Key Points
S100A9+ neutrophils accumulated prominently in the central area of granulomas in humans and guinea pigs.
Granuloma formation was markedly impaired by a treatment with the S100A9 inhibitor, tasquinimod.
Abstract
Macrophages have the potential to undergo cellular transformation into epithelioid cells, and their concentric accumulation in tissues results in the development of granulomas. Although epithelioid cells are an essential and dominant component of granulomas, other cell types have also been detected, which may contribute to the establishment of well-organized granulomas, as observed in human granulomatous diseases. We herein demonstrated that neutrophils may mediate these functions. By taking advantage of the guinea pig pulmonary granuloma model, we obtained a rat monoclonal antibody with unique reactivity to granuloma cells. This antibody, termed G213, reacted with clusters of neutrophils located in the central area of granulomas, and a biochemical analysis identified the G213-reactive antigen as S100A9, a calcium-binding protein of the S100 family, which was expressed abundantly in neutrophils. Consistent with the multifaceted functions attributed to S100A9, including its role in neutrophil extravasation and macrophage activation, the blockade of S100A9 functions with the specific inhibitor, tasquinimod, impaired the formation of organized granulomas with neutrophil cores. These results demonstrate the critical role of neutrophils and the S100A9 protein in granuloma formation. Because intragranuloma S100A9+ neutrophils were also detected in humans, these results indicate the potential of tasquinimod, a new anticancer drug candidate, for manipulating human granulomatous diseases.
Introduction
Acute inflammatory responses in tissues generally resolve within days when irritants are removed from the site of inflammation.1 However, tissue responses may persist in some types of disease-associated inflammation, resulting in the formation of organized, compact aggregates of epithelioid cells, specifically referred to as granulomas.2 Epithelioid cells are activated macrophages that exhibit a morphological resemblance to epithelial cells due to their increased cytoplasmic size and tight membrane interactions with adjacent cells. Besides epithelioid cells, multinucleated giant cells are often detected in granulomas, which are considered to arise as a consequence of the sequential fusion events of macrophages.2,3 The emergence of these transformed macrophages indicates dynamic macrophage activation that persistently occurs within granulomas and is a histopathological hallmark of granulomas formed in tuberculosis, sarcoidosis, Crohn disease, and other human granulomatous disorders.4-7 Therefore, macrophages have been the central target of granuloma research and studied extensively. Nevertheless, the key molecules and cell types that potently elicit and orchestrate the organized collection of macrophages have not yet been identified.
In addition to macrophage-derived epithelioid cells and multinucleated giant cells, other myeloid and nonmyeloid cells have also been detected in granulomas.4,8-10 Of these, neutrophils are the most common and prominently detected cell type in granulomas9,10 ; however, due to their various functions, which are known to be executed in acute, rather than chronic inflammation, the contribution of neutrophils to the formation of granulomas has not yet been elucidated in detail. In the present study, we found that neutrophils expressing the marker protein, S100A9, accumulated in the central part of granulomas generated in vivo in the bacille Calmette-Guérin (BCG)-challenged guinea pig lung. Furthermore, the blockade of S100A9 functions with the specific inhibitor, tasquinimod,11 markedly impaired the formation of organized granulomas. The accumulation of S100A9+ neutrophils in granulomas was also observed in patients with tuberculosis, suggesting their role in human granulomatous diseases. These results demonstrate for the first time the critical role of neutrophils and the S100A9 protein in the formation of granulomas.
Methods
Human tissue samples
Formalin-fixed, paraffin-embedded tissue samples were obtained from the Kyoto University Hospital Archives. This study was approved by the Ethics Committee of the Graduate School of Medicine, Kyoto University. Informed consent was obtained from all patients.
Guinea pig granuloma model
Animal experiments were performed in accordance with institutional guidelines on animal welfare and were approved by the Committee of the Kyoto University Animal Experimentation. Three-week-old female Hartley guinea pigs were purchased from Japan SLC, Inc (Shizuoka, Japan) and housed under specific pathogen-free conditions. Guinea pigs received an intradermal injection of the vaccine strain BCG Tokyo 172 (1 × 108 colony-forming units per animal) for sensitization, and after 6 weeks, an IV injection of BCG (1 × 108 colony-forming units per animal) was performed to induce the formation of granulomas in the lungs. In some experiments, 6 hours before the IV injection of BCG, animals received an intraperitoneal injection of tasquinimod (5 mg per kg body weight) in 10% dimethyl sulfoxide, followed by additional tasquinimod injections every 2 days throughout the granuloma induction period. Formalin-fixed, paraffin-embedded tissue samples derived from Mycobacterium tuberculosis–infected guinea pigs were provided by the Research Institute of Tuberculosis (Kiyose, Tokyo, Japan).
Generation of monoclonal antibodies (mAbs) reactive to granuloma cells
Individual granulomas were surgically isolated from the lungs and emulsified in complete Freund’s adjuvant (Difco, Detroit, MI), followed by an intradermal injection into 8-week-old female Wister rats (Japan SLC, Inc). Three weeks after the immunization, cells were obtained from the draining lymph nodes and fused with SP2/0 myeloma cells using the standard polyethylene glycol method.12 Hybridomas were selected with aminopterin-containing medium, and culture supernatants were screened for their reactivity to granuloma cells by the indirect immunoperoxidase staining of frozen tissue sections. Selected hybridoma cells were subcloned by limiting dilution, and ascites was produced by an intraperitoneal injection of the hybridoma cells into pristane-treated BALB/c-nu/nu mice (Japan SLC, Inc). G213 mAb (rat immunoglobulin G [IgG]2a, κ) was purified by affinity chromatography using the protein G Sepharose column (GE Healthcare, Little Chalfont, United Kingdom) according to the manufacturer’s instructions.
