Key Points
Expression of full-length hBAFF from cDNA in the endogenous murine locus does not improve maturation of human B cells in hu-mice.
mBAFF is not limiting the maturation of human B cells in hu-mice.
Abstract
Hematopoietic humanized mice (hu-mice) have been developed to study the human immune system in an experimental in vivo model, and experiments to improve its performance are ongoing. Previous studies have suggested that the impaired maturation of human B cells observed in hu-mice might be in part due to inefficient interaction of the human B-cell–activating factor (hBAFF) receptor with mouse B-cell–activating factor (mBAFF), as this cytokine is an important homeostatic and differentiation factor for B lymphocytes both in mice and humans. To investigate this hypothesis, we created a genetically engineered mouse strain in which a complementary DNA (cDNA) encoding full-length hBAFF replaces the mBAFF-encoding gene. Expression of hBAFF in the endogenous mouse locus did not lead to higher numbers of mature and effector human B cells in hu-mice. Instead, B cells from hBAFF knock-in (hBAFFKI) hu-mice were in proportion more immature than those of hu-mice expressing mBAFF. Memory B cells, plasmablasts, and plasma cells were also significantly reduced, a phenotype that associated with diminished levels of immunoglobulin G and T-cell–independent antibody responses. Although the reasons for these findings are still unclear, our data suggest that the inefficient B-cell maturation in hu-mice is not due to suboptimal bioactivity of mBAFF on human B cells.
Introduction
Hematopoietic humanized mice (hu-mice) have been developed to study the human immune system in an experimental in vivo model.1-3 These mice bear a transplanted human immune system that can be manipulated and studied with methodologies similar to those used in mice. Major advances in engrafting a human immune system in mice have been achieved using mice with genetic manipulations that lead to severe immunodeficiency and, consequently, minimal rejection of human hematopoietic stem cells (hHSCs) and their differentiated progeny.4-8 One example of these recipients is the BALB/c-Rag2nullIl2rγnull (BRG) strain that, when transplanted with hHSCs, develop human B cells, T cells, and, with varying frequencies, other human hematopoietic cell types.9-17 This strain has been recently modified into BRGS with the introduction of the NOD-derived Sirpa allele (SirpaNOD) that further improves human chimerism.14 In spite of this progress, the maturation of human B cells remains inefficient in hu-mice.7
Human immature B cells develop quite normally in the bone marrow of hu-mice, but the maturation of peripheral B cells and related humoral responses remains defective.6,16,18 Although our studies have indicated that T cells are crucial to B-cell maturation in hu-mice,16 other studies have suggested that the defects in B-cell maturation and antibody responses observed in hu-mice are caused by an inability of mouse B-cell–activating factor (mBAFF) to aid survival and antigen responses of human B cells.19,20 B-cell–activating factor (BAFF)/B-lymphocyte stimulator (BlyS) is an important homeostatic factor for B cells in both mice and humans,21 and a BAFF deficiency in mice and antagonism in humans cause major loss of late transitional and mature B cells.21-24 Together, these studies suggest that the inefficient generation, survival, and responses of mature B cells in hu-mice might be due, at least in part, to low bioactivity of mBAFF on human B lymphocytes.
To test this hypothesis and attempt to improve human B-cell function in hu-mice, we generated a novel genetically engineered mouse strain that expresses human BAFF (hBAFF) in place of mBAFF. We found that expression of hBAFF in hu-mice did not improve B-cell numbers. In fact, the relative proportion of mature B cells was decreased with endogenous expression of hBAFF, a defect that was further reflected by diminished numbers of memory B cells and plasmablasts, and lower antibody responses to a T-cell–independent vaccine. Overall, these data indicate that the impaired maturation of the human B cell observed in hu-mice is not due to a suboptimal interaction with mBAFF.
Materials and methods
Mice, hu-PBL mice, and hu-mice
BALB/c-Rag2nullIL2Rγnull (BRG) and BALB/c-Rag2nullIl2rγnullSirpaNOD (BRGS) have been previously described.9,13,14 Human BAFFKI mice (described in supplemental Methods) were backcrossed into BRG and BRGS genetic backgrounds. All BRG(S) mice were bred and maintained on a diet enriched with Septra under specific pathogen-free and biosafety level 2 conditions at the Biological Resource Center at National Jewish Health (NJH; Denver, CO) or at the University of Colorado Denver Anschutz Medical Center (UCD-AMC) Vivarium (Aurora, CO). To generate hu-PBL mice, peripheral blood mononuclear cells (PBMCs) collected from healthy adult donors in the Clinical Division of NJH were isolated over Ficoll-density gradients. PBMCs were enriched for B cells by depleting CD2+ cells with an Automacs (Miltenyi Biotec) to reach a cell mixture in which B cells were ∼15% of total. Approximately 20 × 106 of these B-cell–enriched PBMCs were injected per mouse intraperitoneally or IV into adult BRGS and BAFFKI-BRGS mice, 3 to 5 hours after sublethal irradiation (250 rad). Hu-PBL mice were analyzed 2 to 3 weeks after transplant and before any visible onset of graft-versus-host disease, which is known to occur in this model.25 For the generation of hu-mice, a model that is not affected by graft-versus-host disease, human umbilical cord blood (CB) samples were obtained from the University of Colorado Cord Blood Bank at ClinImmune Labs (Aurora, CO) as samples that were rejected due to low volume or other reasons. CB CD34+ cells were prepared using the CD34+ selection kit (Miltenyi Biotec) and expanded in culture as previously described.13,16 Approximately 100 000 to 400 000 in vitro–expanded CD34+ cells were injected IV (typically) or intrahepaticly (less frequently) into 1- to 3-day-old BRG(S) or hBAFFKI-BRG(S) that were previously irradiated with 350 rad. Hu-mice were analyzed 23 to 24 weeks after CD34+ cell transplant. Investigators in this study were blinded from donor identities, and the studies were performed in compliance with NJH and University of Colorado Institutional Review Boards and in accordance with the Declaration of Helsinki. Animal procedures were approved by the NJH Animal Care and Use Committee or the UCD-AMC Institutional Animal Care and Use Committee.
Analyses of BAFF expression
Analyses of mBAFF and hBAFF by enzyme-linked immunosorbent assay (ELISA) and of mTnfsf13b and hTNFSF13B by quantitative polymerase chain reaction are described in supplemental Methods.
