Key Points
The addition of sorafenib to chemotherapy for older adults with FLT3-ITD mutated AML is safe and may improve overall survival.
Abstract
The Cancer and Leukemia Group B (CALGB), now part of the Alliance for Clinical Trials in Oncology, conducted a multicenter, single-arm, phase 2 study in patients ≥60 years with FMS-like tyrosine kinase 3 (FLT3)–mutated acute myeloid leukemia (AML). In this study, sorafenib was added to daunorubicin and cytarabine-based induction and consolidation chemotherapy and was also continued for 12 months of maintenance therapy. The primary end point of the study was overall survival (OS) at 1 year in the FLT3 internal tandem duplication (FLT3-ITD) cohort. Fifty-four patients with a median age of 67 years (range, 60.3-82.7 years) were enrolled; 39 were FLT3-ITD patients (71%) and 15 were FLT3-TKD (29%) patients. The observed 1-year OS (95% confidence interval [CI]) was 62% (45%-78%) for the FLT3-ITD patients (meeting the primary end point 62% vs 30% for a historical control group, P < .0001) and 71% (42%-92%) for the FLT3-TKD patients. The median disease-free survival and OS were 12.2 months (95% CI, 5-16.9) and 15.0 months (95% CI, 10.4-20.1), respectively, in the FLT3-ITD group and 9.6 (95% CI, 1.9 to not available [NA]) and 16.2 months (95% CI, 5.0 to NA) for the FLT3-TKD group. This study suggests that the addition of sorafenib to chemotherapy for FLT3-ITD AML is feasible and may improve the survival of older adults with FLT3-mutated AML. This trial was registered at www.clinicaltrials.gov as #NCT01253070.
Introduction
The overall outcome of older adults (≥60 years) with acute myeloid leukemia (AML) has changed little over the past few decades. For patients deemed fit for intensive treatment, induction chemotherapy combining an anthracycline and cytarabine is associated with a lower rate of complete remission (CR; 50%-60%) and higher early mortality (up to 20% at 30 days) compared with younger patients.1-3 Intensification of postremission therapy with high-dose cytarabine is poorly tolerated and has not improved outcomes, with a 3-year disease-free survival (DFS) of ∼10% to 15%.4 The reasons for increased toxicity and decreased survival are incompletely understood and include both unfavorable tumor biology and patient-specific characteristics such as increased comorbidities.
FMS-like tyrosine kinase 3 (FLT3) is a type III receptor tyrosine kinase expressed on both normal CD34+ hematopoietic stem and progenitor cells and AML blasts. Mutations of FLT3 are common in AML, occurring in 20% to 30% of adult patients across the entire age spectrum.2 These mutations occur either as an internal tandem duplication (ITD) of the juxtamembrane domain or as a point mutation in the activation loop of the tyrosine kinase domain (TKD), both of which result in ligand-independent activation and signaling that stimulate the proliferation of AML blasts.5-8 The presence of an FLT3 mutation is associated with a poor prognosis in AML, with an increased rate of relapse and reduced OS.7,9-12 In earlier prospective studies by the Cancer and Leukemia Group B (CALGB; now a part of the Alliance for Clinical Trials in Oncology) analyzing outcomes for 219 previously untreated adults ≥60 years with AML treated with chemotherapy alone, the CR rate for the 72 patients with an FLT3-ITD was 67%, compared with 70% for the 147 with wild-type FLT3 (FLT3-WT).13 However, overall outcomes were markedly different. After 1 year, overall survival (OS) was 31% vs 69%, event-free survival (EFS) was 17% vs 40%, and DFS was 25% vs 53% for patients with FLT3-ITD vs FLT3-WT. FLT3 is therefore an attractive target in AML, and several FLT3 inhibitors are currently in clinical development, including midostaurin, quizartinib, and gilteritinib.14,15
Sorafenib (Nexavar) is an oral multikinase inhibitor approved for the treatment of metastatic renal cell and advanced hepatocellular carcinoma.16,17 Sorafenib was initially developed as an inhibitor of RAF1, a serine-threonine kinase in the mitogen activated protein kinase (MAPK) pathway critical in cellular responses such as cell proliferation, growth, motility, survival, and apoptosis. Sorafenib is a potent inhibitor of the FLT3-ITD, with a 50% inhibitory concentration in the single-digit nanomolar range.18 Although FLT3-TKD mutations are resistant to sorafenib,19 sorafenib blocks the autophosphorylation and activation of other tyrosine kinases, including vascular endothelial growth factor, platelet-derived growth factor receptor B, FGFR1, and KIT. Furthermore, inhibition of the MAPK pathway by sorafenib may also contribute to its clinical activity, as MAPK is a downstream target of FLT3 signaling and can be activated by other factors, including mutations in RAS, which are found in ∼15% of patients with AML.
