Key Points
CML patients can be stratified into 4 subgroups with different risk of blastic transformation based on cytogenetic abnormalities.
TKI therapy mitigates risk of blastic transformation associated with low-risk ACAs or no ACAs but not that associated with HR ACAs.
Abstract
The high fatality of patients with blast phase (BP) chronic myeloid leukemia (CML) necessitates identification of high-risk (HR) patients to prevent onset of BP. Here, we investigated the risk of BP based on additional chromosomal abnormality (ACA) profiles in a cohort of 2326 CML patients treated with tyrosine kinase inhibitors (TKIs). We examined the time intervals from initial diagnosis to ACA emergence (interval 1), from ACA emergence to onset of BP (interval 2), and survival after onset of BP (interval 3). Based on BP risk associated with each ACA, patients were stratified into intermediate-1, intermediate-2, and HR groups, with a median duration of interval 2 of unreached, 19.2 months, and 1.9 months, respectively. There was no difference in durations of intervals 1 or 3 among 3 groups. Including patients without ACAs who formed the standard-risk group, the overall 5-year cumulative probability of BP was 9.8%, 28.0%, 41.7%, and 67.4% for these 4 groups, respectively. The pre-BP disease course in those who developed BP was similar regardless of cytogenetic alterations, and 84.4% of BP patients developed BP within the first 5 years of diagnosis. In summary, interval 2 is the predominant determinant of BP risk and patient outcome. By prolonging the duration of interval 2, TKI therapy mitigates BP risk associated with low-risk ACAs or no ACAs but does not alter the natural course of CML with HR ACAs. Thus, we have identified a group of patients who have HR of BP and may benefit from timely alternative treatment to prevent onset of BP.
Introduction
Chronic myeloid leukemia (CML) is characterized by a natural history of 3 distinct stages: chronic phase (CP), accelerated phase (AP), and blast phase (BP).1 CML usually presents in an indolent CP; without effective therapy it will progress to a fatal BP over 2 to 4 years, with an annual incidence of transformation over 20%.2 Conventional therapies including radiotherapy, busulfan, and hydroxyurea, with the exception of interferon-α, offer only symptomatic alleviation and leave the natural course of the disease unaffected.2,3 The introduction of tyrosine kinase inhibitors (TKIs) has fundamentally altered the disease course of CML.4 With TKI therapy, most patients with CP-CML can have a near-normal life expectancy.5 However, ∼5% of patients will undergo progression with an annual incidence of blastic transformation of 1% to 1.5%.6
Despite the remarkable effects of TKI therapy in patients with CP-CML and thus low incidence of blastic transformation, BP-CML remains a major therapeutic challenge in the TKI era. Although TKIs may exhibit some degree of activity in BP, the rate and duration of response are less favorable than in patients whose disease is in CP, and resistance and relapse are common.7-9 Intensive chemotherapy in BP can be effective, but is less successful than in de novo acute leukemia, and returning to CP is observed in only about 10% of patients, providing a small chance for consolidation with allogeneic hematopoietic stem cell transplantation (allo-HSCT).10 Overall, the median survival in patients with BP-CML is usually <1 year, even in the TKI era.7,11-17
Considering the lack of effective treatment of BP, a potential key to improving patient outcome may lie in preventing the onset of BP. The challenge is to identify patients early who are at a high risk (HR) of developing BP so that an alternative treatment may be offered before the onset of BP. Additional chromosomal abnormalities (ACAs) in Philadelphia chromosome–positive (Ph+) cells are important determinants of patient outcome. Based on their frequency, ACAs have been stratified into major [trisomy 8 (+8), i(17q), trisomy 19 (+19), and an extra copy of Ph (+Ph)] and minor-route ACAs. Currently, major-route ACAs at initial diagnosis and any ACAs in Ph+ cells acquired during therapy are considered criteria for defining AP.18 However, this frequency-based stratification of ACAs into major- and minor-route ACAs may not necessarily reflect the underlying biology or their impact on disease progression. Additionally, not all ACAs acquired during therapy have an equal impact on prognosis. We previously showed that +8, a major-route ACA, emerging initially or during therapy, confers a relatively better prognosis.19 In contrast, patients with 3q26.2 rearrangement or monosomy 7/7q deletion (−7/7q−), 2 minor-route ACAs, have a poor treatment response to TKIs and a dismal survival.20,21 Once the disease progresses to the stage of BP, however, the prognostic impact of ACAs is diminished greatly regardless of the type or the complexity of ACAs, supporting that the impact of ACAs is disease stage–dependent and ACA type–dependent, and that the major role of ACAs lies in promoting BP.22,23 However, it is unknown how each ACA promotes BP differently in the TKI era.