Histochemistry
Chemical reagents were purchased from Nacalai Tesque (Kyoto, Japan) unless otherwise indicated. Guinea pig lungs were isolated, fixed overnight at 4°C with 4% paraformaldehyde, and deep frozen in optimal cutting temperature compound (Sakura Finetechnical, Tokyo, Japan). Cryosections were treated with 1.5% hydrogen peroxide in methanol at room temperature for 30 minutes and incubated at 4°C overnight with either G213 mAb or isotype-matched control Ab (Biolegend, San Diego, CA) at a concentration of 1 μg/mL. Labeled cryosections were then incubated with biotinylated secondary antibodies (Abs; Dako, Carpinteria, CA), followed by chromogenic reactions with 3,3′-diaminobenzidine (DAB) using the ABC horseradish peroxidase (HRP) kit (Vector Laboratories, Burlingame, CA). Tissue samples were counterstained with hematoxylin (Wako Pure Chemical Industries, Osaka, Japan). In experiments to evaluate the effect of tasquinimod on granuloma formation, 2 comparable sections were prepared from the left upper lobe of the lung of each animal, and after staining with hematoxylin and eosin, granulomas with a diameter of 200 μm or more were counted. Some cryosections were also labeled with G213 mAb as described above, and granulomas containing the central cluster of S100A9+ neutrophils were counted.
In order to detect the expression of the S100A9 protein in human pathological samples, formalin-fixed, paraffin-embedded tissue sections were labeled with either rabbit anti-human S100A9 mAb (clone EPR3555; Abcam, Cambridge, United Kingdom) at a dilution of 1:250 or control Ab (clone DA1E; Cell Signaling Technology, Beverly, MA), followed by HRP-DAB–based detection as described above. Stained sections were viewed under the Keyence BZ-X710 microscope (Keyence, Osaka, Japan).
Immunogold-labeled electron microscopy
Lungs isolated from BCG-challenged guinea pigs were fixed with 4% paraformaldehyde and 0.1% glutaraldehyde (Wako Pure Chemical Industries) at 4°C for 4 hours. Sixty-micrometer-thick sections were prepared and incubated with G213 mAb, followed by the 1.4-nm gold particle–conjugated Fab′ fragment of anti-rat IgG Abs (Nanoprobes, Yaphank, NY). Labeled sections were then postfixed in 1% glutaraldehyde, and the attached gold particles were silver-intensified with the HQ silver kit (Nanoprobes). Sections were subsequently placed in 1% osmium tetroxide, dehydrated, and flat-embedded in epoxy resin. Ultrathin sections were prepared from resin-embedded sections using an ultramicrotome (Leica, Heidelberg, Germany) and viewed under an H-7650 electron microscope (Hitachi, Tokyo, Japan).
Immunoblotting and immunoprecipitation
Cells were lysed with 0.5% Triton X-100 in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and a protease inhibitor cocktail [Sigma-Aldrich, St. Louis, MO]) on ice for 30 minutes. Following the removal of insoluble material by centrifugation, cellular proteins (50 μg/lane) were resolved on 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels under reducing conditions and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). The membranes were then incubated with G213 ascites at a dilution of 1:400, followed by peroxidase-conjugated anti-mouse/rat IgG Abs (Jackson ImmunoResearch Laboratories, West Grove, PA). Luminol-based detection was conducted with the ECL Western Blotting Detection Reagent (GE Healthcare) according to the manufacturer’s instructions, and signals were analyzed using the LAS-4000mini image analyzer (GE Healthcare). In order to detect β-actin, all of the attached reagents were removed from the PVDF membranes and reprobed with polyclonal Abs to β-actin (Cell Signaling Technology), followed by peroxidase-conjugated anti-rabbit IgG Abs (Jackson ImmunoResearch Laboratories). In immunoprecipitation experiments, cell lysates were incubated with either G213 or an isotype-matched rat mAb at 4°C for 1 hour, followed by incubation with protein G–Sepharose beads for an additional 1 hour. The immunoprecipitation samples were suspended in an SDS sample buffer containing 1% dithiothreitol (APRO Life Science Institute, Tokushima, Japan) and boiled at 99°C for 5 minutes, followed by resolution on SDS-PAGE and western blotting as described above.