Sorafenib has been tested as a single agent in phase 1 clinical trials for patients with relapsed or refractory AML. Evidence of clinical activity was observed, with CR or complete remission with incomplete hematopoietic recovery (CRi) achieved in 5 (10%) patients and a significant reduction in bone marrow and/or peripheral blood blasts in 17 (34%) additional patients, all of whom had FLT3-ITD mutations.20 Sorafenib has also been tested as a single agent in the compassionate use setting, with case series reporting the development of rapid remissions in patients with FLT3-ITD–mutated relapsed or refractory disease.21,22 Responses were transient, however, with no durable remissions reported. Sorafenib has also been used in combination with idarubicin and cytarabine. In a phase 2 trial, 18 patients with newly diagnosed AML with FLT3-ITD were treated with this regimen, with 17 out of 18 patients (94%) achieving a morphological CR/complete remission with incomplete platelet recovery.23 Phase 3 studies have combined sorafenib with chemotherapy for younger and older adults with AML, with mixed results.24,25 We hypothesized that the addition of sorafenib to induction and postremission therapy in patients >60 years with FLT3-mutated AML would improve the OS of these patients.
Subjects and methods
Description of participants
This CALGB trial (registered at www.clinicaltrials.gov as #NCT01253070) was conducted as a multicenter, single-arm phase 2 study. Eligible subjects were age ≥60 years, diagnosed with AML with a FLT3 mutation, and had no prior chemotherapy for their disease. Subjects with acute promyelocytic leukemia, AML with t(8;21)(q22;q22), RUNXT-RUNX1T1, or inv(16)(p13.1q22), CBFB-MYH11 were excluded from the study. Patients with a history of a myelodysplastic syndrome or myeloproliferative neoplasm were eligible provided that they had not received chemotherapy including lenalidomide, azacitidine, or decitabine for their antecedent hematologic disorder. Institutional review board approval was obtained for the study by all participating institutions.
Treatment plan
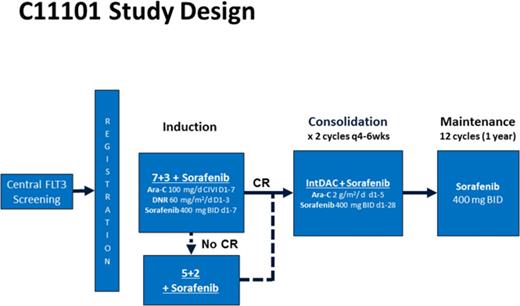
Remission induction therapy consisted of 7+3 (cytarabine 100 mg/m2 per day by continuous intravenous infusion on days 1-7 and daunorubicin 60 mg/m2 per day IV on days 1-3) with sorafenib 400 mg orally every 12 hours on days 1 to 7. A second induction course consisting of 5+2 with sorafenib (cytarabine 100 mg/m2 per day on days 1-5, daunorubicin 60 mg/m2 per day on days 1-2, and sorafenib 400 mg twice daily on days 1-7) was administered to patients not achieving a hypoplastic marrow on a day +14 bone marrow evaluation. Due to a daunorubicin shortage in the United States during the study, substitution of idarubicin 12 mg/m2 day was permitted during induction. Sorafenib for this trial was supplied by the National Cancer Institute and Bayer Pharmaceuticals, Inc.
For patients achieving a CR or CRi, postremission therapy consisted of 2 cycles of intermediate-dose cytarabine 2 g/m2 per day over 3 hours on days 1 to 5, together with sorafenib 400 mg twice daily for 28 days. Maintenance treatment with sorafenib 400 mg twice daily was then administered for up to twelve 28-day cycles. No dose modifications were made for hematologic toxicities during remission induction or consolidation therapy. During maintenance, sorafenib was interrupted and could be dose-reduced for grade 3/4 neutropenia (absolute neutrophil count <1000/μL), thrombocytopenia (platelets <50 000/μL), or clinically significant grade 3/4 nonhematologic toxicities. Patients who achieved a CR/CRi were permitted to undergo an allogeneic hematopoietic cell transplant (allo-HCT) if deemed appropriate by the treating physician. Response to treatment was assessed according to the Revised International Working Group criteria for AML.26
FLT3 analysis
Detection of either the FLT3-ITD or TKD (D835) mutation was performed using standard polymerase chain reaction (PCR) techniques by a Clinical Laboratory Improvement Amendments–approved central laboratory. Genomic DNA was isolated from cells submitted to the Alliance Leukemia Tissue Bank at the Ohio State University using a QIAamp DNA Blood Mini Kit. Exons 14/15 and exon 20 of FLT3 were simultaneously amplified in triplicate with primers described previously.27 For the ITD analysis, PCR was initiated at 95°C for 15 minutes, followed by 35 cycles at 95°C for 30 seconds, 58°C for 55 seconds, 72°C for 90 seconds, and 72°C for 10 minutes. For the TKD D835 mutation, PCR was initiated at 95°C for 15 minutes, followed by 40 cycles at 95°C for 30 seconds, 58°C for 60 seconds, 72°C for 105 seconds, and 72°C for 10 minutes. The TKD amplicons (5 μL) were subjected to digestion with 1 U EcoRV (Invitrogen, Carlsbad, CA) in a 20-μL reaction at 37°C for 1 hour, followed by heat activation at 65°C for 10 minutes. The resultant ITD and EcoRV-digested TKD amplicons were subjected to capillary electrophoresis in a 3130XL Genetic analyzer and analyzed by GeneMapper V4.0 (Applied Biosystems, Foster City, CA). The lower limit of detection was 2.6% (2.169% ± 0.94%) and 4.4% (4.384% ± 0.40%) for ITD and TKD, respectively. The cutoff for ITD and TKD for this clinical trial was 5.0%. The allelic ratio was defined as FLT3-mutant/FLT3-WT.
