4+YFP+ T cells coexpressed a range of cytokines, including IL-17A, IFNγ, TNF, IL-22, and GM-CSF (Figure 2A), with IL-17A, IL-22, GM-CSF, and TNF significantly enriched when compared with CD4+YFP− counterparts (Figure 2B). Like IL-17A, IL-22 expression by CD4+YFP+ T cells decreased significantly over time; however, GM-CSF, IFNγ, and TNF expression was largely maintained with >50% of CD4+YFP+ T cells expressing all 3 cytokines at d21. In contrast, IL-10 secretion was reduced in the CD4+YFP+ population, consistent with an inflammatory phenotype.13 Given the loss of IL-17A/IL-22 expression in the majority of CD4+YFP+ T cells, this suggests a Th17 transition toward a “Th1-like” phenotype. Coexpression of IFNγ and/or GM-CSF is a known hallmark of enhanced Th17 pathogenicity.16,18,30-34 Concordant with RORγt/Tbet coexpression (Figure 1G), 2 distinct populations were present in posttransplant donor Th17 that segregated based on IFNγ coexpression (Figure 2A,C). By comparing IL-17+IFNγ– and IL-17A+IFNγ+ Th17 subsets, we observed significantly elevated GM-CSF and TNF in the IL-17A+IFNγ+ fraction, confirming that this population is significantly more proinflammatory than IL-17A+IFNγ– counterparts (Figure 2C).
Donor-derived Th17 are highly proinflammatory and plastic in their cytokine profile. (A-C) CD4+YFP+ development was assessed in lethally irradiated allogeneic mice transplanted with G-CSF mobilized grafts (B6.IL-17CreRosa26eYFP → B6D2F1). (A) Representative FACS analysis showing IL-17A, IFNγ, IL-22, IL-13, TNF, GM-CSF, and IL-10 cytokine expression posttransplant by splenic CD4+YFP− or CD4+YFP+ populations after short-term in vitro restimulation (d7). (B) Frequencies of cytokine-expressing CD4+ T cells isolated from spleen and assessed at d7 or d21 post-SCT (mean ± SEM, n = ≥5 mice per group, *P < .05, **P < .01, ***P < .001). (C) GM-CSF and TNF coexpression by IL-17A+IFNγ– and IL-17A+IFNγ+ CD4+ T cells at d7 after allo-SCT (n = 6 mice from 1 representative experiment, *P < .0001). DN, double negative (TNF−GM-CSF−).
Donor-derived Th17 are highly proinflammatory and plastic in their cytokine profile. (A-C) CD4+YFP+ development was assessed in lethally irradiated allogeneic mice transplanted with G-CSF mobilized grafts (B6.IL-17CreRosa26eYFP → B6D2F1). (A) Representative FACS analysis showing IL-17A, IFNγ, IL-22, IL-13, TNF, GM-CSF, and IL-10 cytokine expression posttransplant by splenic CD4+YFP− or CD4+YFP+ populations after short-term in vitro restimulation (d7). (B) Frequencies of cytokine-expressing CD4+ T cells isolated from spleen and assessed at d7 or d21 post-SCT (mean ± SEM, n = ≥5 mice per group, *P < .05, **P < .01, ***P < .001). (C) GM-CSF and TNF coexpression by IL-17A+IFNγ– and IL-17A+IFNγ+ CD4+ T cells at d7 after allo-SCT (n = 6 mice from 1 representative experiment, *P < .0001). DN, double negative (TNF−GM-CSF−).
