Case
A 5-year-old with sickle cell anemia (SCA) presents with a history of multiple prior pain and acute chest syndrome (ACS) episodes in the last 12 months, with a similar pattern of multiple prior pain and ACS episodes annually for the last several years. The patient has been on maximum tolerated dose of hydroxyurea (HU) therapy for the last 3 years, has been evaluated, and does not have a diagnosis of asthma.
After multiple discussions with Fitzhugh and Walters, who took the opposite position in the virtual debate, here are the points on which we agree:
In high-income countries, the overall survival rate in children with SCA is >98%, particularly while on HU therapy.
The risk of having a first stroke, the major morbidity for SCA, drops a log-fold (11% to 1%) with routine transcranial Doppler screening, and when children are detected as having an elevated transcranial Doppler (TCD) velocity and treated with regular blood transfusion therapy for at least a year.
Hematopoietic stem cell transplant (HSCT), even with human leukocyte antigen–matched siblings, is still evolving. We do not know the optimal therapy that maximizes treatment and minimizes death and late effects of HSCT.
Transplant biology is advancing quickly, such that we expect improved HSCT outcomes 10 years from now and greater donor availability.
We believe that National Institutes of Health–supported Bone Marrow Transplant–Clinical Trial Network (BMT-CTN) should support future HSCT trials in children and adults with SCA, along with long-term follow-up with central adjudication of organ function (cerebral infarcts, end-stage renal disease, congestive heart failure, pulmonary hypertension, chronic lung disease), quality of life, morbidity, and mortality. The long-term follow-up should be compared with outcomes of contemporary cohorts of children and adults with SCA receiving maximum medical therapy with identical central adjudication of organ function, quality of life, morbidity, and mortality. Without such analyses, opinions without data will be made about benefits of HSCT and other curative therapies.
Single-center HSCT standard protocols for SCA significantly limit our ability to advance the care of this rare disease in high-income countries. Children with SCA that undergo HSCT should be offered a BMT-CTN protocol or at least a regional trial, with a clear upfront discussion communicating that we do not know the optimal HSCT strategy. The family should be informed that they are participating in research with the expectation that we will follow their child for at least a decade to determine the long-term risks and benefits of therapy.
In 2017, in an asymptomatic child with SCA with an HLA-matched sibling donor, we would not offer HSCT as a first option. In families with unique situations, we may consider HSCT for an asymptomatic child with SCA; however, this would be a rare occurrence.
Commentary
Children with sickle cell disease (SCD) in high-income countries who receive high-quality supportive care no longer have a high rate of childhood mortality. The standard use of conjugated vaccines and penicillin prophylaxis has all but eliminated life-threatening childhood infections for children with SCD. HU therapy has dramatically decreased the incidence rate of the most common sequelae of SCD in childhood: acute vaso-occlusive pain events and ACS, a poorly defined pulmonary disease that can be life-threatening. Over the last 40 years, with optimal care, the rate of an initial stroke has dropped a log-fold from 0.67 strokes per year to 0.06 strokes per year with the routine use of TCD measurements in children with SCA.1 Those with abnormal TCD velocities are treated with at least 12 months of regular blood transfusion therapy,2 followed by HU therapy or ongoing regular blood transfusion therapy.3
A lingering misconception in the pediatric HSCT and SCD communities is that children with multiple acute vaso-occlusive pain and ACS episodes will either die early from their disease or have significant and progressive decline in organ function (cerebral infarcts, renal, cardiac, or pulmonary disease) in adulthood, resulting in earlier death. Thus, multiple acute pain or ACS episodes are now indications for HSCT.4 However, evidence that multiple pain or ACS episodes predict early mortality or progressive end-organ disease is lacking. The misinterpretation of the sequelae of multiple acute severe pain and ACS episodes is in part based on outdated clinical experiences from 20 years ago.5 In 1 of the first attempts to predict markers of disease severity in young children with SCD, Miller et al examined an infant cohort in the Cooperative Study for Sickle Cell Disease to identify risk factors for severe disease.5 However, the analysis plan was flawed from the onset because the primary outcome measure for severity was a composite of 4 endpoints with unequal clinical significance: death, stroke, at least 2 acute vaso-occlusive pain episodes per year, and at least 1 ACS event per year. These 4 outcomes had unequal clinical impact, for instance, ACS vs death. Furthermore, the study results are not pertinent to a contemporary cohort of children with SCA because participants were born before routine use of the following: (1) penicillin prophylaxis; (2) conjugated vaccines preventing life-threatening childhood infections; (3) HU therapy in young children; and (4) randomized controlled trials demonstrating the efficacy of HU and regular blood transfusion therapy for primary2 and secondary stroke prevention in children with SCA.3,6 Others have attempted to replicate the predictive model for severity of SCA in childhood without success.7 No model has been published to predict death or organ failure (end-stage renal disease, congestive heart failure, pulmonary hypertension, chronic lung disease) because of these events are infrequent in children with SCA.