Plasma inhibitory assays (PIAs) were performed as previously described.28 Briefly, Molm-14 cells, which harbor an FLT3-ITD mutation, were incubated with patient plasma and subjected to antiphosphotyrosine immunoblotting. Densitometry was performed, and the PIA was calculated by expressing the density as percent decrease from the baseline pretreatment sample.
Statistical considerations
The trial was designed as a 1-stage single-arm phase 2 study. The primary end point of the study was the 1-year OS rate in the FLT3-ITD AML subgroup. Historically, patients in this subgroup who were not treated with sorafenib had CR rates of 67% and 1-year OS rates of 31%.13 The study was designed to detect an improvement in the 1-year OS rate from 30% to 50% using a 1-sided type I error rate of 10%. A sample size of 39 evaluable patients with newly diagnosed FLT3-ITD AML was required based on these assumptions. This sample size also provided 90% power to detect an improvement in the complete remission rates from 67% to 82%.
OS was defined as the time from registration to death from any cause. EFS was defined as the time from registration to failure to achieve CR during induction, relapse after achieving a CR, or death from any cause. DFS was defined for patients who achieved a complete response during induction as the time from CR or CRi to relapse after achieving a CR or CRi or death from any cause. Patients who were event-free for each time-to-event end point were censored at their time of last follow-up. All time-to-event end points were analyzed using the method of Kaplan-Meier. Univariable Cox proportional hazards models for time-to-event end points (OS, DFS, and EFS) were performed to understand the impact of sex, age, race/ethnicity, FLT3 status, and European LeukemiaNet classification on outcomes. All P values reported are 2 sided, except for the primary efficacy analyses, which are 1 sided following the design of the study.
Results
FLT3 mutation screening
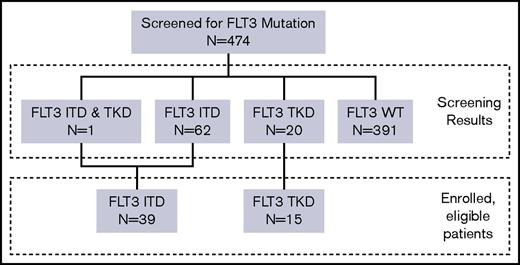
Data were frozen for this analysis as of May 2016. All patients were off active treatment at the time of this analysis. A total of 474 older adults were screened for FLT3 mutations through the central laboratory. The median turn-around time for FLT3 testing from receipt of sample was 46 hours, and 99.1% of cases were returned within 48 hours. FLT3 mutations were identified in 83 subjects (17.5%), including 62 (13.1%) with FLT3-ITD, 20 (4.2%) with FLT3-TKD, and 1 (0.2%) with both ITD and TKD (Figure 1). Between April 2011 and August 2013, 54 patients (39 with FLT3-ITD and 15 with FLT3-TKD) enrolled in the study. This study used a common CALGB screening protocol that enabled patients who were subsequently considered unsuitable for intensive therapy by their treating physician to enroll in an alternative CALGB clinical trial.
Patient characteristics
Baseline characteristics are summarized in Table 1. The median age of the study cohort was 67 years, and 37% of patients (n = 20) were ≥70 years. The majority of patients (85%; n = 46) had de novo disease and either normal or intermediate-risk cytogenetics according to European LeukemiaNet classification.29 The median FLT3 allelic ratio was 0.7 (range, 0.1-7.3) for patients with FLT3-ITD and 0.3 (range, 0.1-2.1) for patients with FLT3-TKD.