Cell staining, flow cytometry, and cell sorting
Cells were stained in staining buffer (phosphate-buffered saline, 1% bovine serum albumin, 0.1% sodium azide) for 15 minutes at 4°C and washed 2 times with the same buffer. For intracellular staining, cells were fixed in 2% formaldehyde and permeabilized and stained in 0.5% saponin (Sigma) as previously described.17 Stained cells were run on a Cyan analyzer (Beckman Coulter) either at NJH or UCD Cancer Center flow cytometry cores, and analyses were performed with FlowJo software (TreeStar). Cell sorting was performed on a FACSAria Fusion (BD Biosciences) at UCD, and purity of the sorted populations was at least 97%. Antibodies used in these analyses are described in supplemental Methods.
Immunizations and ELISA
Immunization of mice and analyses of immunoglobulins by ELISA are described in supplemental Methods.
Statistical analysis
Data were first analyzed to determine whether they fit a normal distribution. Data with normal distribution were analyzed by 2-tailed Student t test. Welch correction was added when comparing groups with unequal variance. Data that were not normally distributed were analyzed with the nonparametric Mann-Whitney U test. Differences were considered significant when P < .05.
Results
hBAFF is detected in hematopoietic hu-mice
Past studies have suggested that the low bioactivity that mBAFF displays toward human B cells contributes to the inefficient generation, survival, and responses of human B lymphocytes observed in immunodeficient mice engrafted with human hematopoietic cells.21-24 Given BAFF is expressed by both nonhematopoietic and hematopoietic cells,21 we first evaluated whether hBAFF might be present within hu-mice, as this has not been previously reported. Polymorphonuclear leukocytes are the major source of BAFF in human blood, but this cytokine is also expressed by lymphocytes,21 which are relatively more frequent in hu-mice.16 Thus, we measured messenger RNA (mRNA) levels of the hBAFF-encoding gene, hTNFSF13B, in human (T and B) lymphocytes and nonlymphocytes from both immunodeficient mice transplanted with hHSCs (hu-mice) and human blood. Human B and T lymphocytes expressed similar levels of hTNFSF3B whether in hu-mice or in human blood, levels that were less than those of polymorphonuclear leukocytes in human blood, but similar to those of nonlymphocytes in hu-mice (Figure 1A). To determine whether the hBAFF protein was present in hu-mice, we developed a quantitative ELISA that distinguishes hBAFF from mBAFF in sera (Figure 1B). With this method, hBAFF was detected in serum of hu-mice at an average concentration of 0.19 ± 0.04 ng/mL (median, 0.082 ng/mL; range, 0.03-0.7 ng/mL; N = 19). This was about 11-fold lower than the BAFF concentration measured in a small cohort of human blood samples (average, 2.21 ± 0.4 ng/mL; median, 2.46 ng/mL; range, 0.85-3.18 ng/mL; N = 5; Figure 1B left graph), which is similar to that reported in sera of healthy humans by others (median range, 0.61-2.49 ng/mL; overall range, 0.39-2.96 ng/mL26-30 ).
Expression of hBAFF and hBAFFR. (A) Relative levels of hTNFSF13B mRNA in indicated human leukocyte subsets isolated from human PBMCs and hu-mice. hu-mice were generated by transplanting human umbilical cord HSCs into BRGS animals. Data were normalized relative to human naive T cells and are representative of 2 independent experiments. (B) hBAFF concentration in sera of BRGS intact mice (N = 5), humans (N = 5), and either BRGS hu-mice (N = 19) on the left or hBAFFKI-BRGS hu-mice (N = 8) on the right graph. The y-axes are on a different scale in the 2 graphs. Each symbol represents an individual mouse. Arithmetic mean ± standard error of the mean (SEM) are indicated for each group. Data were combined from 4 independent experiments. (C) BAFFR geometric mean fluorescence intensity (MFI) on CD20+ B cells from the following tissue of BRG(S) hu-mice: bone marrow (BM; N = 12), spleen (SP; N = 18), and lymph nodes (LN; N = 10). CD20− cells (N = 18) were included as a reference negative control. Bars indicate mean ± SEM. Data were combined from 3 independent experiments. (D) Relative levels of mBAFF (left) and hBAFF (right) in sera of heterozygous (M/H) and homozygous (H/H) hBAFFKI (BALB/c) mice and littermate control wild-type (M/M) mice as measured by ELISA. Data of each graph were combined from 3 independent experiments with 2 mice per group for mBAFF ELISA and 1 to 2 mice per group for hBAFF ELISA, per experiment. The y-axis shows optical density (O.D.; 405 nm) values that were normalized relative to the average values obtained with M/M sera for mBAFF or with H/H sera for hBAFF in each experiment. *P < .05; **P < .01; ***P < .001; ****P < .0001. B, B cell; PMN, polymorphonuclear leukocyte; T, T cell.
Expression of hBAFF and hBAFFR. (A) Relative levels of hTNFSF13B mRNA in indicated human leukocyte subsets isolated from human PBMCs and hu-mice. hu-mice were generated by transplanting human umbilical cord HSCs into BRGS animals. Data were normalized relative to human naive T cells and are representative of 2 independent experiments. (B) hBAFF concentration in sera of BRGS intact mice (N = 5), humans (N = 5), and either BRGS hu-mice (N = 19) on the left or hBAFFKI-BRGS hu-mice (N = 8) on the right graph. The y-axes are on a different scale in the 2 graphs. Each symbol represents an individual mouse. Arithmetic mean ± standard error of the mean (SEM) are indicated for each group. Data were combined from 4 independent experiments. (C) BAFFR geometric mean fluorescence intensity (MFI) on CD20+ B cells from the following tissue of BRG(S) hu-mice: bone marrow (BM; N = 12), spleen (SP; N = 18), and lymph nodes (LN; N = 10). CD20− cells (N = 18) were included as a reference negative control. Bars indicate mean ± SEM. Data were combined from 3 independent experiments. (D) Relative levels of mBAFF (left) and hBAFF (right) in sera of heterozygous (M/H) and homozygous (H/H) hBAFFKI (BALB/c) mice and littermate control wild-type (M/M) mice as measured by ELISA. Data of each graph were combined from 3 independent experiments with 2 mice per group for mBAFF ELISA and 1 to 2 mice per group for hBAFF ELISA, per experiment. The y-axis shows optical density (O.D.; 405 nm) values that were normalized relative to the average values obtained with M/M sera for mBAFF or with H/H sera for hBAFF in each experiment. *P < .05; **P < .01; ***P < .001; ****P < .0001. B, B cell; PMN, polymorphonuclear leukocyte; T, T cell.
To assess the ability of B cells in hu-mice to respond to BAFF, we measured the expression of the main BAFF receptor, BAFFR. Human B cells begin expressing BAFFR in the bone marrow of hu-mice and increase expression with cell maturation in spleen and lymph nodes (Figure 1C), indicating a progressive ability to respond to this prosurvival cytokine. Preliminary analyses, moreover, showed that hTNFSF13B mRNA levels were higher in spleen of hu-mice in which the proportion of mature B cells was >40% as compared with those with lower mature B-cell frequencies (supplemental Figure 1).