Expression plasmids and transfection
Total RNA was extracted from guinea pig bone marrow cells, and oligo(dT)-primed reverse transcription was performed with PrimeScript reverse transcriptase (Takara Bio, Inc, Otsu, Japan) as described previously.13 Full-length complementary DNAs (cDNAs) encoding S100A8 and S100A9 were obtained by polymerase chain reaction using bone marrow–derived cDNAs as a template. The primers used were: 5′-GCG CTC GAG CTG ACT GAA CTG GAG AAA GCC-3′ (sense) and 5′-ATA GCG GCC GCC TCT ACT AGT GTT TGT GGC-3′ (antisense) for S100A8, and 5′-ACG CTC GAG GCA GCC AAT AAG TCG CAG CTG-3′ (sense) and 5′-ATA GCG GCC GCT TAA TGG CAA TGG CCT TTC-3′ (antisense) for S100A9. Following digestion with NotI and XhoI, S100A8 and S100A9 cDNAs were cloned into pCAG-HA and pCAG-FLAG expression vectors (gifts from Tadatsugu Taniguchi, Tokyo University), respectively. HEK293T cells were transfected with either pCAG-HA/S100A8 or pCAG-FLAG/S100A9 by lipofection using the Lipofectamine 2000 transfection reagents (Gibco, Carlsbad, CA). After 24 hours, cells were harvested and lysed with 0.5% Triton X-100 in lysis buffer. Cellular proteins were resolved on SDS-PAGE gels, and blotting with G213 and Abs to β-actin was performed as described above. In order to detect the FLAG-tagged S100A9 protein, PVDF membranes were incubated with anti-FLAG mAb (clone M2; Sigma-Aldrich), followed by peroxidase-conjugated anti-mouse/rat IgG Abs (Jackson ImmunoResearch Laboratories), whereas the HA-tagged S100A8 protein was detected directly with peroxidase-conjugated anti-HA mAb (clone 3F10; Roche, Mannheim, Germany).
Proteomic analysis
G213-reactive proteins were immunoprecipitated from guinea pig bone marrow cells as described above and resolved on a 15% SDS-PAGE gel, followed by silver staining. The 15-kDa band representing the protein species recognized by G213 was excised and sent to the APRO Life Science Institute, where in-gel digestion, peptide extraction, and mass spectrometric analysis were conducted. Mass spectrometry data were analyzed for protein identification using the Mascot server (Matrix Science, Boston, MA).
Statistical analysis
An unpaired 2-tailed Student t test was used for all statistical analyses.
Results
Isolation of a mAb clone exhibiting unique reactivity to granuloma cells
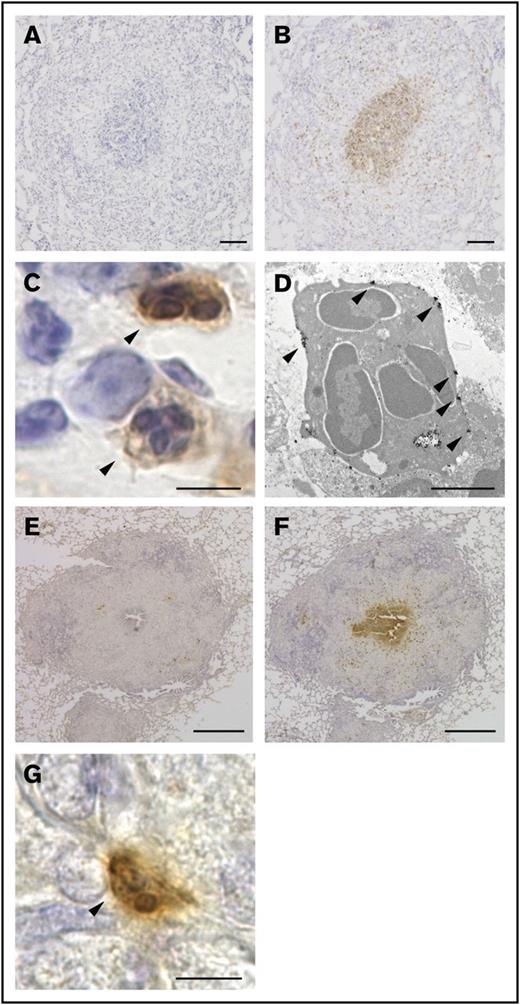
In our guinea pig granuloma model, well-organized granulomas emerged in the lung as early as 1 week after the IV administration of BCG. These granulomas contained 2 essential cell types, epithelioid cells and multinucleated giant cells. Furthermore, central necrosis was readily detectable in some granulomas, demonstrating that guinea pig granulomas resembled the prototypic granulomas that form in human tuberculosis.14 Therefore, this model system provided a valuable opportunity for us to experimentally analyze the pathophysiology of granuloma formation. We first attempted to obtain an array of rat mAbs exhibiting unique reactivity to granuloma cells. To this end, individual granulomas were surgically isolated from the lungs and homogenized in complete Freund’s adjuvant, followed by their inoculation into rat skin. Cells collected from the draining lymph nodes were then fused with myeloma cells, and supernatants from the culture of each hybridoma clone were screened for their reactivity to guinea pig granuloma cells on tissue sections. Among the 344 hybridoma clones tested, a mAb clone, named G213, attracted our attention because of its unique staining pattern. G213 mAb preferentially labeled cells located in the central area of granulomas (Figure 1B), whereas isotype-matched mAb did not show any significant reactivity to granuloma cells (Figure 1A). Magnified light microscopic views indicated that G213-labeled cells were polymorphonuclear cells (PMNs) (Figure 1C arrowheads) even though their cytoplasmic granules were not readily detectable by hematoxylin (Figure 1C) or Giemsa staining (not shown). Therefore, we performed immunogold-labeled electron microscopy and confirmed that G213-labeled cells were PMNs with multilobulated nuclei but apparently lacked cytoplasmic granules (Figure 1D), suggesting that these cells undergo exhaustive degranulation. These electron micrographs also indicated that the molecules recognized by G213 mAb were preferentially associated with the plasma membrane (Figure 1D, indicated with arrowheads). Importantly, the accumulation of G213-labeled PMNs at the core of granulomas was observed not only in our experimental granuloma model elicited by the IV administration of BCG but also in the authentic guinea pig model of human tuberculosis, in which animals were infected with aerosolized M tuberculosis. G213 mAb (Figure 1F), but not isotype-matched control mAb (Figure 1E), reacted to cells located in the central area of granulomas, and these G213-labeled cells were PMNs (Figure 1G). To the best of our knowledge, clusters of apparently activated (degranulated) PMNs were not noted previously in any granuloma studies. Furthermore, their unique central localization within granulomas led us to speculate that these G213-reactive PMNs, most likely neutrophils, may influence granuloma formation. In order to gain insights into their roles, we attempted to determine the molecular identity of the target antigen recognized by G213 mAb.