Alloantigen-induced Th17 transition toward IFNγ production is dependent upon IL-12p40 and IFNγ
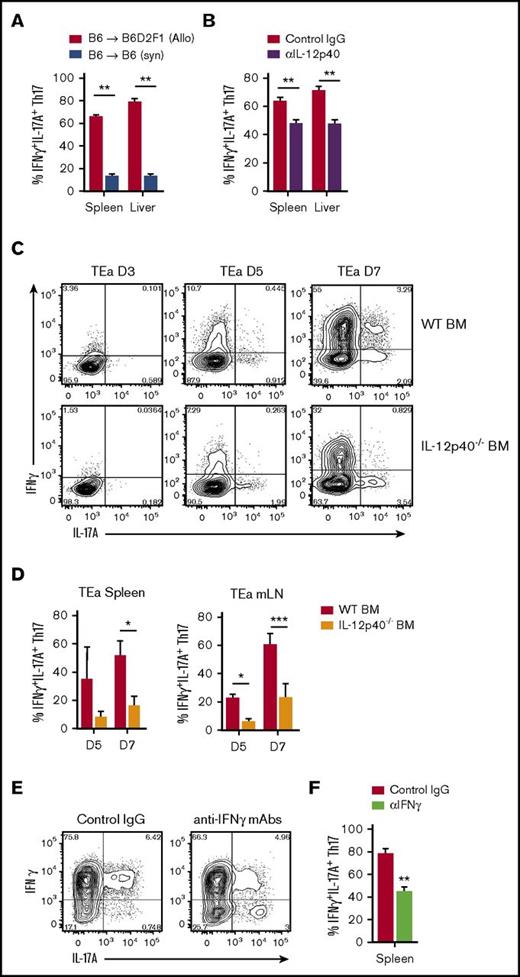
We next confirmed that proinflammatory Th17 differentiation was dependent upon the presence of alloantigen, because the proportion of IL-17A+IFNγ+ Th17 remained low in syngeneic recipients (Figure 3A). Previous studies have reported that Th17 cytokine plasticity is dependent upon both IL-12 and IL-23.8,16,18,31-35 We therefore examined Th17 development in the presence of IL-12p40 blocking antibodies after allo-SCT. Although overall Th17 frequencies were unchanged (data not shown), we observed a significant reduction in IFNγ-producing Th17 (Figure 3B). To explore this further, we used alloreactive CD4+ transgenic T cells (TEa that recognize host-derived (Balb/c) I-Ed peptide when presented within donor I-Ab major histocompatibility complex II.22 Mice were transplanted with WT or IL-12p40-deficient donor grafts (d0), followed by transgenic TEa T cells (d12) and Th17 differentiation within alloantigen-specific T cells assessed. In both spleen and mesenteric LNs, we observed IL-17A+ T cells arising at d5, which in the presence of donor-derived IL-12p40, transitioned toward an IL-17A+IFNγ+ donor T-cell phenotype by d7 (Figure 3C-D). We have previously demonstrated that IFNγ regulates both Th17 and Tc17 frequencies after allo-SCT,1,2 and here, we find that IFNγ also supports the expression of IFNγ in Th17 cells (Figure 3E-F).
Proinflammatory donor Th17 are induced by alloantigen, IL-12/23, and IFNγ. (A-F) Donor CD4+ T-cell cytokine expression was examined after (A) syngeneic SCT (WT.B6 → WT.B6) or (A-F) allo-SCT, (A-B,E-F) WT.B6 → B6D2F1 (G-CSF mobilized grafts) or (C-D) WT.B6 or IL-12p40–/– BM, WT.B6 T-cells and B6.TEa T-cells → Balb/c. (A) Proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after either allo-SCT or syngeneic transplant (mean ± SEM, n = ≥6 mice per group, **P < .01). (B) Proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after allo-SCT in the presence or absence of IL-12p40 blocking antibody treatment (mean ± SEM, n = ≥9 mice per group from 2 independent experiments, **P < .01). (C) Representative FACS analysis of donor TEa transgenic T cells harvested on d3, d5, and d7 after adoptive transfer. (D) Proportion of splenic and mLN CD4+IL-17A+ TEa cells coexpressing IFNγ d5 and d7 after allo-SCT in the presence and absence of donor BM-derived IL-12p40 (mean ± SD, n = 3 mice per group per time point, *P < .05, ***P < .001). (E) Representative FACS analysis and (F) proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after allo-SCT in the presence or absence of IFNγ blocking antibody treatment (mean ± SEM, n = 6 mice per group pooled from 2 independent experiments, **P < .01).
Proinflammatory donor Th17 are induced by alloantigen, IL-12/23, and IFNγ. (A-F) Donor CD4+ T-cell cytokine expression was examined after (A) syngeneic SCT (WT.B6 → WT.B6) or (A-F) allo-SCT, (A-B,E-F) WT.B6 → B6D2F1 (G-CSF mobilized grafts) or (C-D) WT.B6 or IL-12p40–/– BM, WT.B6 T-cells and B6.TEa T-cells → Balb/c. (A) Proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after either allo-SCT or syngeneic transplant (mean ± SEM, n = ≥6 mice per group, **P < .01). (B) Proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after allo-SCT in the presence or absence of IL-12p40 blocking antibody treatment (mean ± SEM, n = ≥9 mice per group from 2 independent experiments, **P < .01). (C) Representative FACS analysis of donor TEa transgenic T cells harvested on d3, d5, and d7 after adoptive transfer. (D) Proportion of splenic and mLN CD4+IL-17A+ TEa cells coexpressing IFNγ d5 and d7 after allo-SCT in the presence and absence of donor BM-derived IL-12p40 (mean ± SD, n = 3 mice per group per time point, *P < .05, ***P < .001). (E) Representative FACS analysis and (F) proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after allo-SCT in the presence or absence of IFNγ blocking antibody treatment (mean ± SEM, n = 6 mice per group pooled from 2 independent experiments, **P < .01).