Overall, these data indicate that hu-mice produce low levels of hBAFF and suggest that increasing the production of this cytokine might improve maturation of human B cells in mice.
Generation and initial characterization of hBAFF knock-in mice
To increase hBAFF levels in hu-mice, we created a novel genetically engineered BALB/c knock-in (KI) mouse strain, hBAFFKI, which expresses hBAFF in place of mBAFF (supplemental Figure 2A-D). Quantitative polymerase chain reaction analyses confirmed that homozygous hBAFFKI mice expressed hTNFSF13B, but not mTnfsf13b, mRNA transcripts (supplemental Figure 2E), indicating that the hTNFSF13B gene cassette replaces the mTnfsf13b gene in the targeted allele. Moreover, hBAFF and not mBAFF was detected by ELISA in serum of homozygous hBAFFKI mice, whereas heterozygous mice produced hBAFF at half these levels (Figure 1D), demonstrating that the hTNFSF13B gene cassette is functional. Importantly, BALB/c mice bearing the hBAFFKI allele harbored higher frequencies of mouse B cells that expressed higher levels of CD21 (supplemental Figure 3) and similar to BAFF transgenic (BAFFTg) mice.31 This indicates the hBAFF expressed in hBAFFKI mice is functional.
hBAFFKI mice were bred onto the BRGS genetic background to generate hBAFFKI-BRGS animals. hBAFFKI-BRGS and control mice were transplanted with hHSCs to generate hu-mice and their hBAFF levels were measured by ELISA. hBAFF concentration in sera of hBAFFKI hu-mice was 10.91 ± 1.36 ng/mL on average (median, 9.5 ng/mL; range, 7.1-18.8 ng/mL; N = 8; Figure 1B right graph). This was approximately fivefold higher than human sera (Figure 1B and Mariette et al,26 Matsushita et al,27 Hamzaoui et al,28 Knight et al,29 and Kreuzaler et al30 ), and 50-fold higher than BRGS hu-mouse sera (Figure 1B).
These data demonstrate that homozygous hBAFFKI mice express a functional hBAFF instead of mBAFF and at a concentration adequate for human B-cell function.
Contribution of hBAFF to human B cells in hu-PBL mice
A past study showed that recovery of human B cells from immunodeficient mice transplanted with human PBMCs (ie, hu-PBL mice) improved with daily administration of hBAFF.19 Thus, we initially setup hu-PBL mice to determine whether endogenous expression of hBAFF would impact on B-cell numbers. Human PBMCs from 4 different donors were injected into both BRGS and hBAFFKI-BRGS animals that were analyzed 2 to 3 weeks posttransplantation. Frequencies and absolute B-cell numbers, as quantified by flow cytometry (Figure 2A), were similar in the spleen of hu-PBL mice that expressed mBAFF or hBAFF (Figure 2B). To establish whether endogenous production of hBAFF altered the distribution of major B-cell subsets, we analyzed the expression of CD38 and CD19 on CD19+ B cells (Figure 2C). The CD19highCD38−/low subset includes mature (naive and memory) B cells, whereas the CD19lowCD38high subset comprises plasmablasts and plasma cells.32-34 Interestingly, the fraction of CD19lowCD38high B cells was considerably larger in the spleen of hu-PBL mice relative to human PBMCs (Figure 2C), and because the numbers of recovered CD38high cells were much higher than those injected, these cells had likely differentiated from naive B cells in vivo. We found that hBAFF affected the distribution of B cells in the CD38 subsets, with a higher fraction of B cells that were CD38−/low and, reciprocally, a lower fraction that were CD38high in hBAFFKI recipients (Figure 2D). The absolute CD38low and CD38high cell numbers (Figure 2E) as well as the serum immunoglobulin M (IgM) and IgG concentrations (Figure 2F) were similar in the 2 groups of hu-PBL mice (P > .5), but showed significant variations among animals. To better understand the effect of hBAFF on B-cell distribution, we compared the absolute numbers of CD38low and CD38high cells in each animal and performed paired Student t test analyses. Although in hu-PBL animals expressing mBAFF there was a significant net increase of CD38high relative to CD38low cells, numbers of the 2 cell populations were unchanged in hu-PBL hBAFFI mice (Figure 2E).
Analysis of B cells and immunoglobulin production in hu-PBL mice expressing either mBAFF or hBAFF. Hu-PBL mice expressing either mBAFF (M) or hBAFF (H) were analyzed 2 to 3 weeks after transplantation of human PBMCs into BRGS and hBAFFKI-BRGS mice, respectively. (A) Representative analysis of CD19 expression on hCD45+ cells. Numbers indicate the frequency of CD19+ B cells. (B) Frequency of CD19+ B cells within the hCD45+ leukocyte cell population (left) and absolute CD19+ cell numbers (right) in individual hu-PBL mice, with mean and SEM in each group of mice. (C) Representative flow cytometric analysis of CD19 and CD38 expression on CD19+ cells from human PBMCs and spleen of hu-PBL mice. Gates were drawn around CD19+CD38low mature B cells (naive and memory) and CD19lowCD38high plasmablasts and plasma cells. Numbers indicate frequency of each cell population. (D) Frequency of CD19+CD38low mature (naive and memory) B cells (left) and of CD19lowCD38high plasmablasts and plasma cells (PBs&PCs; right) within the CD19+ cell population of each hu-PBL mouse, with mean and SEM for each group of mice. (E) CD19+CD38low and CD19lowCD38high absolute cell numbers in the spleen of individual hu-PBL mice expressing mBAFF (left) or hBAFF (right). Each symbol represents an individual mouse and lines connect values obtained from the same animal. Statistical differences were analyzed by paired Student t test. (F) Serum IgM and IgG levels in hu-PBL mice expressing mBAFF (M) or hBAFF (H). Data in all graphs were combined from 4 independent experiments (with 4 donor PBMCs) and a total of 8 to 13 hu-PBL mice per group. *P < .05. ns, not significant.