Baseline characteristics
| . | FLT3 ITD (n = 39) . | FLT3 TKD (n = 15) . | Total (N = 54) . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 67.6 (60.3-82.7) | 65.4 (60.8-82.1) | 67.4 (60.3-82.7) |
| ≥70 | 17 (44) | 3 (20) | 20 (37) |
| Sex | |||
| Male | 20 (51) | 10 (67) | 30 (56) |
| Female | 19 (49) | 5 (33) | 24 (44) |
| Onset of AML | |||
| De novo | 32 (82) | 14 (93) | 46 (85) |
| Therapy-related myeloid | 2 (5) | 1 (7) | 3 (6) |
| MDS related | 5 (13) | 0 (0) | 5 (9) |
| ELN classification | |||
| Intermediate I/CN-AML | 21 (54) | 6 (40) | 27 (50) |
| Intermediate II | 12 (31) | 5 (33) | 17 (32) |
| ELN adverse | 1 (3) | 2 (13) | 3 (6) |
| Cytogenetics insufficient for evaluation | 5 (13) | 2 (13) | 7 (13) |
| Allelic ratio | |||
| Median (range) | 0.7 (0.1-7.3) | 0.3 (0.1-2.1) | |
| WBC count | |||
| Median (range) | 22 (0.8-344) | 16 (0.7-60) | 18 (0.7-344) |
| Bone marrow blast, % | |||
| Median (range)* | 68 (0-96) | 58 (31-96) | 60 (0-96) |
| ECOG performance status | |||
| 0 | 18 (46) | 5 (33) | 23 (43) |
| 1 | 17 (44) | 10 (67) | 27 (50) |
| 2 | 4 (10) | 0 | 4 (7) |
| . | FLT3 ITD (n = 39) . | FLT3 TKD (n = 15) . | Total (N = 54) . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 67.6 (60.3-82.7) | 65.4 (60.8-82.1) | 67.4 (60.3-82.7) |
| ≥70 | 17 (44) | 3 (20) | 20 (37) |
| Sex | |||
| Male | 20 (51) | 10 (67) | 30 (56) |
| Female | 19 (49) | 5 (33) | 24 (44) |
| Onset of AML | |||
| De novo | 32 (82) | 14 (93) | 46 (85) |
| Therapy-related myeloid | 2 (5) | 1 (7) | 3 (6) |
| MDS related | 5 (13) | 0 (0) | 5 (9) |
| ELN classification | |||
| Intermediate I/CN-AML | 21 (54) | 6 (40) | 27 (50) |
| Intermediate II | 12 (31) | 5 (33) | 17 (32) |
| ELN adverse | 1 (3) | 2 (13) | 3 (6) |
| Cytogenetics insufficient for evaluation | 5 (13) | 2 (13) | 7 (13) |
| Allelic ratio | |||
| Median (range) | 0.7 (0.1-7.3) | 0.3 (0.1-2.1) | |
| WBC count | |||
| Median (range) | 22 (0.8-344) | 16 (0.7-60) | 18 (0.7-344) |
| Bone marrow blast, % | |||
| Median (range)* | 68 (0-96) | 58 (31-96) | 60 (0-96) |
| ECOG performance status | |||
| 0 | 18 (46) | 5 (33) | 23 (43) |
| 1 | 17 (44) | 10 (67) | 27 (50) |
| 2 | 4 (10) | 0 | 4 (7) |
Data are presented as n (%) of patients unless otherwise indicated.
CN, cytogenetically normal; ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet; MDS, myelodysplastic syndrome; WBC, white blood cell.
One patient was enrolled with multiple myeloid sarcomas but no increase in bone marrow blasts.
Remission induction
Following 1 or 2 courses of induction chemotherapy, 74% of patients (40 of 54) achieved a CR. No patient had a CRi. A total of 31 patients (57%) achieved CR after the first induction course, and 9 (17%) required a second cycle of induction chemotherapy. The rate of CR was similar between patients with FLT3-ITD (74%; 95% confidence interval [CI], 58%-87%) and FLT3-TKD (73%; 45%-92%). The CR rate by age categorization was 86% for patients 60 to 69 years (19 of 22) and 59% for those ≥70 years (10 of 17) in the FLT3-ITD subgroup; the CR rate was 67% for patients 60 to 69 years (8 of 12) vs 100% for those ≥70 years (3 of 3) in the FLT3TKD subgroup. Five deaths (9%) occurred during the initial 30 days of treatment. All early deaths occurred in subjects aged ≥69 years (range, 69-81 years).
Consolidation and maintenance