Inflammatory Th17 are regulated by immunosuppression in both clinical and preclinical settings
Calcineurin inhibitors such as CSA are the most widely used immunosuppressive therapy after clinical allo-SCT, and both IL-12/23 and IFNγ responses are known to be diminished in its presence.2,36 Therefore, we assessed the influence of CsA treatment on Th17 cytokine plasticity by exposing mice to increasing doses of CsA early posttransplant. Although calcineurin inhibition is known to have broad immunosuppressive effects on T-cell responses, including a significant reduction in overall Th17 frequencies,2 we found that CsA treatment successfully reduced the transition of IL-17A+IFNγ– Th17 toward an IFNγ−producing phenotype (Figure 4A-B). Because we have previously demonstrated that donor antigen-presenting cells (APCs) are a major source of IL-12 posttransplant,37 we examined the effect of CsA using IL-12p40-YFP reporter mice. Using multiphoton confocal imaging of whole LN explants, we observed a significant in vivo reduction in IL-12p40-producing YFP+ cells in recipients of allo-SCT and CsA treatment (Figure 4C-D). To determine if this was a direct effect on APCs, we compared cytokine secretion by BMDCs in response to toll-like receptor ligation in the presence and absence of CsA. We confirmed that CsA can directly downregulate APC responses to stimulation and specifically observed reduced IL-12 and IL-23 secretion in vitro (Figure 4E). In contrast, IL-6 was not suppressed by CSA, consistent with recent in vivo data.2 Other immunosuppressants are known to target different T-cell activation pathways, such as the mammalian target of rapamycin inhibitor rapamycin. To explore the influence of such treatment, we assessed IL-17A+IFNγ+ Th17 development in the presence of rapamycin, where we observed similar effects to that of CsA (Figure 4F-H). Notably, rapamycin suppression of Th17 plasticity and proinflammatory cytokine production in non-Th17 CD4+ T cells appeared inferior to the effects observed in CsA-treated animals.
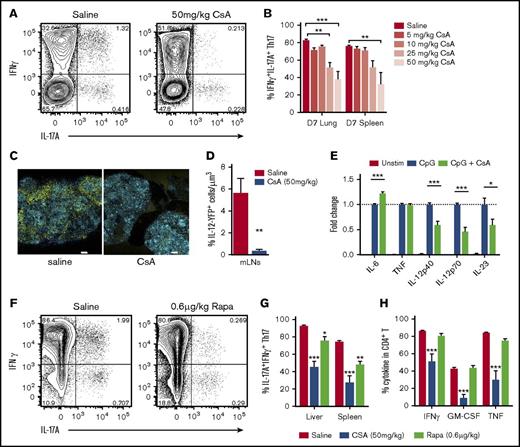
Donor Th17 plasticity and proinflammatory profile is limited by immunosuppression. (A-D,F-H) Donor CD4+ T-cell cytokine expression was examined after allo-SCT (A-B,E-F) WT.B6 → B6D2F1 (G-CSF mobilized grafts) or (C-D) WT.B6 or IL-12p40−/− BM, WT.B6 T-cells → Balb/c. (A) Representative FACS analysis of splenic CD4+ T cells and (B) proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after allo-SCT in the presence or absence of increasing doses of CsA (mean ± SEM, n = 8 mice per group, pooled from 2 independent experiments, ***P < .001). (C) Representative images and (D) quantitative analysis of IL-12p40-YFP+ cells in mLN explants at d12 posttransplant, unstained tissues were captured by multiphoton confocal microscopy (20× objective) with spectral detection and linear unmixing (unmixed channels are shown: blue = CFP; yellow = YFP; scale bars = 300 μm). (E) Cytokine production by BMDCs was assessed in response to overnight stimulation in the presence and absence of CsA. Data are expressed as fold change relative to CpG-only stimulation (mean ± SEM, data pooled from 2 independent experiments, ***P < .001). (F) Representative FACS analysis of splenic CD4+ T cells, (G) proportion of CD4+IL-17A+ T cells coexpressing IFNγ, (H) frequency of cytokine-positive CD4+ T cells d7 after allo-SCT in the presence or absence of CsA (50 mg/kg) or Rapa (rapamycin) (0.6 μg/kg) (mean ± SEM, n = 6 mice per group, *P < .05, **P < .01, ***P < .001).