Analysis of B cells and immunoglobulin production in hu-PBL mice expressing either mBAFF or hBAFF. Hu-PBL mice expressing either mBAFF (M) or hBAFF (H) were analyzed 2 to 3 weeks after transplantation of human PBMCs into BRGS and hBAFFKI-BRGS mice, respectively. (A) Representative analysis of CD19 expression on hCD45+ cells. Numbers indicate the frequency of CD19+ B cells. (B) Frequency of CD19+ B cells within the hCD45+ leukocyte cell population (left) and absolute CD19+ cell numbers (right) in individual hu-PBL mice, with mean and SEM in each group of mice. (C) Representative flow cytometric analysis of CD19 and CD38 expression on CD19+ cells from human PBMCs and spleen of hu-PBL mice. Gates were drawn around CD19+CD38low mature B cells (naive and memory) and CD19lowCD38high plasmablasts and plasma cells. Numbers indicate frequency of each cell population. (D) Frequency of CD19+CD38low mature (naive and memory) B cells (left) and of CD19lowCD38high plasmablasts and plasma cells (PBs&PCs; right) within the CD19+ cell population of each hu-PBL mouse, with mean and SEM for each group of mice. (E) CD19+CD38low and CD19lowCD38high absolute cell numbers in the spleen of individual hu-PBL mice expressing mBAFF (left) or hBAFF (right). Each symbol represents an individual mouse and lines connect values obtained from the same animal. Statistical differences were analyzed by paired Student t test. (F) Serum IgM and IgG levels in hu-PBL mice expressing mBAFF (M) or hBAFF (H). Data in all graphs were combined from 4 independent experiments (with 4 donor PBMCs) and a total of 8 to 13 hu-PBL mice per group. *P < .05. ns, not significant.
These data indicated that endogenous expression of hBAFF in hu-PBL mice does not improve overall B-cell numbers, whereas it negatively affects the generation or survival of plasmablasts.
Contribution of hBAFF to human B-cell maturation in hu-mice
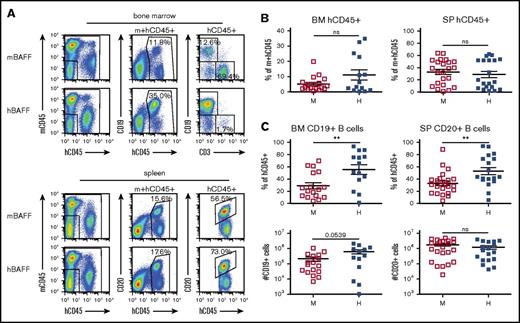
To further investigate whether endogenous expression of hBAFF aids human B-cell maturation in mice, we analyzed the B-cell population of hu-mice in which B cells develop from HSCs.16 For these studies, CD34+ cells from 4 different CB samples were transplanted into groups of recipient mice that expressed either mBAFF or hBAFF. Bone marrow and spleen from hBAFFKI and control hu-mice were evaluated by flow cytometry to quantify human hematopoietic cells and B cells as shown in Figure 3A. The level of human hematopoietic chimerism (percentage of human CD45+ [hCD45+] divided by percentage of hCD45+ plus mouse CD45+ [mCD45+] cells) was not statistically different in the 2 groups of mice (Figure 3B), but the hCD45+ cells of hBAFFKI hu-mice displayed a higher proportion of B cells in both tissues (Figure 3C top). Absolute B-cell numbers, however, were similar between the 2 groups of hu-mice, although they trended to be higher in the developing bone marrow B-cell population of hBAFFKI animals (Figure 3C bottom).
The B-cell population in hu-mice expressing mBAFF or hBAFF. hu-mice were generated by transplanting CB CD34+ cells into BRGS or hBAFFKI-BRGS mice and these animals were analyzed 23 to 24 weeks after transplantation. (A) Representative flow cytometric analysis of bone marrow (top) and spleen (bottom) cells from BRGS (mBAFF) and hBAFFKI-BRGS (hBAFF) hu-mice. The human leukocyte (hCD45+) population was measured within the combined mouse and hCD45+ cell fraction of both bone marrow and spleen. The frequency of human B cells (CD19+ in bone marrow and CD20+ in spleen) was measured within the hCD45+ cell fraction. (B) Frequencies of hCD45+ cells in the combined mouse and human leukocyte population of bone marrow and spleen of BRGS (□) and hBAFFKI-BRGS (▪) hu-mice. (C) Frequencies of B cells within the hCD45+ cell populations (top) and absolute numbers (bottom) in bone marrow and spleen of BRGS and hBAFFKI-BRGS hu-mice. Each symbol in panels B and C represents a mouse; bars are mean and SEM. N = 14 to 23 mice per group and 3 to 4 different CB groups. **P < .01.
The B-cell population in hu-mice expressing mBAFF or hBAFF. hu-mice were generated by transplanting CB CD34+ cells into BRGS or hBAFFKI-BRGS mice and these animals were analyzed 23 to 24 weeks after transplantation. (A) Representative flow cytometric analysis of bone marrow (top) and spleen (bottom) cells from BRGS (mBAFF) and hBAFFKI-BRGS (hBAFF) hu-mice. The human leukocyte (hCD45+) population was measured within the combined mouse and hCD45+ cell fraction of both bone marrow and spleen. The frequency of human B cells (CD19+ in bone marrow and CD20+ in spleen) was measured within the hCD45+ cell fraction. (B) Frequencies of hCD45+ cells in the combined mouse and human leukocyte population of bone marrow and spleen of BRGS (□) and hBAFFKI-BRGS (▪) hu-mice. (C) Frequencies of B cells within the hCD45+ cell populations (top) and absolute numbers (bottom) in bone marrow and spleen of BRGS and hBAFFKI-BRGS hu-mice. Each symbol in panels B and C represents a mouse; bars are mean and SEM. N = 14 to 23 mice per group and 3 to 4 different CB groups. **P < .01.
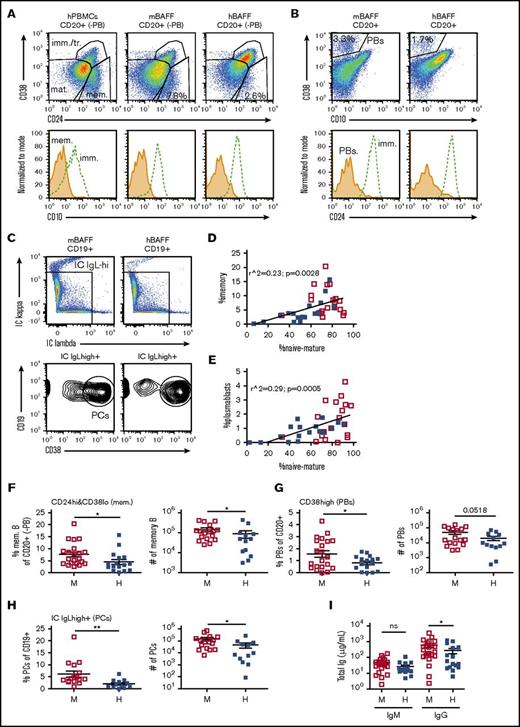
Flow cytometry was used to further characterize human B-cell maturation. Mature B cells were detected by first gating out cells expressing very high CD38 (plasmablasts) from CD20+ gated cells (Figure 4A left column), and then by gating the remaining CD20+ cells as either CD10− (Figure 4A second column) or CD24lowCD38low (Figure 4A third column). Surprisingly, the proportion of B cells that were mature was significantly decreased in the splenic B-cell population of hBAFFKI hu-mice relative to control (Figure 4B). When we measured absolute cell numbers, those of immature B cells were slightly higher and those of mature B cells were slightly lower in hBAFFKI hu-mice (Figure 4C), but these differences were not statistically significant between the 2 groups of hu-mice. Because there was great variation in each group of mice, we performed a paired analysis where we compared the numbers of immature and mature B cells in each mouse. This analysis showed that although there was a significant population expansion from the immature to the mature B-cell compartments in hu-mice expressing mBAFF, this expansion did not occur in hBAFFKI hu-mice (Figure 4D).