Of the 40 patients who achieved a CR, 33 received consolidation with intermediate-dose cytarabine plus sorafenib. Subsequently, 21 patients (16 with FLT3-ITD and 5 with FLT3-TKD) started maintenance therapy, receiving a median of six 28-day cycles of therapy (range, 1-12 cycles). During sorafenib maintenance, the most commonly reported treatment-related adverse events were grade 1 diarrhea, fatigue, and transaminitis and grade 2 palmar-plantar erythrodysesthesia. Three patients discontinued sorafenib secondary to adverse events (including 2 cases involving a rash).
Survival
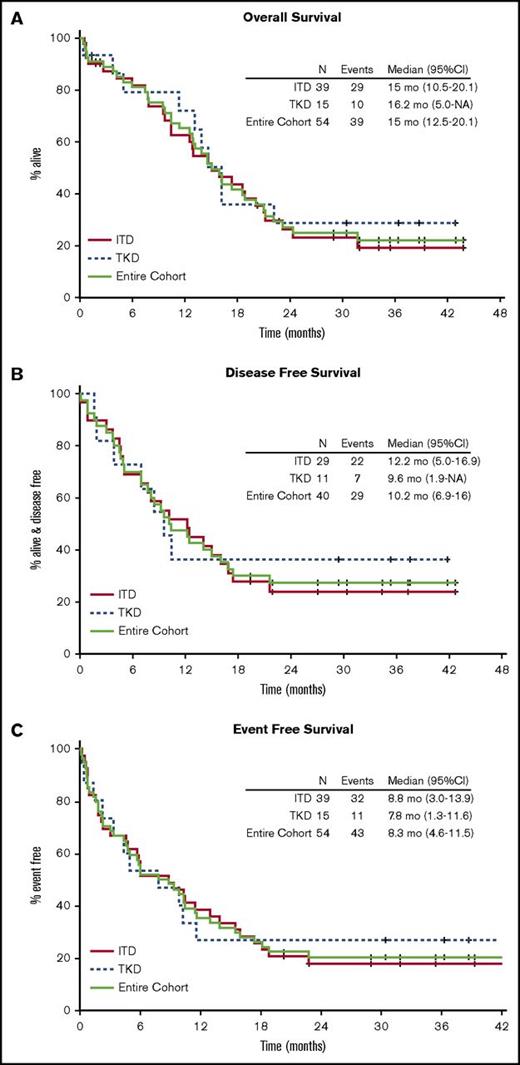
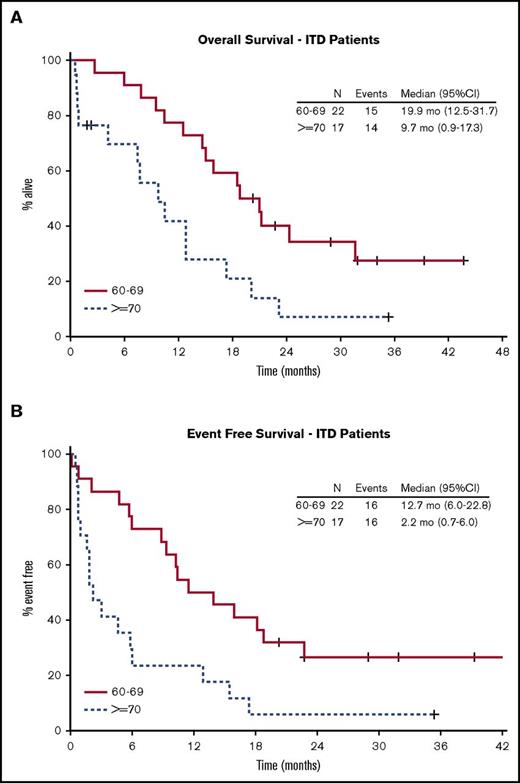
With a median follow-up of 34.8 months for surviving patients, the Kaplan-Meier estimate for median OS was 15 (range, 10.4-20.1) months for patients with FLT3-ITD and 16.2 (range, 5.0 to not available [NA]) months for FLT3-TKD (Figure 2A). The observed 1-year OS rate was 62% (95% CI, 45%-78%) in the FLT3-ITD group and 71% (95% CI, 42%-92%) in the FLT3-TKD group. This study thus met the protocol-defined criteria for improvement in the 1-year OS rate (62% vs 30% [our historical experience]; 1-sided P < .0001). The median and 1-year DFS were 12.2 months (95% CI, 5.0-16.9) and 52% (95% CI, 36%-74%) for FLT3-ITD patients and 9.6 months (95% CI, 1.9 to NA) and 36% (95% CI, 17%-80%) for FLT3-TKD patients (Figure 2B). For ITD and TKD patients, the median EFS was 8.8 months (95% CI, 3.0-13.9) and 7.8 months (95% CI, 1.3-11.6), respectively, with a 1-year EFS of 39% (95% CI, 26-57) and 27% (95% CI, 12-62), respectively (Figure 2C). For all subjects, the 2-year OS and EFS rates were 27% (95% CI, 17%-43%) and 20% (95% CI, 12%-34%), respectively. In univariable Cox regression analysis of the FLT3-ITD population, only age ≥70 years was associated with statistically significant differences in OS (hazard ratio [HR], 2.6; 95% CI, 1.2-5.5; P = .01), DFS (HR, 2.5; 95% CI, 1.05-5.9; P = .04), and EFS (HR, 2.7; 95% CI, 1.3-5.5; P = .01) (Table 2; Figure 3).
Overall outcomes of FLT3-mutated AML treated with sorafenib. Kaplan-Meier curves for OS (A), DFS (B), and EFS (C).
Overall outcomes of FLT3-mutated AML treated with sorafenib. Kaplan-Meier curves for OS (A), DFS (B), and EFS (C).
Univariable Cox proportional hazards models for OS, EFS, and DFS for the FLT3-ITD subgroup
| . | n (events) . | HR . | 95% CI . | 2-sided P value . |
|---|---|---|---|---|
| OS | ||||
| Race/ethnicity (non-Hispanic white vs other) | 39 (29) | 0.538 | 0.226-1.280 | .16 |
| Sex (female vs male) | 39 (29) | 1.289 | 0.620-2.682 | .50 |
| Age (≥70 y vs <70 y) | 39 (29) | 2.600 | 1.237-5.469 | .01 |
| ELN risk group (CN-AML vs intermediate II) | 34 (24) | 1.230 | 0.504-3.002 | .65 |
| ELN risk group (ELN adverse vs intermediate II) | 34 (24) | 1.764 | 0.211-14.782 | .60 |
| Allelic ratio (≥0.7 vs <0.7) (cutoff at median) | 39 (29) | 1.793 | 0.845-3.807 | .13 |
| EFS | ||||
| Race/ethnicity (non-Hispanic white vs other) | 39 (32) | 0.766 | 0.327-1.787 | .54 |
| Sex (female vs male) | 39 (32) | 1.338 | 0.665-2.691 | .41 |
| Age (≥70 y vs <70 y) | 39 (32) | 2.738 | 1.340-5.596 | .01 |
| ELN risk group (CN-AML vs intermediate II) | 34 (27) | 1.302 | 0.565-3.00 | .54 |
| ELN risk group (ELN adverse vs intermediate II) | 34 (27) | 3.789 | 0.441-32.554 | .22 |