G213 reacted with PMNs located at the core of granulomas. (A-B) Sections of lungs obtained from the BCG-challenged guinea pig granuloma model were labeled with either isotype-matched negative control mAb (A) or G213 mAb (B), and HRP-DAB-based detection was performed. Sections were counterstained with hematoxylin. Scale bars, 100 μm. (C) A magnified view of G213-reactive cells (arrowheads) observed in panel B indicated that the stained cells were PMNs. Scale bar, 5 μm. (D) An immunoelectron micrograph of PMN cells positively labeled with G213 is shown. Gold particles representing the expression of the G213 antigen are indicated with arrowheads. Scale bar, 2 μm. (E-F) Paraffin-embedded lung sections derived from M tuberculosis–infected guinea pigs were labeled with either isotype-matched negative control mAb (E) or G213 mAb (F), and HRP-DAB-based detection was performed. G213-positive cells were prominently found in the central area of pathological granulomas. Scale bars, 1 mm. (G) A magnified view of a G213-positive cell (arrowhead) observed in panel F exhibited a typical PMN morphology. Scale bar, 5 μm.
G213 reacted with PMNs located at the core of granulomas. (A-B) Sections of lungs obtained from the BCG-challenged guinea pig granuloma model were labeled with either isotype-matched negative control mAb (A) or G213 mAb (B), and HRP-DAB-based detection was performed. Sections were counterstained with hematoxylin. Scale bars, 100 μm. (C) A magnified view of G213-reactive cells (arrowheads) observed in panel B indicated that the stained cells were PMNs. Scale bar, 5 μm. (D) An immunoelectron micrograph of PMN cells positively labeled with G213 is shown. Gold particles representing the expression of the G213 antigen are indicated with arrowheads. Scale bar, 2 μm. (E-F) Paraffin-embedded lung sections derived from M tuberculosis–infected guinea pigs were labeled with either isotype-matched negative control mAb (E) or G213 mAb (F), and HRP-DAB-based detection was performed. G213-positive cells were prominently found in the central area of pathological granulomas. Scale bars, 1 mm. (G) A magnified view of a G213-positive cell (arrowhead) observed in panel F exhibited a typical PMN morphology. Scale bar, 5 μm.
Identification of the G213-reactive protein as S100A9
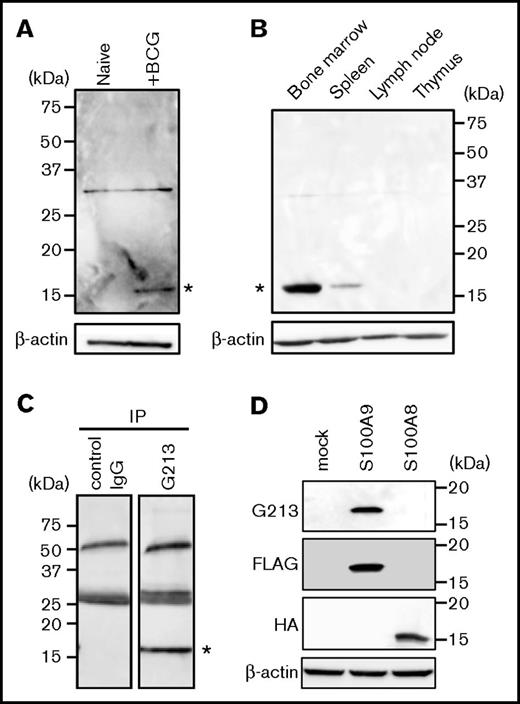
An immunohistochemical analysis revealed that G213-labeled cells were not readily detectable in the lungs of untreated animals (data not shown); therefore, lysates of lung tissues derived from BCG-challenged and naive guinea pigs were run side by side on an SDS-PAGE gel, and blotting patterns with G213 mAb were compared (Figure 2A). A protein species with an apparent molecular mass of ∼15 kDa was specifically detected for granuloma lungs (right lane, indicated with an asterisk), but not for unaffected lungs (left lane). Lymphoid organs, such as lymph nodes and the thymus, also lacked the expression of the 15-kDa species; however, its protein expression was readily detectable in the bone marrow of untreated animals and in the spleen, albeit less prominently than in bone marrows (Figure 2B, indicated with an asterisk). These expression patterns were consistent with neutrophils being the major cell type for G213 antigen expression and also indicative of G213-reactive cells in granulomas originating from the bone marrow. By using bone marrow containing a large pool of the G213-reactive protein, lysates of normal bone marrow cells were prepared, and immunoprecipitation was performed with either G213 or isotype-matched control Ab, followed by western blotting with G213. The 15-kDa species was detected in the G213 immunoprecipitation sample (Figure 2C, right lane), but not in the control sample (left lane), leading to the conclusion that the 15-kDa protein was the antigen recognized by G213 mAb. In order to determine the molecular identity of the 15-kDa species, the corresponding band was excised from the gel, digested with trypsin, and subjected to mass spectrometry. An analysis of tandem mass spectrometry data by the MASCOT algorithm resulted in the identification of 2 mutually related proteins: S100A9 (82% coverage) and S100A8 (37% coverage). In order to identify the molecular species recognized directly by G213, FLAG-tagged S100A9 and HA-tagged S100A8 cDNAs were individually expressed in HEK293T cells by transfection, and cellular proteins were resolved on SDS-PAGE, followed by western blotting. The expression of both tagged proteins was observed at similar levels, and only S100A9, but not S100A8, was detected with G213 mAb (Figure 2D). Therefore, we concluded that G213 mAb interacted directly with S100A9, a 15-kDa protein known to be expressed primarily in neutrophils.15 S100A9 is expressed not only as a free form but also as a heterodimer complexed with S100A8.16,17 Biochemical and mass spectrometric results obtained above indicated that G213 mAb may recognize both forms. S100A9 expressed in the cytosol can potentially be recruited to the plasma membrane upon neutrophil activation18 ; therefore, the preferential association of S100A9 with the plasma membrane of granuloma core neutrophils (Figure 1D) may indicate the cells being activated.