Analysis of B-cell maturity in hu-mice. (A) Representative flow cytometric analysis of immature and mature B cells and of T cells in the spleen of BRGS (top) and hBAFFKI-BRGS (bottom) hu-mice. Plasmablasts (PB), identified as CD38 very-high (CD38vhigh) and CD10−, were gated by Boolean gate on CD20+ (and hCD45+) cells to remove them from the rest of the analysis (left column). Remaining CD20+ cells (-PB) were analyzed for CD10, CD24, and CD38 (second and third columns) to establish the proportion of immature/transitional (imm./tr.; CD10+ and CD24highCD38high) and mature (CD24lowCD38low) or mature/memory (mat./mem.; CD10−) B cells. hCD45+ cells were analyzed to establish frequencies of CD3+ T cells (right column). (B) Frequencies of mature B cells (CD10−, left; CD24lowCD38low, right) within the CD20+ (-PB) spleen B-cell fraction of individual BRGS (□) and hBAFFKI-BRGS (▪) hu-mice. (C) Absolute numbers of immature (CD24highCD38high) B cells (left) and of mature (CD24lowCD38low) B cells (right). (D) Correlation between immature (CD24highCD38high) and mature (CD24lowCD38low) B-cell numbers in the spleen of each hu-mouse. Statistical differences were analyzed by paired Student t test. (E) Scatter plot analysis of the percentage of CD10− mature/memory B cells within the CD20+ (-PB) cell population (y-axis) relative to the frequency of CD3+ T cells within the hCD45+ cell population (x-axis) in the spleen of each hu-mouse. Linear regression analyses are shown separately for BRGS and hBAFFKI-BRGS groups of hu-mice. (F) Frequencies of CD3+ T cells in the hCD45+ spleen cell population (left) and absolute cell numbers (right) in BRGS and hBAFFKI-BRGS hu-mice. (G) Frequencies of T cells (left) and of mature B cells (right) in a subset of BRGS and hBAFFKI-BRGS hu-mice with comparable proportion of T cells (>30% and <54% within hCD45+). Each symbol in panels B through G represents a mouse; bars are mean and SEM. N = 16 to 21 mice per group (in B-G) and 4 different CB groups. *P < .05; ***P < .001.
Analysis of B-cell maturity in hu-mice. (A) Representative flow cytometric analysis of immature and mature B cells and of T cells in the spleen of BRGS (top) and hBAFFKI-BRGS (bottom) hu-mice. Plasmablasts (PB), identified as CD38 very-high (CD38vhigh) and CD10−, were gated by Boolean gate on CD20+ (and hCD45+) cells to remove them from the rest of the analysis (left column). Remaining CD20+ cells (-PB) were analyzed for CD10, CD24, and CD38 (second and third columns) to establish the proportion of immature/transitional (imm./tr.; CD10+ and CD24highCD38high) and mature (CD24lowCD38low) or mature/memory (mat./mem.; CD10−) B cells. hCD45+ cells were analyzed to establish frequencies of CD3+ T cells (right column). (B) Frequencies of mature B cells (CD10−, left; CD24lowCD38low, right) within the CD20+ (-PB) spleen B-cell fraction of individual BRGS (□) and hBAFFKI-BRGS (▪) hu-mice. (C) Absolute numbers of immature (CD24highCD38high) B cells (left) and of mature (CD24lowCD38low) B cells (right). (D) Correlation between immature (CD24highCD38high) and mature (CD24lowCD38low) B-cell numbers in the spleen of each hu-mouse. Statistical differences were analyzed by paired Student t test. (E) Scatter plot analysis of the percentage of CD10− mature/memory B cells within the CD20+ (-PB) cell population (y-axis) relative to the frequency of CD3+ T cells within the hCD45+ cell population (x-axis) in the spleen of each hu-mouse. Linear regression analyses are shown separately for BRGS and hBAFFKI-BRGS groups of hu-mice. (F) Frequencies of CD3+ T cells in the hCD45+ spleen cell population (left) and absolute cell numbers (right) in BRGS and hBAFFKI-BRGS hu-mice. (G) Frequencies of T cells (left) and of mature B cells (right) in a subset of BRGS and hBAFFKI-BRGS hu-mice with comparable proportion of T cells (>30% and <54% within hCD45+). Each symbol in panels B through G represents a mouse; bars are mean and SEM. N = 16 to 21 mice per group (in B-G) and 4 different CB groups. *P < .05; ***P < .001.
In a previous study, we indicated that the level of maturity of the human B-cell population in the spleen of BRG hu-mice correlates with the frequency of T cells, and that T cells contribute to B-cell maturation.16 A significant and similar correlation between T cells (identified as shown in Figure 4A right column) and mature B cells was also present in the spleen of BRGS hu-mice, independent of which BAFF species was expressed (Figure 4E). Percentages and numbers of splenic T cells were lower, on average, in hu-mice expressing hBAFF (Figure 4F). To remove the potential effect of T-cell differences, we assessed the maturity of the B-cell population in hu-mice in which the frequency (and absolute numbers, not shown) of T cell was similar and below 55% in both groups (Figure 4G left). The frequency of mature B cells in animals with similar T-cell frequencies was still significantly lower in hBAFFKI hu-mice relative to controls (Figure 4G right). Consequently, this difference was at least partly independent of variations of T-cell frequencies and numbers.
Overall, these data show that the maturation of B cells in hu-mice does not improve with the expression of hBAFF and thus, conversely, it is not restricted by the expression of mBAFF.