In this study, we investigated the value of ACAs in predicting blastic transformation of CML in a large cohort of 2326 patients treated with TKIs. Based on the risk of blastic transformation associated with each common ACA, we stratified patients into 4 different groups and identified a group of patients who are at HR of rapid blastic transformation and require timely alternative treatment to prevent the onset of BP.
Patients and methods
Patient selection
CML patients that met the following selection criteria were included in this study: 1, receiving TKIs; 2, age at diagnosis ≥18 years; and 3, presence of t(9;22)(q34,q11.2) or variant translocations detected by conventional chromosomal analysis and confirmed by fluorescence in situ hybridization (FISH) and molecular analyses. We excluded patients with cryptic Ph and patients with isolated myeloid sarcoma without concurrent BP in peripheral blood or bone marrow (BM) because isolated myeloid sarcoma in CML patients confers a prognosis distinct from that of medullary BP,24,25 and chromosomal analysis and disease monitoring were not routinely performed on myeloid sarcoma tissue. BP was defined as ≥20% blasts in peripheral blood or BM. The study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center and conducted in accordance with the Declaration of Helsinki.
Karyotyping, FISH, and molecular analyses
Conventional chromosomal analysis was performed on G-banded metaphase cells prepared from cultured BM aspirates. FISH analysis was performed on cultured BM aspirate or direct BM smears. Molecular analyses for BCR-ABL1 transcript subtype and quantification were performed as previously described.23 For initial diagnosis and follow-up disease monitoring, karyotyping, FISH, and molecular analyses were performed generally in line with the European LeukemiaNet recommendations and National Comprehensive Cancer Network guidelines.26,27 The clonality of an ACA was defined according to the International System for Human Cytogenetic Nomenclature (2016).28 Only cytogenetic abnormalities in Ph+ cells were considered ACAs. Variant Ph translocations and −Y were not considered as ACAs.29-32 We only focused on the first ACAs in each patients.
Statistical analysis
Time-to-event data, including overall survival (OS) and time to the onset of BP, was determined by the Kaplan-Meier method. The median follow-up time was estimated using the reverse Kaplan-Meier method,33 and differences between groups were evaluated by the log-rank test. OS was calculated from 3 different start points: (1) date of initial CML diagnosis; (2) date of emergence of first ACAs; and (3) date of BP. The end point was the date of last follow-up or death.
Results
Clinicopathologic characteristics
A total of 2326 patients were included in this study, including 1322 men and 1004 women with a median age of 49 years (range, 18-88 years) at initial diagnosis of CML (Table 1). All patients received TKI therapy per the selection criteria. Additionally, 254 patients (10.9%) underwent allo-HSCT. The median follow-up time was 109.8 months (range, 0.7-221.6 months).