Identification of the G213-reactive protein as S100A9. (A) Tissue lysates derived from the lungs of untreated (left lane) and BCG-challenged (right lane) guinea pigs were resolved on SDS-PAGE, and western blotting was conducted with G213 (upper panel) and Ab to β-actin (lower panel). The 15-kDa species specifically recognized by G213 is indicated with an asterisk. The 30-kDa bands observed on both lanes may represent nonspecific signals. (B) Tissue lysates derived from the bone marrow, spleen, lymph nodes, and thymus of an untreated guinea pig were prepared, and western blotting was performed as in panel A. The 15-kDa species specifically recognized by G213 is indicated with an asterisk. (C) Bone marrow cells obtained from an untreated guinea pig were lysed, and immunoprecipitation (IP) was performed with either control IgG (left lane) or G213 (right lane). Samples were resolved on an SDS-PAGE gel, followed by immunoblotting with G213. The 15-kDa species specifically recognized by G213 is indicated with an asterisk. (D) Cell lysates derived from HEK293T cell transfectants expressing either FLAG-tagged S100A9 or HA-tagged S100A8 as well as mock-treated cells were resolved on SDS-PAGE gels, and immunoblotting with Abs to FLAG, HA, and β-actin as well as G213 mAb was conducted.
Identification of the G213-reactive protein as S100A9. (A) Tissue lysates derived from the lungs of untreated (left lane) and BCG-challenged (right lane) guinea pigs were resolved on SDS-PAGE, and western blotting was conducted with G213 (upper panel) and Ab to β-actin (lower panel). The 15-kDa species specifically recognized by G213 is indicated with an asterisk. The 30-kDa bands observed on both lanes may represent nonspecific signals. (B) Tissue lysates derived from the bone marrow, spleen, lymph nodes, and thymus of an untreated guinea pig were prepared, and western blotting was performed as in panel A. The 15-kDa species specifically recognized by G213 is indicated with an asterisk. (C) Bone marrow cells obtained from an untreated guinea pig were lysed, and immunoprecipitation (IP) was performed with either control IgG (left lane) or G213 (right lane). Samples were resolved on an SDS-PAGE gel, followed by immunoblotting with G213. The 15-kDa species specifically recognized by G213 is indicated with an asterisk. (D) Cell lysates derived from HEK293T cell transfectants expressing either FLAG-tagged S100A9 or HA-tagged S100A8 as well as mock-treated cells were resolved on SDS-PAGE gels, and immunoblotting with Abs to FLAG, HA, and β-actin as well as G213 mAb was conducted.
S100A9 expression in a human granulomatous disease
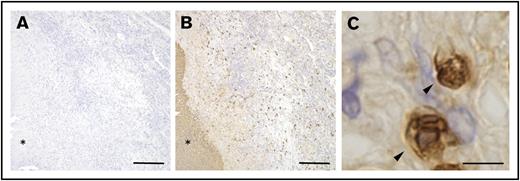
The results presented above demonstrated that S100A9+ neutrophils accumulated in the central area of granulomas in the BCG-elicited guinea pig granuloma model. Similar results were also obtained in guinea pigs infected with aerosolized pathogenic mycobacteria (Figure 1). Guinea pigs have been used over the past several decades as the most reliable small animal model of human tuberculosis because of their histopathology comparable to that in humans,19,20 and we predicted that similar neutrophil responses may also be observed in humans. In order to address this directly, pathological lung specimens derived from patients with tuberculosis were labeled with commercial mAb reactive to human S100A9. As shown in Figure 3B, human tuberculosis–associated pulmonary granulomas were found to contain S100A9+ cells in the periphery of central necrosis (shown with an asterisk), whereas no significant staining was noted on a sequential section labeled with control Ab (Figure 3A). Magnified views of these S100A9+ cells indicated that they were PMNs (Figure 3C). These histopathological features were similar to those observed in the lungs of BCG-challenged guinea pigs (Figure 1B). Therefore, our guinea pig granuloma model appeared to recapitulate human granulomatous diseases, allowing us to examine the impact of the neutrophil S100A9 protein on granuloma formation using the specific inhibitor, tasquinimod.