Development of terminally differentiated B cells and antibodies
Memory B cells, plasmablasts, and plasma cells are also regulated by BAFF (and by a proliferation-inducing ligand [APRIL]) binding to its receptors BAFFR, transmembrane activator and calcium-modulating ligand interactor (TACI), and B-cell maturation antigen (BCMA).21,35-37 To determine whether expression of hBAFF impacted the distribution of these effector B cells in hu-mice, we used flow cytometry to quantify cells with this phenotype (Figure 5A-C). Memory B cells and plasmablasts, identified as CD20+CD24highCD38−CD10− and CD20+CD38vhighCD10−CD24–, respectively, were proportional to mature (naive) B cells in each hu-mouse, as shown by linear regression analyses (Figure 5D-E). However, both cell populations were significantly decreased in frequencies and numbers in hu-mice expressing hBAFF compared with control hu-mice (Figure 5F-G). Plasma cells with the phenotype of high intracellular IgL chain expression (κ or λ) and CD19+CD38vhigh expression (Figure 5C) were also decreased in both frequencies and numbers in hBAFFKI hu-mice relative to control (Figure 5H). In accordance with these differences, the total serum levels of IgG was, on average, lower in hu-mice expressing hBAFF relative to those expressing mBAFF (Figure 5I).
Memory B cells, plasmablasts, and serum immunoglobulins in hu-mice expressing mBAFF or hBAFF. (A) Representative flow cytometric analysis of CD38 and CD24 expression (top plots) on hCD45+CD20+ from human PBMCs and from spleen of BRGS and hBAFFKI-BRGS hu-mice (after gating out CD38vhigh PBs by Boolean gate as shown in Figure 4A). The plots show the gating of CD38lowCD24high memory B cells, CD38highCD24high immature/transitional B cells, and CD38lowCD24low naive mature B cells. The bottom histograms confirm low CD10 expression on memory B cells relative to the high expression on immature/transitional B cells. (B) Representative flow cytometric analysis of CD38 and CD10 expression (top plots) on total hCD45+CD20+ spleen cells showing the gating of CD38vhighCD10− plasmablasts. The bottom histogram confirms no CD24 expression on the gated plasmablasts (PBs) relative to the high expression on immature/transitional B cells (gated as CD38highCD24high). (C) Representative flow cytometric analysis to identify plasma cells in the spleen of hu-mice. Splenic hCD45+CD19+ cells were first gated for high expression of intracellular (IC) Igκ or Igλ (top plots, shown with biexponential axes). These latter (IC IgL-high+) cells were then gated for high expression of CD38 (bottom plots). (D-E) Scatter plot analyses of the percentage of CD38lowCD24low naive mature B cells (x-axis) relative to the percentage of CD38lowCD24high memory B cells (y-axis) within the CD20+ (-PB) cell population (D), and of the percentage of CD10– mature B cells (as in Figure 4A) within CD20+ (-PB) cells (x-axis) relative to the percentage of CD38vhighCD10− plasmablasts (y-axis) within all CD20+ cells (E) in individual BRGS (□) and hBAFFKI-BRGS (▪) hu-mice. The linear regression analyses were performed on all data. (F) Frequencies of memory B cells (identified as in panel A) in the CD20+ (-PB) splenic B-cell population (left) and absolute cell numbers (right) in individual BRGS and hBAFFKI-BRGS hu-mice. (G) Frequencies of plasmablasts (identified as in panel B) within CD20+ splenic cells (left) and absolute cell numbers (right). (H) Frequencies of plasma cells (identified as in panel C) within CD19+ splenic cells (left) and absolute cell numbers (right). (I) Total IgM and IgG concentrations in serum of BRGS and hBAFFKI-BRGS hu-mice. Each symbol in panels D through I represents a mouse; bars are mean and SEM. N = 16 to 23 mice per group and 4 different CB groups. *P < .05; **P < .01.
Memory B cells, plasmablasts, and serum immunoglobulins in hu-mice expressing mBAFF or hBAFF. (A) Representative flow cytometric analysis of CD38 and CD24 expression (top plots) on hCD45+CD20+ from human PBMCs and from spleen of BRGS and hBAFFKI-BRGS hu-mice (after gating out CD38vhigh PBs by Boolean gate as shown in Figure 4A). The plots show the gating of CD38lowCD24high memory B cells, CD38highCD24high immature/transitional B cells, and CD38lowCD24low naive mature B cells. The bottom histograms confirm low CD10 expression on memory B cells relative to the high expression on immature/transitional B cells. (B) Representative flow cytometric analysis of CD38 and CD10 expression (top plots) on total hCD45+CD20+ spleen cells showing the gating of CD38vhighCD10− plasmablasts. The bottom histogram confirms no CD24 expression on the gated plasmablasts (PBs) relative to the high expression on immature/transitional B cells (gated as CD38highCD24high). (C) Representative flow cytometric analysis to identify plasma cells in the spleen of hu-mice. Splenic hCD45+CD19+ cells were first gated for high expression of intracellular (IC) Igκ or Igλ (top plots, shown with biexponential axes). These latter (IC IgL-high+) cells were then gated for high expression of CD38 (bottom plots). (D-E) Scatter plot analyses of the percentage of CD38lowCD24low naive mature B cells (x-axis) relative to the percentage of CD38lowCD24high memory B cells (y-axis) within the CD20+ (-PB) cell population (D), and of the percentage of CD10– mature B cells (as in Figure 4A) within CD20+ (-PB) cells (x-axis) relative to the percentage of CD38vhighCD10− plasmablasts (y-axis) within all CD20+ cells (E) in individual BRGS (□) and hBAFFKI-BRGS (▪) hu-mice. The linear regression analyses were performed on all data. (F) Frequencies of memory B cells (identified as in panel A) in the CD20+ (-PB) splenic B-cell population (left) and absolute cell numbers (right) in individual BRGS and hBAFFKI-BRGS hu-mice. (G) Frequencies of plasmablasts (identified as in panel B) within CD20+ splenic cells (left) and absolute cell numbers (right). (H) Frequencies of plasma cells (identified as in panel C) within CD19+ splenic cells (left) and absolute cell numbers (right). (I) Total IgM and IgG concentrations in serum of BRGS and hBAFFKI-BRGS hu-mice. Each symbol in panels D through I represents a mouse; bars are mean and SEM. N = 16 to 23 mice per group and 4 different CB groups. *P < .05; **P < .01.