Donor Th17 plasticity and proinflammatory profile is limited by immunosuppression. (A-D,F-H) Donor CD4+ T-cell cytokine expression was examined after allo-SCT (A-B,E-F) WT.B6 → B6D2F1 (G-CSF mobilized grafts) or (C-D) WT.B6 or IL-12p40−/− BM, WT.B6 T-cells → Balb/c. (A) Representative FACS analysis of splenic CD4+ T cells and (B) proportion of CD4+IL-17A+ T cells coexpressing IFNγ d7 after allo-SCT in the presence or absence of increasing doses of CsA (mean ± SEM, n = 8 mice per group, pooled from 2 independent experiments, ***P < .001). (C) Representative images and (D) quantitative analysis of IL-12p40-YFP+ cells in mLN explants at d12 posttransplant, unstained tissues were captured by multiphoton confocal microscopy (20× objective) with spectral detection and linear unmixing (unmixed channels are shown: blue = CFP; yellow = YFP; scale bars = 300 μm). (E) Cytokine production by BMDCs was assessed in response to overnight stimulation in the presence and absence of CsA. Data are expressed as fold change relative to CpG-only stimulation (mean ± SEM, data pooled from 2 independent experiments, ***P < .001). (F) Representative FACS analysis of splenic CD4+ T cells, (G) proportion of CD4+IL-17A+ T cells coexpressing IFNγ, (H) frequency of cytokine-positive CD4+ T cells d7 after allo-SCT in the presence or absence of CsA (50 mg/kg) or Rapa (rapamycin) (0.6 μg/kg) (mean ± SEM, n = 6 mice per group, *P < .05, **P < .01, ***P < .001).
To characterize Th17 in the clinical setting, we assessed IL-17A and IFNγ responses in peripheral blood CD4+ T cells derived from healthy adults and CsA-treated allogeneic transplant patients (Figure 5A-B). These data demonstrated a significant bias in the Th17 population toward “single positive” IL-17A+IFNγ– T cells in healthy adults, where ∼10% to 20% of Th17 were found to coexpress IFNγ (Figure 5B). Because Th17 pathogenicity has only been described in the context of autoimmunity, and here, alloreactivity, it is likely IL-17A+IFNγ+ CD4+ T cells in healthy adults represent pathogen-specific memory T cells. Two weeks posttransplant, a similar proportion (∼10% to 15%) of Th17 cells were found to coexpress IFNγ, suggesting that early CsA administration is sufficient to suppress inflammatory Th17 differentiation as seen in CsA-treated mice. Importantly, this population persisted and expanded in number within peripheral blood for up to 9 months after transplant (Figure 5B-C). TNF was also produced in significantly higher frequencies by IL-17+IFNγ+ T cells in patients at all time points tested (Figure 5D), consistent with our preclinical modeling. Although no significant correlation was observed between IFNγ and IL-17A levels in plasma of healthy adults (Figure 5E), allo-SCT recipients display increasingly dysregulated IL-17A and IFNγ late after transplant during the period when patients typically are undergoing CSA taper and/or develop breakthrough GVHD (∼d100).25 Importantly, cross-correlation analysis over time demonstrated a highly significant link between the expression of these 2 cytokines after transplant, consistent with the murine data (Figure 5F).
Proinflammatory Th17 in patients following allo-SCT. (A) Representative FACS analysis and (B) proportion of peripheral blood CD4+IL-17A+ T cells expressing IFNγ in healthy donors and d14, d120, and d240 after clinical allo-SCT and CsA treatment (∼d0-d100) (mean ± SEM, d14, n = 11 patients, d240, n = 12 patients). (C) Total IL-17A+IFNγ+ concentrations in human peripheral blood d14 and d240 after clinical allo-SCT and CsA treatment (mean ± SEM, d14, n = 10 patients, d240, n = 10 patients, ***P < .001). (D) Proportion of human peripheral blood IL-17A+IFNγ+ and IL-17A+IFNγ– CD4+ T cells coexpressing TNF on d14, d120, and d240 posttransplant (paired analyses, d14, n = 6 patients, d120, n = 3 patients, and d240, n = 12 patients, *P < .05, **P < . 01, ***P < .001). (E) Pearson correlation analysis of systemic IL-17A and IFNγ concentrations in healthy donor plasma. (F) Cross-correlation analysis of systemic IL-17A, IFNγ, and TNF concentrations in patient plasma over time derived from allo-SCT recipients on days −7, 0, 7, 14, 30, 60, 90, 120, 180, 240, and 360 (n = 53 patients, ***P < .001). n.s., not significant; TNF+ve, tumor necrosis factor positive.