| Allelic ratio (≥ 0.7 vs < 0.7) (cutoff at median) | 39 (32) | 1.665 | 0.823-3.366 | .16 |
| DFS | ||||
| Race/ethnicity (non-Hispanic white vs other) | 29 (22) | 0.426 | 0.167-1.082 | .07 |
| Sex (female vs male) | 29 (22) | 1.466 | 0.631-3.405 | .37 |
| Age (≥70 y vs <70 y) | 29 (22) | 2.479 | 1.046-5.876 | .04 |
| ELN risk group (CN-AML vs intermediate II)* | 25 (18) | 2.105 | 0.691-6.410 | .19 |
| Allelic ratio (≥0.7 vs <0.7) (cutoff at median) | 29 (22) | 1.247 | 0.537-2.897 | .61 |
| . | n (events) . | HR . | 95% CI . | 2-sided P value . |
|---|---|---|---|---|
| OS | ||||
| Race/ethnicity (non-Hispanic white vs other) | 39 (29) | 0.538 | 0.226-1.280 | .16 |
| Sex (female vs male) | 39 (29) | 1.289 | 0.620-2.682 | .50 |
| Age (≥70 y vs <70 y) | 39 (29) | 2.600 | 1.237-5.469 | .01 |
| ELN risk group (CN-AML vs intermediate II) | 34 (24) | 1.230 | 0.504-3.002 | .65 |
| ELN risk group (ELN adverse vs intermediate II) | 34 (24) | 1.764 | 0.211-14.782 | .60 |
| Allelic ratio (≥0.7 vs <0.7) (cutoff at median) | 39 (29) | 1.793 | 0.845-3.807 | .13 |
| EFS | ||||
| Race/ethnicity (non-Hispanic white vs other) | 39 (32) | 0.766 | 0.327-1.787 | .54 |
| Sex (female vs male) | 39 (32) | 1.338 | 0.665-2.691 | .41 |
| Age (≥70 y vs <70 y) | 39 (32) | 2.738 | 1.340-5.596 | .01 |
| ELN risk group (CN-AML vs intermediate II) | 34 (27) | 1.302 | 0.565-3.00 | .54 |
| ELN risk group (ELN adverse vs intermediate II) | 34 (27) | 3.789 | 0.441-32.554 | .22 |
| Allelic ratio (≥ 0.7 vs < 0.7) (cutoff at median) | 39 (32) | 1.665 | 0.823-3.366 | .16 |
| DFS | ||||
| Race/ethnicity (non-Hispanic white vs other) | 29 (22) | 0.426 | 0.167-1.082 | .07 |
| Sex (female vs male) | 29 (22) | 1.466 | 0.631-3.405 | .37 |
| Age (≥70 y vs <70 y) | 29 (22) | 2.479 | 1.046-5.876 | .04 |
| ELN risk group (CN-AML vs intermediate II)* | 25 (18) | 2.105 | 0.691-6.410 | .19 |
| Allelic ratio (≥0.7 vs <0.7) (cutoff at median) | 29 (22) | 1.247 | 0.537-2.897 | .61 |
CN, cytogenetically normal; ELN, European LeukemiaNet.
No patients were ELN adverse.
Kaplan-Meier curves for OS and EFS for FLT3-ITD patients by age. (A) Patients aged 60 to 69 years. (B) Patients aged ≥70 years.
Kaplan-Meier curves for OS and EFS for FLT3-ITD patients by age. (A) Patients aged 60 to 69 years. (B) Patients aged ≥70 years.
Effect of allo-HCT
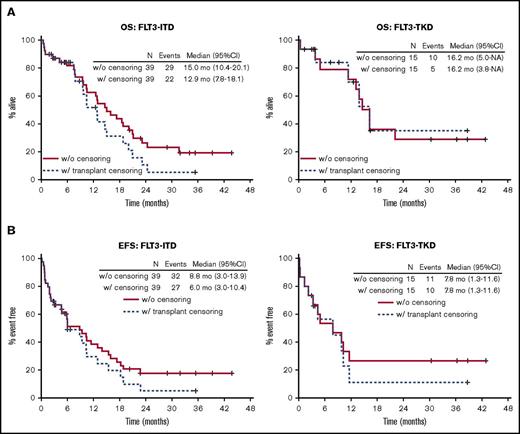
In this study, 22 patients (14 with FLT3-ITD [36%] and 8 with FLT3-TKD [53%]) underwent allo-HCT; 18 of the transplants occurred during CR1 (13 ITD and 5 TKD), 2 were performed in CR2 (2 TKD), and 2 (1 ITD and 1 TKD) were performed during first relapse. The median time to transplant for all patients was 164 days (range, 82-802). In an attempt to separate the effect of allo-HCT on patient outcomes, we conducted a secondary sensitivity analysis in which we censored subjects at the time of transplant (Figure 4A-B). After censoring at the time of transplant, the 1-year rates of OS, DFS, and EFS for the FLT3-ITD population were 52% (95% CI, 35%-76%), 40% (95% CI, 22%-71%), and 29% (95% CI, 16%-54%), respectively, and 70% (95% CI, 45%-100%), 16% (95% CI, 3%-96%), and 11% (95% CI, 2%-70%), respectively, for the FLT3-TKD population.
Sensitivity analysis for effect of allo-HCT. Kaplan-Meier curves for OS (A) and EFS (B) with and without censoring for allo-HCT by FLT3-mutated subgroups.