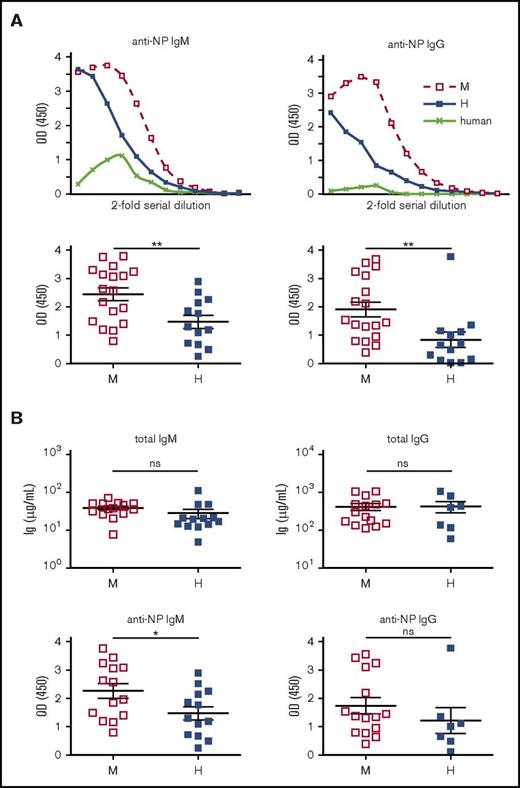
Previous studies have reported that thymus-independent antibody responses to the Pneumovax vaccine were increased by hBAFF treatment in both hu-PBL and hu-mice.19,20 To evaluate T-cell–independent responses, we immunized hu-mice between 18 and 22 weeks with 4-hydroxy-3-nitrophenyl acetyl (NP)–Ficoll and found that T-cell–independent NP-specific IgM and IgG responses were significantly reduced in hu-mice expressing hBAFF relative to control (Figure 6A). To determine whether this was the result of differences in total immunoglobulin levels, we compared NP-specific responses in hu-mice with similar IgM and IgG levels (Figure 6B top). The difference in NP-specific IgM was still significantly lower in hBAFFKI hu-mice (Figure 6B bottom).
T-cell–independent antibody responses. (A) BRGS and hBAFFKI-BRGS hu-mice were immunized with NP-Ficoll and antibody responses to NP were measured in serially diluted serum by ELISA. Human serum was used as negative control (top graphs). NP-specific IgM and IgG responses are indicated as O.D. (405 nm) values. Top graphs are representative ELISA curves after the subtraction of background signal. Data from 3 independent experiments were combined in the bottom graphs. Each experiment (set) showed similar differences between mBAFF and hBAFF samples. The graph reports O.D. values corresponding to the same serum dilution for every sample within a set of ELISAs and within the linear part of the ELISA curves. The serum dilution in each of the 3 sets was selected based on the condition that the average O.D. of the mBAFF group was similar between sets. (B) Bottom graphs display anti-NP IgM and IgG O.D. (405 nm) values for a subset of hu-mice with similar total IgM and IgG levels (top graphs). *P < .05; **P < .01.
T-cell–independent antibody responses. (A) BRGS and hBAFFKI-BRGS hu-mice were immunized with NP-Ficoll and antibody responses to NP were measured in serially diluted serum by ELISA. Human serum was used as negative control (top graphs). NP-specific IgM and IgG responses are indicated as O.D. (405 nm) values. Top graphs are representative ELISA curves after the subtraction of background signal. Data from 3 independent experiments were combined in the bottom graphs. Each experiment (set) showed similar differences between mBAFF and hBAFF samples. The graph reports O.D. values corresponding to the same serum dilution for every sample within a set of ELISAs and within the linear part of the ELISA curves. The serum dilution in each of the 3 sets was selected based on the condition that the average O.D. of the mBAFF group was similar between sets. (B) Bottom graphs display anti-NP IgM and IgG O.D. (405 nm) values for a subset of hu-mice with similar total IgM and IgG levels (top graphs). *P < .05; **P < .01.
In summary, endogenous expression of hBAFF in hu-mice does not improve the generation of effector B cells and of T-cell–independent antibody responses.
Discussion
Human B cells developing from hHSCs within immunodeficient hosts exhibit defective maturation.16 This study was undertaken to determine whether this defect is (at least partly) due to the inability of mBAFF to regulate human B cells. To address this, we developed a novel immunodeficient mouse strain (hBAFFKI) that expresses hBAFF in place of mBAFF and transplanted these animals with either human PBMCs or hHSCs. Results from these studies reveal that endogenous expression of hBAFF increases neither the recovery of B cells in animals transplanted with human PBMCs (hu-PBL mice) nor B-cell numbers and maturation in animals transplanted with hHSCs (hu-mice). Consequently, we conclude that mBAFF is not responsible for the impaired ability of human B cells to survive and mature in hu-mice.
Several studies have shown that the deletion of the gene encoding BAFF in mice and the antibody-mediated neutralization of BAFF in humans lead to a similar loss of late transitional and naive mature B cells,22-24,38-42 demonstrating that BAFF is required for the maintenance of mature B cells in both species. Moreover, supraphysiological levels of serum BAFF as achieved in BAFFTg mice, lead to higher numbers of mature B cells,43-45 indicating that B-cell numbers are regulated by BAFF levels. Whether this is also true in humans is difficult to verify because the consumption of BAFF by B cells leads to an inverse correlation between BAFF levels and B-cell numbers.30,46,47 Hence, in the absence of injecting BAFF into humans, the relationship between BAFF levels and the numbers of mature human B cells remains uncertain. However, increased recovery of human B cells has been reported in hu-PBL mice injected daily with hBAFF,19 suggesting an analogous dependency between BAFF levels and human B-cell numbers. More importantly, this latter study showed that hBAFF leads to higher NF-κB activity in human B cells relative to mBAFF,19 which has led to the proposition that mBAFF has reduced bioactivity on human cells, a premise for our study. Based on these multiple observations, we strongly anticipated finding improved numbers of human B cells, with a higher proportion of mature B cells, in hu-mice expressing endogenous hBAFF. This was not the case.