Clinical characteristics of patients with different types of additional chromosomal abnormalities
| . | 3q26.2, n = 25 . | −7/7q−, n = 13 . | i(17q), n = 22 . | +8, n = 45 . | +Ph, n = 42 . | Other single, n = 186 . | High-risk complex, n = 74 . | Other complex, n = 163 . | No ACA, n = 1756 . |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male | 20 | 10 | 14 | 27 | 29 | 108 | 46 | 99 | 969 |
| Female | 5 | 3 | 8 | 18 | 13 | 78 | 28 | 64 | 787 |
| Age at Dx, y | |||||||||
| Median | 52 | 57 | 44 | 46 | 48 | 48 | 52 | 48 | 49 |
| Range | 21-76 | 26-70 | 22-73 | 21-75 | 22-86 | 18-81 | 20-78 | 19-88 | 18-87 |
| Interval 1, mo* | |||||||||
| Median | 15.2 | 8.3 | 33.3 | 12.9 | 33.6 | 5.8 | 14.0 | 14.6 | N/A |
| Range | 0-122 | 0-111 | 0-112 | 0-100 | 0-182 | 0-159 | 0-143 | 0-203 | N/A |
| Interval 2, mo† | |||||||||
| Median | 2.8 | 0 | 32.6 | NR | NR | NR | 0 | 19.2 | N/A |
| BP phenotype | |||||||||
| Myeloid | 21 | 4 | 14 | 7 | 7 | 47 | 46 | 66 | 64 |
| Lymphoid | 0 | 6 | 2 | 5 | 3 | 23 | 14 | 24 | 52 |
| Mixed phenotype | 0 | 1 | 0 | 0 | 1 | 5 | 0 | 3 | 3 |
| No BP | 4 | 2 | 6 | 33 | 31 | 111 | 14 | 70 | 1637 |
| ACA emergence | |||||||||
| Before BP | 12 | 2 | 9 | 5 | 7 | 21 | 15 | 24 | N/A |
| At BP | 6 | 6 | 2 | 4 | 2 | 36 | 32 | 47 | N/A |
| After BP | 3 | 3 | 5 | 3 | 2 | 18 | 13 | 22 | N/A |
| Response‡ | |||||||||
| CCyR or deeper | 5 | 2 | 3 | 18 | 19 | 82 | 12 | 43 | 1249 |
| MMR or deeper | 6 | 1 | 3 | 18 | 13 | 62 | 9 | 37 | 1112 |
| Status at last F/U | |||||||||
| Alive | 3 | 2 | 3 | 24 | 30 | 95 | 19 | 60 | 1434 |
| Dead | 22 | 11 | 19 | 21 | 12 | 91 | 55 | 103 | 322 |
| Allo-HSCT | 12 | 3 | 3 | 8 | 6 | 33 | 26 | 35 | 128 |
| . | 3q26.2, n = 25 . | −7/7q−, n = 13 . | i(17q), n = 22 . | +8, n = 45 . | +Ph, n = 42 . | Other single, n = 186 . | High-risk complex, n = 74 . | Other complex, n = 163 . | No ACA, n = 1756 . |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male | 20 | 10 | 14 | 27 | 29 | 108 | 46 | 99 | 969 |
| Female | 5 | 3 | 8 | 18 | 13 | 78 | 28 | 64 | 787 |
| Age at Dx, y | |||||||||
| Median | 52 | 57 | 44 | 46 | 48 | 48 | 52 | 48 | 49 |
| Range | 21-76 | 26-70 | 22-73 | 21-75 | 22-86 | 18-81 | 20-78 | 19-88 | 18-87 |
| Interval 1, mo* | |||||||||
| Median | 15.2 | 8.3 | 33.3 | 12.9 | 33.6 | 5.8 | 14.0 | 14.6 | N/A |
| Range | 0-122 | 0-111 | 0-112 | 0-100 | 0-182 | 0-159 | 0-143 | 0-203 | N/A |
| Interval 2, mo† | |||||||||
| Median | 2.8 | 0 | 32.6 | NR | NR | NR | 0 | 19.2 | N/A |
| BP phenotype | |||||||||
| Myeloid | 21 | 4 | 14 | 7 | 7 | 47 | 46 | 66 | 64 |
| Lymphoid | 0 | 6 | 2 | 5 | 3 | 23 | 14 | 24 | 52 |
| Mixed phenotype | 0 | 1 | 0 | 0 | 1 | 5 | 0 | 3 | 3 |
| No BP | 4 | 2 | 6 | 33 | 31 | 111 | 14 | 70 | 1637 |
| ACA emergence | |||||||||
| Before BP | 12 | 2 | 9 | 5 | 7 | 21 | 15 | 24 | N/A |
| At BP | 6 | 6 | 2 | 4 | 2 | 36 | 32 | 47 | N/A |
| After BP | 3 | 3 | 5 | 3 | 2 | 18 | 13 | 22 | N/A |
| Response‡ | |||||||||
| CCyR or deeper | 5 | 2 | 3 | 18 | 19 | 82 | 12 | 43 | 1249 |
| MMR or deeper | 6 | 1 | 3 | 18 | 13 | 62 | 9 | 37 | 1112 |
| Status at last F/U | |||||||||
| Alive | 3 | 2 | 3 | 24 | 30 | 95 | 19 | 60 | 1434 |
| Dead | 22 | 11 | 19 | 21 | 12 | 91 | 55 | 103 | 322 |
| Allo-HSCT | 12 | 3 | 3 | 8 | 6 | 33 | 26 | 35 | 128 |
CCyR, complete cytogenetic response; Dx, diagnosis; F/U, follow-up; MMR, major molecular response; N/A, not applicable; NR, not reached.