S100A9-expressing PMNs found in human pathological granulomas. (A-B) Sections of formalin-fixed, paraffin-embedded pathological lung tissues derived from patients with tuberculosis were labeled with either anti-human S100A9 (B) or control mAb (A), followed by HRP-DAB-based detection as described in “Methods.” Asterisks indicate the necrotic area at the center of the granuloma. Scale bars, 200 μm. (C) A magnified view of G213-reactive cells (arrowheads) observed in panel B indicated that the stained cells were PMNs. Scale bar, 5 μm.
S100A9-expressing PMNs found in human pathological granulomas. (A-B) Sections of formalin-fixed, paraffin-embedded pathological lung tissues derived from patients with tuberculosis were labeled with either anti-human S100A9 (B) or control mAb (A), followed by HRP-DAB-based detection as described in “Methods.” Asterisks indicate the necrotic area at the center of the granuloma. Scale bars, 200 μm. (C) A magnified view of G213-reactive cells (arrowheads) observed in panel B indicated that the stained cells were PMNs. Scale bar, 5 μm.
Effect of tasquinimod on granuloma formation
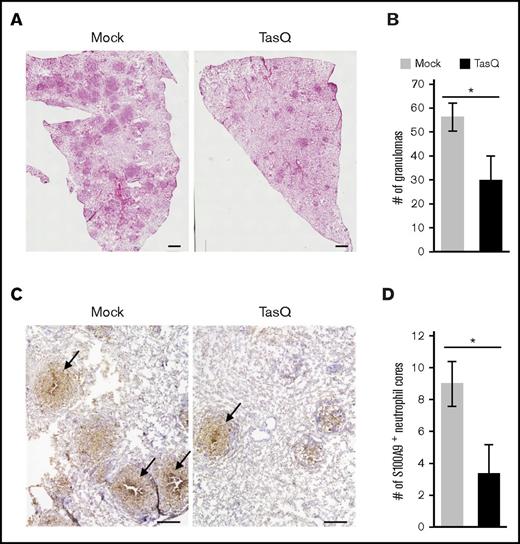
Previous findings suggested that S100A9 plays a role in the pathogenesis of inflammatory disorders.21-23 However, its precise role in granuloma formation remains unknown. Therefore, the effect of tasquinimod on granuloma formation was evaluated using the guinea pig granuloma model. The inhibition of S100A9 functions with tasquinimod resulted in a decrease in the number of lung granulomas (Figure 4A-B). Furthermore, whereas the accumulation of S100A9+ neutrophils in the central area of granulomas was readily detectable in mock-treated animals (Figure 4C right panel, indicated with arrows), the number of granulomas containing S100A9+ neutrophil cores was significantly reduced by the treatment with tasquinimod (Figure 4C right panel and 4D). These results indicate that the centrally located neutrophils and the S100A9 protein that they abundantly produce have critical functions in dictating the organized construction of granulomas.
The effect of tasquinimod on granuloma formation. The effect of tasquinimod (TasQ) on granuloma formation was evaluated using the guinea pig granuloma model. (A) Lung sections derived from TasQ-treated (right panel) and mock-treated (left panel) guinea pigs were stained with hematoxylin and eosin, and representative micrographs obtained from 2 independent experiments are shown. Scale bars, 1 mm. (B) Granulomas with a diameter of >200 μm were counted for 3 animals in each group. Numbers of granulomas per section are shown as the mean ± standard error of the mean. *P < .01. (C) Lung sections derived from TasQ-treated and mock-treated guinea pigs were labeled with G213 mAb, and HRP-DAB-based detection was performed. Representative micrographs obtained from 2 independent experiments are presented with granulomas containing the central accumulation of S100A9+ neutrophils indicated with arrows. Scale bars, 500 μm. (D) Granulomas with S100A9+ neutrophil cores were counted per section for 3 animals in each group, and data are shown as the mean ± standard error of the mean. *P < .01.
The effect of tasquinimod on granuloma formation. The effect of tasquinimod (TasQ) on granuloma formation was evaluated using the guinea pig granuloma model. (A) Lung sections derived from TasQ-treated (right panel) and mock-treated (left panel) guinea pigs were stained with hematoxylin and eosin, and representative micrographs obtained from 2 independent experiments are shown. Scale bars, 1 mm. (B) Granulomas with a diameter of >200 μm were counted for 3 animals in each group. Numbers of granulomas per section are shown as the mean ± standard error of the mean. *P < .01. (C) Lung sections derived from TasQ-treated and mock-treated guinea pigs were labeled with G213 mAb, and HRP-DAB-based detection was performed. Representative micrographs obtained from 2 independent experiments are presented with granulomas containing the central accumulation of S100A9+ neutrophils indicated with arrows. Scale bars, 500 μm. (D) Granulomas with S100A9+ neutrophil cores were counted per section for 3 animals in each group, and data are shown as the mean ± standard error of the mean. *P < .01.