Our analyses of hu-mice (generated with transplantation of CD34+ CB cells) revealed that endogenous expression of hBAFF did not increase the proportion of mature B cells relative to immature B cells and, thus, did not correct the defect we set out to address. In fact, the opposite was true. The proportion of mature B cells in the splenic B-cell population was significantly decreased in hBAFFKI hu-mice relative to control. Of note, mature B cells were present in the spleen of hBAFFKI hu-mice, and although their average numbers were similar to those of control mice, paired analyses indicated an inability to expand relative to immature B-cell numbers. These findings raise the question of whether the hBAFF cytokine expressed in hBAFFKI mice is functional and is present at sufficient concentrations to promote B-cell survival and maturation. Our findings suggest that this is the case. First, we show that the serum concentration of hBAFF is at least 50-fold higher in hBAFFKI hu-mice relative to control hu-mice. The concentration of circulating hBAFF in the blood of hBAFFKI hu-mice appears to be also approximately fivefold higher relative to human blood and, so, it should be sufficient for normal B-cell survival. Second, there were lower levels of surface BAFFR on human B cells from hBAFFKI hu-mice relative to control hu-mice (supplemental Figure 4). Given hBAFFR levels decrease in proportion to binding BAFF,48 this confirms the increased production of hBAFF in hBAFFKI hu-mice and demonstrates the ability of the recombinant hBAFF to bind the hBAFFR. Third, when hBAFFKI mice were analyzed on the wild-type BALB/c genetic background in the presence of mouse B cells, there was a significant increase in mouse B-cell frequencies and the level of surface CD21 these B cells expressed (supplemental Figure 3A-B). These findings are similar to what has previously been described in BAFFTg mice that exhibit supraphysiological levels of soluble BAFF,30,31,43,45,49,50 and are also in line with the ability of hBAFF to associate with the mBAFFR.30,35 Together, we consider these data to demonstrate that the hBAFF expressed in hBAFFKI mice is bioactive and functional.
Our analyses further show that B cells of hu-mice express BAFFR, which is the receptor responsible for translating binding BAFF into mature B-cell survival.51,52 Consequently, B cells that develop in hu-mice have the ability to respond to BAFF. It is puzzling, therefore, that endogenous expression of hBAFF led to decreased numbers of mature B cells in hu-mice. B cells of CB display increased susceptibility to apoptosis relative to those of adult blood.53 Thus, the defective B-cell maturation in our hu-mice may be related to the use of CB HSCs. However, previous studies have shown that the survival of CB B cells, like that of adult B cells, is dependent on BAFF.53 Moreover, although there are only a handful of studies that compared B cells in hu-mice generated with CB or adult CD34+ cells, defects in B-cell differentiation have been observed in this animal model irrespective of the source of CD34+ cells.7,54 An alternative explanation is that the hBAFF transgene we introduced in mice encodes only the full-length form of BAFF and does not allow expression of alternative forms.55,56 Although this has not been an issue for mouse B cells in BAFFTg mice, it is possible that human B cells have different requirements.
BAFF is also required for the maintenance of memory B cells and contributes, together with APRIL, to the survival of plasmablasts and plasma cells.21,35-37 Based on this, we also anticipated observing increased numbers of these effector B-cell populations in hBAFFKI hu-mice, a hypothesis supported by studies in which injection of hBAFF in hu-PBL and hu-mice improved thymus-independent antibody responses to the Pneumovax vaccine.19,20 Our linear regression analyses comparing naive B cells and memory B cells or plasmablasts in hu-mice indicated a correlation between effector and naive cell populations. However, although the numbers of naive mature B cells were not significantly different in hBAFFKI and control hu-mice, numbers of memory B cells, plasmablasts, and plasma cells were actually decreased in hBAFFKI hu-mice. These findings were analogous to those in hu-PBL mice where we found reduced proportion and expansion of CD19lowCD38high plasmablasts/plasma cells relative to naive B cells in hBAFFKI recipients relative to control. Reduced total human IgG levels, as well as IgM and IgG antibody responses to the thymus-independent antigen NP-Ficoll, were also observed in hBAFFKI hu-mice. Overall, these data demonstrate that endogenous expression of hBAFF does not improve the size of the effector B-cell compartments and the magnitude of T-cell–independent antibody responses. Instead, humanized hBAFFKI mice display a defect in the differentiation of naive B cells into effector B-cell populations.
We have previously shown that the relative proportion of mature B cells in hu-mice correlated with and depended on the frequency of T cells in the human leukocyte population,16 and we observed a similar correlation in the present study. This correlation occurred also in hu-mice with endogenous hBAFF, though hBAFFKI hu-mice exhibited lower numbers of splenic T cells. In order to assess B-cell maturation without the differences in T cells, we compared groups of hBAFFKI and control hu-mice with similar frequencies of T cells. We found that the proportion of mature B cells in hBAFFKI mice was still lower relative to mBAFF-expressing animals. Thus, although likely playing a role, decreased T-cell numbers were not the sole cause of reduced B-cell maturation in hBAFFKI hu-mice.
The lack of expansion of mature and effector B cells in our hBAFFKI hu-mice remains unexplained. Although we cannot exclude that hBAFF might improve human B-cell maturation and function in hu-mice in the presence of additional factors, the most simple explanation of our data is that the presumed low bioactivity that mBAFF has been shown to exercise on human B cells19 is not the (main) factor responsible for limiting human B-cell maturation in hu-mice. It follows that other factors are largely accountable for this phenomenon.
In summary, we show that, the defective maturation human B cells exhibit when developing in immunodeficient mice is not corrected with the expression of endogenous hBAFF, suggesting that this defect is not caused by suboptimal function of mBAFF. Thus, we conclude that other factors, such as those expressed by T cells, exist to regulate maturation and survival of B cells in hu-mice.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the National Jewish Health (NJH) and University of Colorado Anschutz Medical Center (AMC) cancer center flow cytometry facilities for assistance with cell sorting and analysis, and the Biological Resource Center at NJH and the Vivarium at the University of Colorado AMC for assistance with mouse husbandry. The authors are very grateful to all members of their laboratories for numerous useful discussions. The authors are grateful to Donna Bratton and the Clinical Division of NJH for providing human PBMC samples and to ClinImmune Labs (University of Colorado) for providing human umbilical CB samples. The authors thank Hélène Strick-Marchand (Institut Pasteur, Paris, France) for facilitating the shipment of BRGS mice to them.
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases grants R21-AI105523 (R.P.), R01-AI124474 (R.P.), R01-AI052310 (R.P.), and R01-052157 (R.M.T.), and by National Cancer Institute Cancer Center support grant P30CA046934 for shared resources. J.P.D.S. was supported by grants from European Commission Seventh Framework Programme no. 305578 (PathCO), Institut Pasteur, and INSERM.
Authorship
Contribution: J.L., B.Z., J.L.M., and R.P. designed research; J.L., B.Z., M.K., J.N.P., and J.B. performed research; J.L. B.Z., J.L.M., R.M.T., and R.P. analyzed and interpreted data; B.M.F. and J.P.D.S. contributed essential reagents; R.P. wrote the paper; and J.L., R.M.T., J.P.D.S., and R.P. edited the paper.
Conflict-of-interest disclosure: J.P.D.S. is a stakeholder in AXENIS (founder, member of the executive board). The remaining authors declare no competing financial interests.
Correspondence: Roberta Pelanda, School of Medicine, University of Colorado, 12800 East 19th Ave, Mail Stop 8333, Aurora, CO 80045-2508; e-mail: roberta.pelanda@ucdenver.edu.