Another misperception, relevant to the case presentation, is that multiple prior acute severe pain and ACS episodes are associated with future chronic lung disease. In the most rigorous cohort study to date, the incidence rates of prior lifetime episodes of acute severe pain and ACS episodes did not predict abnormal spirometry results in children with SCA.8
Currently, the only consensus indication for HSCT in children with SCA is for those with strokes receiving regular blood transfusion therapy, a palliative intervention, where at least 45% will have infarct recurrence in ∼5 years.9 We believe that HSCT is superior to regular blood transfusion therapy for secondary stroke prevention, but we do not have strong evidence to support this position. A clinical trial is long overdue to directly compare the 2 treatment strategies (HSCT vs regular blood transfusion therapy) for secondary stroke prevention.
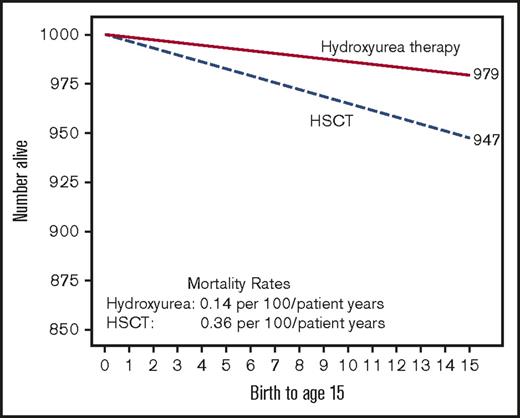
Our colleagues, who advocate for offering HSCT to children without strokes, or progressive decline in renal, cardiac, or lung function, argue that HSCT is at least no worse than standard care therapies. In a recent review, Walters and colleagues compare the Kaplan-Meier estimates of 15-year survival rates of children with SCA who are not on HU therapy to HSCT. The authors conclude that 15-year survival after HSCT compared to supportive care (no hydroxyurea therapy) was not significantly different (0.36 and 0.38 deaths per 100 patient years, respectively).4 The main challenge with interpreting their conclusion is the omission of the fact that in the same cohort children receiving HU therapy had a statistically significant lower mortality rate than those receiving HSCT, 0.14 and 0.36 deaths per 100 patient-years, respectively (P = .01), leading to a better 15-year survival rate (Figure 1).10
Estimated childhood for survival for children with SCA based on therapy. Hypothetical cohort of 1000 children with SCA followed for 15 years receiving either HU therapy (children with severe disease) or HSCT. Mortality rates are based on the Belgium cohort study.10
Estimated childhood for survival for children with SCA based on therapy. Hypothetical cohort of 1000 children with SCA followed for 15 years receiving either HU therapy (children with severe disease) or HSCT. Mortality rates are based on the Belgium cohort study.10
Our colleagues Fitzhugh and Walters justify their decision to perform HSCTs in children with multiple acute pain or ACS episodes by stating that “HSCT prevents inevitable progressive decline in end-organ function in adulthood and has a low mortality rate for HLA-matched sibling donor.” However, as we mentioned earlier, no evidence is available showing that multiple pain and ACS episodes in childhood is associated with future disease. Further, their assertion omits the well-established bias in small single-center trials that consistently overestimate the treatment effect when compared with multicenter trials.11 They also do not reference the experience of aggregate data demonstrating a 5-year Kaplan-Meier estimate of death rate of ∼5% and chronic graft-versus-host disease rate of ∼13% for children <16 years of age in which severe chronic graft-versus-host disease may provide a worse quality of life than severe SCA.12 Often, the more recent French multicenter experience is referenced for the most favorable overall survival rate of 97% in HLA-matched sibling donors in HSCT. However, the median follow-up time was only 3 years,13 and as many as 10% of children receiving HSCT may die 5 years after the procedure.12 Thus, a 3-year median follow-up after HSCT, in a chronic disease with a very low mortality, such as SCA, is simply too short of a period to use when compared with overall survival of at least 15 years with HU therapy. Furthermore, clinicaltrials.gov has >20 open HSCT trials for individuals with SCD, providing clear evidence that HSCT is not currently standard care in SCD.