Interval 1: time interval from initial diagnosis of CML to the emergence of ACAs.
Interval 2: time interval from emergence of ACAs to blastic transformation.
Response after emergence of ACAs but before blastic transformation. All ACAs, regardless of the emerging time, were included.
In total, 570 patients (24.5%) had ACAs: 333 patients (58.4%) had single ACAs and 237 (41.6%) had ≥2 ACAs (or complex ACAs) at emergence of first ACAs. The most common single ACAs was +8 (n = 45), followed by +Ph (n = 42), 3q26.2 rearrangement (n = 25), i(17q) (n = 22), and −7/7q− (n = 13). Of 237 patients with complex ACAs, 18 had 3q26.2 rearrangement, 19 had −7/7q−, and 30 had i(17q) as a component of complex ACAs, respectively. Seven patients had 2 of these 3 ACAs in different combinations. Given the adverse prognostic value of these 3 chromosomal abnormalities in myeloid neoplasms, these complex ACAs were grouped together as HR complex ACAs. Complex ACAs without these 3 abnormalities were designated as other complex ACAs.
Frequency and lineage distribution of blastic transformation
In total, 418 patients developed BP, including 91 patients diagnosed in BP at initial presentation and 327 patients who developed BP later after a median follow-up time of 23.4 months (range, 0.4-205 months). By immunophenotype, 276 patients (66.0%) had myeloid BP (MyBP), 129 (30.9%) had lymphoid BP (LyBP), and 13 (3.1%) had mixed-phenotype BP (Table 2).
Characteristics of patients with BP-CML
| . | Myeloid, n = 276 . | Lymphoid, n = 129 . | Mixed phenotype, n = 13 . |
|---|---|---|---|
| Sex | |||
| Male | 163 | 91 | 7 |
| Female | 113 | 38 | 6 |
| Age at BP, y | |||
| Median | 49 | 48 | 50 |
| Range | 20-88 | 18-81 | 23-70 |
| Latency, mo* | |||
| Median | 19.2 | 8.9 | 1.0 |
| Range | 0-205 | 0-141 | 0-73 |
| OS after BP, mo | |||
| Median | 10.2 | 18.3 | 17.8 |
| Range | 0.03-183 | 0.03-181 | 0.2-126 |
| Status at last F/U | |||
| Alive | 61 | 45 | 3 |
| Dead | 215 | 84 | 10 |
| ACAs | |||
| No | 64 | 52 | 3 |
| Single | 100 | 39 | 7 |
| Complex | 112 | 38 | 3 |
| ACA emergence | |||
| Before BP | 79 | 15 | 1 |
| At BP | 86 | 41 | 8 |
| After BP | 47 | 21 | 1 |
| . | Myeloid, n = 276 . | Lymphoid, n = 129 . | Mixed phenotype, n = 13 . |
|---|---|---|---|
| Sex | |||
| Male | 163 | 91 | 7 |
| Female | 113 | 38 | 6 |
| Age at BP, y | |||
| Median | 49 | 48 | 50 |
| Range | 20-88 | 18-81 | 23-70 |
| Latency, mo* | |||
| Median | 19.2 | 8.9 | 1.0 |
| Range | 0-205 | 0-141 | 0-73 |
| OS after BP, mo | |||
| Median | 10.2 | 18.3 | 17.8 |
| Range | 0.03-183 | 0.03-181 | 0.2-126 |
| Status at last F/U | |||
| Alive | 61 | 45 | 3 |
| Dead | 215 | 84 | 10 |
| ACAs | |||
| No | 64 | 52 | 3 |
| Single | 100 | 39 | 7 |
| Complex | 112 | 38 | 3 |
| ACA emergence | |||
| Before BP | 79 | 15 | 1 |
| At BP | 86 | 41 | 8 |
| After BP | 47 | 21 | 1 |
Abbreviations are explained in Table 1.