Sensitivity analysis for effect of allo-HCT. Kaplan-Meier curves for OS (A) and EFS (B) with and without censoring for allo-HCT by FLT3-mutated subgroups.
FLT3 PIAs
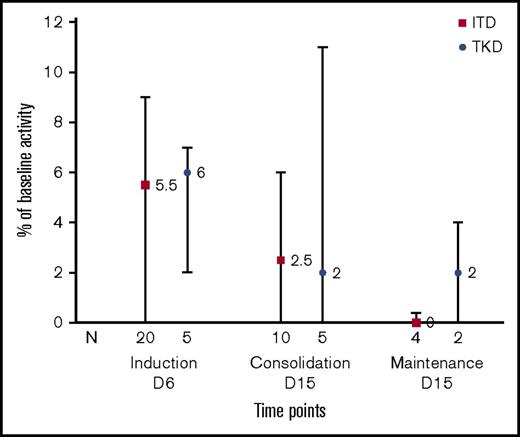
To measure the degree of FLT3 inhibition occurring in vivo during treatment with sorafenib, we measured the PIA for FLT3 in patient plasma samples. The PIA is a surrogate for measuring target inhibition by FLT3 inhibitors.28 In this study, the median result of this assay (the percentage of baseline FLT3 autophosphorylation) in all patients was 6% of pretreatment baseline activity (range, 0-32) (n = 25) on day 6 of induction, 2% (range, 0-24) (n = 15) on day 15 of consolidation, and 0% (range, 0-4) (n = 6) on day 15 of maintenance, demonstrating robust inhibition of FLT3 in vivo (Figure 5).
FLT3 PIA. PIA was measured on day +6 of induction, cycle 1 day +15 of consolidation therapy, and cycle 1 day +15 of maintenance sorafenib. Results are expressed as percent change in FLT3 autophosphorylation from baseline. Bars plot median and interquartile range for subjects with FLT3-ITD or FLT3-TKD.
FLT3 PIA. PIA was measured on day +6 of induction, cycle 1 day +15 of consolidation therapy, and cycle 1 day +15 of maintenance sorafenib. Results are expressed as percent change in FLT3 autophosphorylation from baseline. Bars plot median and interquartile range for subjects with FLT3-ITD or FLT3-TKD.
Discussion
In this paper, we describe the outcome of older adults with FLT3-mutated AML treated with the combination of sorafenib and chemotherapy. Our data suggest an improvement for both OS and DFS and compare favorably to the outcomes we observed for older FLT3-ITD–mutated AML patients in previous CALGB clinical trials. The potential benefit was primarily observed in individuals aged 60 to 69 years who had an EFS and OS of 12.7 and 19.9 months, respectively, compared with 2.2 and 9.7 months for those ≥70 years. Moreover, toxicities were not increased above those expected from a 7+3 regimen in older adults.
When comparing this study to historical CALGB data, there are several important limitations in the interpretation of these results. As the role of allo-HCT in this population evolves, the rates of transplantation for both older adults and those with FLT3-mutated AML have increased. Increased rates of transplantation combined with overall improvements in reducing treatment-related mortality through better supportive care may partly account for the improvement in OS. However, when we censored the analysis for transplantation and examined the EFS of the population, these current results still compare favorably to the median EFS of 0.4 years and 1-year EFS of 17% (95% CI, 9-26) observed in earlier CALGB studies.11 This suggests that differences in outcome remain and that the improved outcomes may be in part due to a reduction in relapse from the addition of sorafenib.
Previous studies of FLT3 inhibitors in AML have had mixed results. Agents such as midostaurin possess modest activity against FLT3 and are relatively nonspecific. As single agents, these drugs have demonstrated only transient decreases in blast counts, which is consistent with the finding that mutations in FLT3 occur relatively late in leukemogenesis and frequently present in a subclone. In a randomized phase 3 study of lestaurtinib conducted in relapsed and refractory AML patients, measurement of FLT3 inhibition by PIA was highly correlated with remission rate, but target inhibition on day 15 to <15% of baseline activity was achieved in only 58% of patients receiving lestaurtinib.30 In the current study, we observed a median inhibition of >90%, which was sustained throughout induction and consolidation. These earlier studies paved the way for more potent inhibitors such as sorafenib and also support the rationale for combining these agents with chemotherapy.
Recently, the Study Alliance Leukemia (SAL) in Germany reported the results of 2 randomized, placebo-controlled, phase 2 studies of sorafenib plus chemotherapy in newly diagnosed AML patients regardless of FLT3 mutation status. In the SORAML study conducted in younger adults (<60 years old), the median EFS improved from 9 months to 21 months in the sorafenib group, corresponding to a 3-year EFS of 22% in the placebo group vs 40% in the sorafenib group (P = .013).24 This study aimed to transplant every eligible patient, and there was no significant difference in OS. In addition, the benefit was not restricted to the FLT3-ITD cohort. In contrast, in a second trial designed for older adults (age >60 years), the addition of sorafenib did not result in improvement in either EFS or OS.25 Notably, in older adults, the combination of sorafenib and chemotherapy was associated with an increased risk of early death (17% vs 7%). In both studies, no differences were observed in outcome based on FLT3 mutation status. However, the power to detect a difference in the older adult study may be limited due to the relatively low numbers of FLT3-ITD patients in that study (n = 28).