Discussion
S100A9 is a small calcium-binding protein of the S100 family that is expressed, in most biological settings, as a heterodimer complexed with its partner, S100A8.16,17 These proteins are abundantly expressed in neutrophils, comprising up to 40% of cytosolic proteins,15 and are proposed to control multifaceted functions attributed to neutrophils.24 S100A9 also exists as a secreted form, and high levels of serum S100A9 have been implicated in some inflammatory diseases; however, the precise molecular mechanisms for its secretion are largely unknown.25-28 As a general principle, extracellular S100A9 stimulates innate immune cells and endothelial cells, via interactions with the receptor for advanced glycation end products29 and Toll-like receptor-4 (TLR4),30 to elicit and augment proinflammatory responses; however, its expression in granulomas as well as its role in granuloma formation have never been addressed. In the present study, we found that neutrophils expressing S100A9 accumulated in the central area of granulomas, and that the S100A9 protein may serve as a key molecule for granuloma formation. Therefore, the fixed concept that the accumulation of locally activated macrophages results in granuloma formation needs to be modified in order to incorporate the potential contribution of S100A9+ neutrophils.
Granulomas are formed as a consequence of sequential, well-disciplined events involving cellular activation, movement, and collection.9,31 The neutrophil S100A9 protein may be involved in the initial steps of granuloma formation, including the recruitment of peripheral blood neutrophils to the site of inflammation. The S100A9-mediated pathway leading to the transendothelial migration of neutrophils has recently been elucidated at the molecular level.32,33 The ligation of P-selectin glycoprotein-1 by E-selectin expressed on the luminal surface of inflamed endothelial cells stimulates neutrophils to secrete S100A9, which, in turn, transmits activation signals via TLR4 in an autocrine manner. The TLR4-mediated cell activation results in the upregulated expression of high-affinity β2 integrins on neutrophils that interact with ICAM-1 expressed on endothelial cells, thereby allowing circulating neutrophils to be trapped on the luminal surface of the endothelium. As a result of this S100A9-dependent crosstalk between neutrophils and endothelial cells, neutrophils swiftly extravasate and accumulate in inflammation foci.32 Therefore, impaired neutrophil recruitment to the site of granuloma formation in tasquinimod-treated animals may be attributed, at least in part, to the specific blockade of the S100A9 autocrine loop.
After extravasation, neutrophils rapidly accumulate in lung tissue and appear to establish a niche favoring the initiation of granuloma formation. Conceptually, this may be similar to the proposed role for neutrophils in tumor metastasis.34,35 S100A9+ neutrophils have been shown to infiltrate premetastatic lungs and support the initiation of metastasis by secreting soluble factors, such as leukotrienes, that can promote tumorigenesis.34 Given the capacity of the intracellular S100A9 protein to capture and transport arachidonic acid,24,36 a primary substrate for the leukotriene-generating Alox5 enzyme,37 S100A9 may be directly involved in the metastasis-initiating activity exhibited by neutrophils. Similarly, S100A9+ neutrophils clustering in the initiation phases of granuloma formation may provide a scaffold or microenvironment, assisting the subsequent construction of organized granulomas. Leukotriene B4, for example, functions as a potent chemoattractant and activator for not only neutrophils but also macrophages.38,39 Furthermore, S100A9 itself has been shown to attract macrophages40 ; therefore, S100A9 deposited at the central area of granulomas may serve to recruit macrophages and activate them continuously via receptor for advanced glycation end products and TLR to produce proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1β, and interleukin-6, as well as chemokines, including macrophage inflammatory protein-1α and monocyte chemoattractant protein-1.22 The prolonged exposure of macrophages to these highly proinflammatory conditions may lead to their transformation into epithelioid cells and multinucleated giant cells.
Besides these activated macrophages, we also noted that centrally located S100A9+ neutrophils lacked cytoplasmic granules, which is indicative of them being continuously activated or even exhausted. Extracellular S100A9 is known to induce the phosphorylation of MAPKs, such as p38, and JNK in human neutrophils, leading to degranulation41 ; thus, the release of S100A9 from neutrophils and its condensed local accumulation in the central area of granulomas may result in exhaustive degranulation in an autocrine manner. Alternatively, neutrophil degranulation may be facilitated by environmental factors. For example, the core of granulomas is known to maintain a state of hypoxia,42 and neutrophils sense and respond to hypoxia via the prolyl hydroxylase/hypoxia-inducible factor pathway, which is associated with the prolonged survival of cells and upregulation of degranulation.43,44
The S100A9 inhibitor, tasquinimod, is a quinolone-3-carboxamide derivative, capable of suppressing the growth and metastasis of tumor cells, and its clinical efficacy for controlling metastatic castration-resistant prostate cancer has been positively evaluated in a phase 2 randomized controlled trial.45 Because of the pleiotropic functions exerted by S100A9, the precise mechanisms by which tumors are controlled by tasquinimod remain to be determined; however, growing evidence has underscored that tasquinimod suppresses the local recruitment and function of myeloid-derived suppressor cells (MDSCs), thereby disrupting the immunosuppressive tumor microenvironment.46 Given that human and mouse MDSCs express high levels of S100A9,47,48 it is intriguing to predict that the granuloma-resident S100A9+ neutrophils may be equipped with MDSC functions that are sensitive to tasquinimod. Immune suppressive microenvironments established in the central area of granulomas may allow pathogenic microbes to infect chronically as proposed for M tuberculosis, the causative microorganism of human tuberculosis. These interesting possibilities should be addressed in future studies.