Two other cohort studies provide direct evidence of better long-term survival of children with SCA, particularly children with HU therapy, than long-term survival of children treated with matched sibling donor HSCT. In a cohort from East London, United Kingdom, Telfer et al demonstrated a 16-year Kaplan-Meier estimate of survival of 99% for infants followed at their tertiary care medical center.14 In another pediatric cohort, data were collected from a region, not a single hospital. After providing electronic access to standard care guidelines for the management of children with SCD, the 5-year Kaplan-Meier estimate of survival was 99%.15 Taken together, results of these 3 cohort studies provide compelling evidence that children with SCA have close to a 99% chance of living at least through their 16th birthday.
In the United States, a new standard has emerged for institutional review boards to evaluate the merits of HSCT or other curative trials for children with SCA. Now, members of institutional review boards must consider the combined evidence of these 3 recent cohort studies when deciding whether “the relation of the anticipated benefit to the risk of HSCT is at least as favorable to the subjects as that presented by available alternative approaches” for greater than minimal risk research involving children.16 The position of Fitzhugh and Walters to perform HSCT based on “the best interest of the child” is not a governing institutional review board standard for approving clinical research in the United States.
We support offering multicenter clinical trial for HSCT trial to fully informed adults with SCD and to older adolescents who are capable of weighing the risks and benefits for themselves. However, regardless of the wishes of the parents, pediatricians are ethically obligated not to inflict avoidable harm on children who cannot make their own informed decision (primum non nocere). We believe that HSCT in a multicenter peer-reviewed clinical trial should only be offered to children with SCA that have progressive decline in organ function. At this point, only children with the following criteria should be considered for HSCT: (1) strokes treated after receiving regular blood or HU therapy; (2) progressive cerebral infarcts in children with silent strokes after receiving regular blood transfusion or HU therapy; or (3) progressive decline, in function of kidneys, heart, or lungs. The now very low expected mortality rate (<1%) in children with SCA, living in high-income countries receiving standard medical care, means the bar has been set very high before HSCT can be offered as an alternative.
In summary, we believe to advance care in children with SCD, as is the case in pediatric oncology 50 years ago, the community of families, investigators, and other stakeholders must come together to ensure the most compelling questions about the role of HSCT. The National Institutes of Health–sponsored BMT-CTN is arguably in the only position to fill this void in the United States. The absence of an organized national strategy to establish cures for SCD has resulted in dozens of open single- or limited-institution SCD HSCT or curative therapy trials competing for the same limited number of eligible children. Surely, the SCD community is now poised to follow the well-established example of our pediatric oncology peers with the tradition of National Cancer Institute–supported pediatric oncology consortium to address the most compelling questions to cure SCD in a systematic and rigorous fashion.
Acknowledgments
The authors thank the members of the Vanderbilt Meharry Center of Excellence Research Laboratory for reviewing the manuscript prior to submission.
Authorship
Contribution: M.R.D. and E.W.C. conceived and drafted the manuscript, revised the manuscript, and approved the final version to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Vanderbilt University School of Medicine, 2200 Children’s Way, 11206 DOT, Nashville, TN 37232-9000; e-mail: m.debaun@vanderbilt.edu.