Latency: time interval from initial diagnosis of CML to blastic transformation.
To determine the roles of ACAs in blastic transformation, the 570 ACAs were divided into 4 groups according to the time of ACA emergence: group 1, ACAs acquired before BP (n = 95); group 2, ACAs detected at diagnosis of BP (n = 135); group 3, ACAs acquired after diagnosis of BP (n = 69); and group 4, without developing BP (n = 271). Because group 3 ACAs emerged after diagnosis of BP, patients with these ACAs were grouped with patients without ACAs in further analyses of the impact of ACAs in blastic transformation.
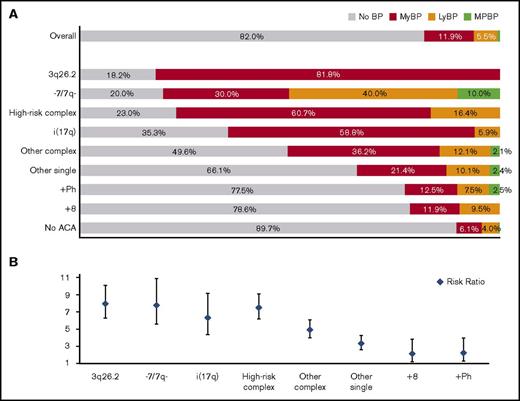
We first analyzed the frequency of BP associated with each common ACA (Figure 1A). Most patients with isolated 3q26.2 rearrangement, −7/7q−, or i(17q) and most patients with HR complex ACAs developed BP with an incidence of 81.8%, 80.0%, 64.7%, and 77.0%, respectively. About half of patients with other complex ACAs developed BP. A significant minority of patients with +8, +Ph, and other single ACAs developed BP with an incidence of 21.4%, 22.5%, and 33.9%, respectively. About 10.3% of patients without ACAs developed BP. The risk ratio is listed in Figure 1B.
ACA-dependent incidence, lineage distribution, and risk ratio of blastic transformation. (A) Incidence and lineage distribution of BP in patients with different types of ACAs acquired before or at blastic transformation and in patients without ACAs. The incidence of mixed-phenotype BP (MPBP) was 0.6% overall and 0.2% in the patients with no ACAs. (B) Risk ratio associated with different types of ACAs.
ACA-dependent incidence, lineage distribution, and risk ratio of blastic transformation. (A) Incidence and lineage distribution of BP in patients with different types of ACAs acquired before or at blastic transformation and in patients without ACAs. The incidence of mixed-phenotype BP (MPBP) was 0.6% overall and 0.2% in the patients with no ACAs. (B) Risk ratio associated with different types of ACAs.
ACAs correlated with the blast lineage of BP: 3q26.2 rearrangement, i(17q), and complex ACAs were associated with a higher risk of developing MyBP, whereas −7/7q− was associated with a higher risk of developing LyBP. MyBP and LyBP appeared equally distributed in patients with +8 or +Ph (Figure 1A).
ACA type–dependent blastic transformation
To determine the impact of ACAs in blastic transformation, we studied the time course of blastic transformation after emergence of each common ACA. Given the time interval in patient follow-up, ACAs detected at diagnosis of BP (group 2) could emerge before or simultaneously with the onset of BP. Parallel analyses including group 2 patients were performed to validate the robustness of the findings.