Proinflammatory Th17 in patients following allo-SCT. (A) Representative FACS analysis and (B) proportion of peripheral blood CD4+IL-17A+ T cells expressing IFNγ in healthy donors and d14, d120, and d240 after clinical allo-SCT and CsA treatment (∼d0-d100) (mean ± SEM, d14, n = 11 patients, d240, n = 12 patients). (C) Total IL-17A+IFNγ+ concentrations in human peripheral blood d14 and d240 after clinical allo-SCT and CsA treatment (mean ± SEM, d14, n = 10 patients, d240, n = 10 patients, ***P < .001). (D) Proportion of human peripheral blood IL-17A+IFNγ+ and IL-17A+IFNγ– CD4+ T cells coexpressing TNF on d14, d120, and d240 posttransplant (paired analyses, d14, n = 6 patients, d120, n = 3 patients, and d240, n = 12 patients, *P < .05, **P < . 01, ***P < .001). (E) Pearson correlation analysis of systemic IL-17A and IFNγ concentrations in healthy donor plasma. (F) Cross-correlation analysis of systemic IL-17A, IFNγ, and TNF concentrations in patient plasma over time derived from allo-SCT recipients on days −7, 0, 7, 14, 30, 60, 90, 120, 180, 240, and 360 (n = 53 patients, ***P < .001). n.s., not significant; TNF+ve, tumor necrosis factor positive.
Discussion
Although there is little doubt that GVHD is mediated by a range of inflammatory T-cell populations, including Th1/Tc1, IL-17-producing T cells also play a significant role in both acute and chronic GVHD pathogenesis.1-4,38,39 In nontransplant settings, Th17 have in turn been described as protective in response to fungal infections and other pathogens,40 pathogenic in multiple human autoimmune diseases,41 and regulatory during inflammation resolution.13 This heterogeneity in Th17 function has been attributed to their high degree of plasticity such that Th17 have been reported to transdifferentiate between these functional roles.13,14 In this study, we used a murine IL-17A “fate-mapping” reporter model to characterize donor Th17 development during GVHD and examine the potential for Th17 plasticity in this context. We found that Th17 differentiation was initiated early posttransplant in response to alloantigen and was significantly impaired in the presence of IL-6R blockade. Despite ongoing Rorc and Il17a gene expression, the frequency of IL-17A protein expression was significantly lower d21 posttransplant in “fate-mapped” Th17, as was IL-22, demonstrating a significant shift in cytokine production during the progression of GVHD. Because IFNγ and TNF production was maintained over time, Th17 acquired a “Th1-like” cytokine profile late posttransplant; however, these cells remained clearly distinguishable by their enhanced propensity for GM-CSF expression.
Numerous studies describe differential pathogenic capacity within the Th17 subset in autoimmunity, corresponding with a transition toward a Th1-like phenotype.17 This enhanced pathogenicity has been attributed to cytokine plasticity, and in particular, IFNγ and Tbet expression by Th17.16-18,29 Our analyses revealed 2 subsets of Th17 in GVHD that clearly segregate based on IFNγ and Tbet expression, in which IFNγ+Th17 correlated with significantly higher cytokine coexpression, and therefore, a more proinflammatory phenotype overall. In non-GVHD settings, both IL-23 and IL-12 have been reported to drive this shift toward proinflammatory Th17 subtypes.8,35 We saw clear evidence of a sequential development process in which donor BM-derived IL-12/23 drives IFNγ expression in alloantigen-specific Th17. Autocrine signaling in Th1 cells by IFNγ/IFNγR is a known mechanism for early Tbet induction and subsequent positive feedback signaling of IL-12 to stabilize IFNγ/Tbet expression.42 Similarly, IFNγ signaling was required by inflammatory Th17, because IFNγ blocking treatment resulted in reduced frequencies of IFNγ+IL-17A+ CD4+ T cells in vivo.