Several factors may account for the differences between our current study and the SAL studies. First, the induction mortality of 9.3% observed in the current study was less than the 17% observed in the older adult SAL sorafenib study. In the older adult SAL study, sorafenib was administered from day 10 of induction until 3 days before the first day of the subsequent chemotherapy course, compared with only on days 1 to 7 of induction in our study. It is possible that prolonged exposure to sorafenib during induction chemotherapy may account for the increased early mortality. Second, the beneficial effect in the SORAML study was observed irrespective of the FLT3 genotype. Recently, CALGB study 10603 (RATIFY) demonstrated that the addition of midostaurin to standard chemotherapy for younger adults (<60 years) with FLT3-mutated AML results in a significant survival advantage over a placebo control in a phase 3 trial.31 Whether the activity of sorafenib and other FLT3 inhibitors in AML is specifically due to effects on FLT3 or on other kinase targets remains an open question.
Our understanding of the role of FLT3 mutations in AML continues to evolve. As more comprehensive genomic studies are performed on patients with AML, it has become clear that AML is a dynamic disease with mutations variably distributed between founding clones and subclones.32 Mutations in FLT3 frequently occur in a subclone and also co-occur with other mutations such as NPM1 and DNMT3A. This can result in complex interactions that influence clinical outcomes.33,34 Integrating genomic information both at diagnosis and throughout therapy will be critical to understand the impact of treatments such as FLT3 inhibitors.
In conclusion, our study demonstrates the feasibility of conducting genotype-directed trials in the initial treatment of AML in the US cooperative groups. We were able to deliver a rapid turnaround time, with results returned to the treating physician within 48 hours, allowing this study to be conducted without significantly delaying therapy.
Older adults have been an attractive patient group for the development of novel therapies in AML due the historically poor outcomes and the large need unmet by currently available therapies. With any new agent, the potential for increased toxicity by adding the agent to a standard regimen may negate any clinical benefits. This appears to be particularly true for older adults, for whom the safety margin for a 7+3 regimen is low. Nevertheless, this study clearly adds to the growing literature supporting the addition of tyrosine kinase inhibitors to chemotherapy in AML and justifies further testing in a randomized phase 3 study.
Acknowledgments
Michael J. Kelly and Samantha Sublett served as protocol coordinators. Scott Smith served as the Executive Officer for the Alliance Leukemia Committee.
This study was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology); U10CA180833, U10CA180836, U10CA180850, U10CA180867, and K23CA140707 (G.L.U.); and P50CA100632 (M.J.L.). Additional support funded in part by the National Cancer Institute grants U10CA031983, U10CA032291, U10CA033601, U10CA003927, U10CA041287, and U10CA077440.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions participated in this study: Dana-Farber/Partners CancerCare Lead Academic Participating Site (LAPS) (Boston, MA), Harold Burstein (U10CA032291 and U10CA180867); Dartmouth College, Norris Cotton Cancer Center LAPS (Lebanon, NH), Konstantin Dragnev (U10CA004326 and U10CA180854); Delaware/Christiana Care NCI Community Oncology Research Program (Newark, DE), Gregory Masters (U10CA045418 and UG1CA189819); Eastern Maine Medical Center Cancer Care (Brewer, ME), Thomas Openshaw; Northwell Health NCORP (Manhasset, NY), Daniel Budman (U10CA035279 and UG1CA189850); The Ohio State University Comprehensive Cancer Center LAPS (Columbus, OH), Richard Goldberg (U10CA077658 and U10CA180850); Southeast Clinical Oncology Research Consortium NCORP (Winston-Salem, NC), James Atkins (U10CA045808 and UG1CA189858); University of Chicago Comprehensive Cancer Center LAPS (Chicago, IL), Hedy Kindler (U10CA041287 and U10CA180836); University of Maryland/Greenebaum Cancer Center (Baltimore, MD), Martin Edelman (U10CA031983); University of Vermont College of Medicine (Burlington, VT), Claire Verschraegen (10CA077406); VCU Massey Cancer Center Minority Underserved NCORP (Richmond, VA), Steven Grossman (U10CA052784 and UG1CA189869); Wake Forest University Health Sciences (Winston-Salem, NC), Heidi Klepin (U10CA003927); Washington University, Siteman Cancer Center LAPS (Saint Louis, MO), Nancy Bartlett (U10CA077440 and U10CA180833); and Weill Medical College of Cornell University (New York, NY), Scott Tagawa (U10CA007968).
Authorship
Contribution: G.L.U. and R.A.L. were responsible for study conception and design; G.L.U., G.M., W.Z., M.J.L., H.D.K., M.R.B., B.L.P., P.W., D.J.D., W.S., W.G.B., C.D.B., R.M.S., and R.A.L. acquired data; G.L.U., R.A.L., S.J.M., K.L., and B.S. analyzed and interpreted the data; G.L.U. drafted the manuscript; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Geoffrey L. Uy, Division of Oncology, Washington University School of Medicine, 660 S Euclid Ave, CB 8007, St. Louis, MO 63110; e-mail: guy@wustl.edu.
References
Competing Interests
Presented in abstract form at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5-8 December 2015.