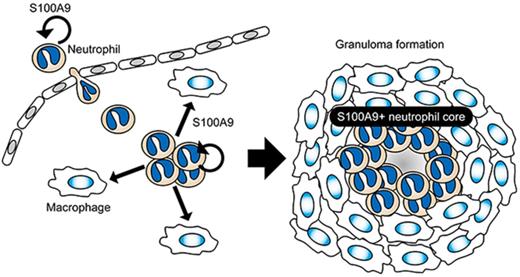
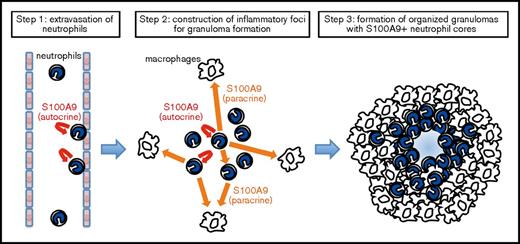
Neutrophils are regarded as a major cell type that quickly infiltrates insulted tissues to take first-aid actions in acute inflammation.34,49 The present study now points to their role in granulomatous responses that represent chronic, rather than acute, inflammation. As shown in Figure 5, neutrophils quickly extravasate and accumulate at sites destined for granuloma formation, in which cells serve to construct a core of granulomas. Besides its critical function in assisting the transendothelial migration of circulating neutrophils,32 S100A9 mediates multifaceted functions as a ligand for innate immune receptors.29,30 High concentrations of the S100A9 protein in the core of developing granulomas may persistently stimulate neutrophils in an autocrine or paracrine manner in order to promote the secretion of proinflammatory cytokines for the establishment of a niche for granuloma formation. Furthermore, S100A9 may upregulate macrophage chemotaxis either directly or indirectly by inducing the production of macrophage chemoattractants. The continuous activation of macrophages by S100A9 in cooperation with other proinflammatory cytokines may assist their transformation into epithelial cells, resulting in the formation of organized granuloma. Besides the proposed role of S100A9 in MDSC-mediated tissue responses described above, each of these proinflammatory functions should also be evaluated directly by optimized cellular assays in future studies, thereby pinpointing the mode of S100A9 action in granuloma formation. The results of the present study imply that neutrophils and the S100A9 protein play the role of a conductor at the center of the stage to orchestrate the functions of the individual players.
A proposed model for S100A9-dependent granuloma formation. The neutrophil S100A9 protein may play essential roles in multiple steps of granuloma formation, including the recruitment of neutrophils from peripheral blood (step 1) and the construction of a niche for the development of a granuloma (step 2). S100A9 released from neutrophils stimulates neutrophils and macrophages in autocrine (U-shaped arrows in red) and paracrine (straight arrows in orange) manners, thereby augmenting focal cellular responses via the secretion of proinflammatory cytokines and macrophage-recruiting chemokines as well as other inflammatory mediators. Prolonged focal inflammation with S100A9+ neutrophils at the core may contribute to the concentric expansion of the surrounding macrophage layer, resulting in the formation of organized granulomas (step 3). Chronic exposure to S100A9 deposited in the central area of granulomas, as well as the hypoxic and acidic microenvironments, may be highly stressful to cells, potentially serving to generate exhausted (degranulated) neutrophils and central necrosis.
A proposed model for S100A9-dependent granuloma formation. The neutrophil S100A9 protein may play essential roles in multiple steps of granuloma formation, including the recruitment of neutrophils from peripheral blood (step 1) and the construction of a niche for the development of a granuloma (step 2). S100A9 released from neutrophils stimulates neutrophils and macrophages in autocrine (U-shaped arrows in red) and paracrine (straight arrows in orange) manners, thereby augmenting focal cellular responses via the secretion of proinflammatory cytokines and macrophage-recruiting chemokines as well as other inflammatory mediators. Prolonged focal inflammation with S100A9+ neutrophils at the core may contribute to the concentric expansion of the surrounding macrophage layer, resulting in the formation of organized granulomas (step 3). Chronic exposure to S100A9 deposited in the central area of granulomas, as well as the hypoxic and acidic microenvironments, may be highly stressful to cells, potentially serving to generate exhausted (degranulated) neutrophils and central necrosis.
Acknowledgments
The authors thank Haruyasu Kouda and Keiko Furuta (Center for Anatomical, Pathological, and Forensic Medical Research, Graduate School of Medicine, Kyoto University, Kyoto, Japan) for their technical assistance in performing electron microscopy. The authors also thank Tadashi Udagawa and Norio Doi (Research Institute of Tuberculosis, Japan Anti-Tuberculosis Association, Tokyo, Japan) for preparing tissue samples derived from M tuberculosis–infected guinea pigs.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (26860038 [T.M.] and 15H04869 [M.S.]), and by grants from the Tokyo Biochemical Research Foundation (T.M.) and Shimizu Foundation (T.M.).
Authorship
Contributions: Y.Y., T.M., T.T., and M.S. designed the research; Y.Y., T.M., H.I., and D.M. generated G213 mAb and identified S100A9 as the antigen recognized by this mAb; T.T., Y.Y., T.M., and M.S. analyzed human histopathology; Y.Y., T.M., S. Murata, A.M., T.A., and M.S. established the guinea pig granuloma model; H.Y. and Y.H. provided M tuberculosis–infected guinea pig lungs for the immunohistochemical analysis; S. Mizuta chemically synthesized tasquinimod; M.S. supervised the overall project; and T.M., Y.Y., and M.S. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tatsuaki Mizutani, Laboratory of Cell Regulation, Institute for Virus Research, Kyoto University, 53 Shogoin Kawahara-cho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: mizutani@virus.kyoto-u.ac.jp.