Combinational immunosuppression is standard clinical practice to limit effector T-cell responses and critical for the prevention of GVHD. Thus, recipients almost universally receive calcineurin inhibitors, such as CsA, after allo-SCT. CsA functions by suppressing calcineurin phosphatase activity and thereby inhibits activation of the transcription factor NFAT and critical downstream cytokine pathways that govern T-cell proliferation.43 However, it is clear that despite these effects, T-cell–mediated responses and GVHD break through this prophylactic suppression regimen in about half of patients. We therefore examined the effects of CsA treatment on Th17 differentiation after allo-SCT and found that Th17 plasticity was significantly limited by CsA, such that escalating doses of CsA treatment resulted in reduced proportions of Th17 coexpressing IFNγ+ after transplant. Because calcineurin inhibition has also been reported to modulate APC function, we examined the effect of CsA treatment on IL-12 and IL-23 expression to determine if there may be indirect mechanisms by which CsA may limit Th17 plasticity. Using both in vitro and in vivo systems, we observed a significant reduction in IL-12/23 production consistent with the critical requirement for these cytokines in driving inflammatory Th17 differentiation. Alternative immunosuppressive therapies, such as mammalian target of rapamycin inhibition, have been reported to have wide ranging effects on dendritic cell function, including downregulation of both APC maturation and antigen presentation.44,45 Although we observed a significant reduction in Th17 plasticity in the presence of rapamycin, this was not as striking as that observed in CsA-treated mice, consistent with its general inferiority as an immunosuppressant in transplant settings.46,47
Multiple studies have now reported correlations between IFNγ+Th17 frequencies and human disease, including juvenile idiopathic arthritis and Crohn disease.17,35 We confirmed the presence of IFNγ+Th17 in peripheral blood mononuclear cells derived from CsA-treated patients following allo-SCT, which share a similar proinflammatory cytokine profile to that observed in mice. Circulating IFNγ+ Th17 cells persisted in patients for up to 9 months following transplant and significantly increased in number over time. Over this same period, we observed a significant correlation between systemic dysregulation of IL-17A and IFNγ in transplant patients not seen in healthy adults.
Emerging evidence suggests that glucocorticoid resistance may be a feature of pathogenic Th17, in which steroid refractory autoimmune disease states are maintained by IFNγ+IL-17A+ CD4+ T cells.17,48 Because steroid treatment is the mainstay of management for chronic GVHD, this clearly warrants further investigation in allo-SCT recipients, and serendipitously, CsA treatment has been proposed to successfully treat this steroid-resistant pathogenic Th17 subtype in autoimmunity.17,48 In the setting of acute GVHD, a recent study found that acute GVHD breaking through immune suppression was typified by T-cell persistence, as well as pro-inflammatory and Th/Tc17 transcriptional biases.49 Here, we describe a T-cell lineage highly concordant with these observations, sharing the hallmarks of persistence, highly proinflammatory phenotype, and the Th17 transcriptional program.
In summary, this study demonstrates that Th17 differentiation in mice is initiated by IL-6 with the subsequent disruption of lineage fidelity by IL-12/23 and IFNγ dysregulation, leading to the generation of promiscuous proinflammatory T cells. Although this lineage is initially suppressed by calcineurin-based immune suppression, its expansion late after clinical transplant will require additional therapeutic approaches based on the critical molecular cues defined here.
Acknowledgments
The authors thank Brigitta Stockinger for providing reagents and acknowledge the assistance of the Flow Cytometry & Imaging facility at QIMR Berghofer.
This work was supported by grants from the Rio Tinto Ride to Conquer Cancer and the National Health and Medical Research Council. G.R.H. and M.J.S. are National Health and Medical Research Council Fellows.
Authorship
Contribution: K.H.G. designed, performed experiments and wrote the manuscript; A.V., M.K., R.J.R., K.A.M., and K.P.A.M. designed and performed experiments; K.C., A.N.W., M.A.U., R.D.K., N.C.R., S.D.O., K.E.L., B.E.T., and M.C. performed experiments; D.S. provided statistical analysis; M.W.L.T. and M.J.S. provided essential reagents and discussion; S.-K.T. enrolled and provided clinical care of patients in the study; and G.R.H. designed experiments and helped write the manuscript.
Conflict-of-interest disclosure: G.R.H. has received funding from Roche Ltd for clinical trial studies and has served as a paid consult for Amgen Inc. The remaining authors declare no competing financial interests.
Correspondence: Kate H. Gartlan, Immunology Department, QIMR Berghofer Medical Research Institute, Level 9 CBCRC, 300 Herston Rd, Herston, QLD 4006, Australia; e-mail: kate.gartlan@qimrberghofer.edu.au.