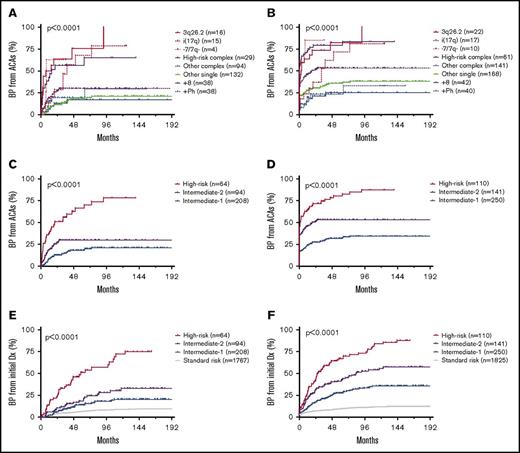
Patients with isolated 3q26.2 rearrangement, −7/7q−, or i(17q) and patients with HR complex ACAs had a significantly shorter time interval to blastic transformation (Figure 2). The median duration from ACA emergence to the onset of BP was 11.0, 8.1, 37.9, and 15.6 months, respectively, when group 2 patients were excluded (Figure 2A), or 2.8, 0, 32.6, and 0 months, respectively, when group 2 patients were included (Figure 2B). In contrast, the median duration from ACA emergence to the onset of BP was not reached in patients with +8, +Ph, or other single ACAs. In patients with other complex ACAs, the median duration was not reached when group 2 patients were excluded, and 19.2 months when group 2 patients were included. The 5-year cumulative probability of blastic transformation was 75.7%, 62.5%, 67.8%, 56.4%, 30.0%, 17.0%, 19.1%, and 17.9% for patients with 3q26.2 rearrangement, −7/7q−, i(17q), HR complex ACAs, other complex ACAs, +8, +Ph, and other single ACAs, respectively, when group 2 patients were excluded (Figure 2A), or 82.3%, 85.0%, 71.6%, 79.3%, 53.4%, 25.0%, 23.2%, and 35.5%, respectively, when group 2 patients were included (Figure 2B). The time course of blastic transformation from initial CML diagnosis by ACA type is shown in supplemental Figure 1.
ACA-dependent blastic transformation of CML. (A-B) Time course of blastic transformation from emergence of ACAs by ACA type. Patients with ACAs detected at blastic transformation were excluded in panel A but included in panel B. (C-D) Time course of blastic transformation from emergence of ACAs by risk group. Patients with ACAs detected at blastic transformation were excluded in panel C but included in panel D. (E-F) Time course of blastic transformation from initial diagnosis of CML by risk group. Patients with ACAs detected at blastic transformation and those who had BP at initial diagnosis were excluded in panel E but included in panel F.
ACA-dependent blastic transformation of CML. (A-B) Time course of blastic transformation from emergence of ACAs by ACA type. Patients with ACAs detected at blastic transformation were excluded in panel A but included in panel B. (C-D) Time course of blastic transformation from emergence of ACAs by risk group. Patients with ACAs detected at blastic transformation were excluded in panel C but included in panel D. (E-F) Time course of blastic transformation from initial diagnosis of CML by risk group. Patients with ACAs detected at blastic transformation and those who had BP at initial diagnosis were excluded in panel E but included in panel F.
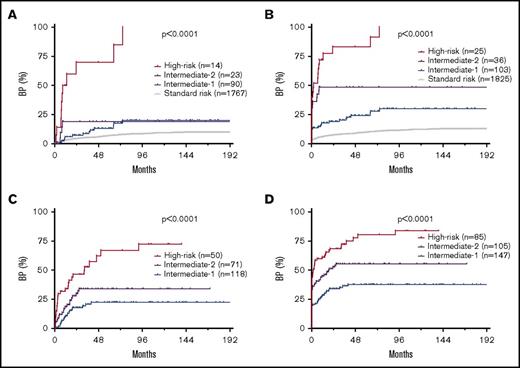
Based on the frequency of and latency to BP associated with each ACA, patients were stratified into 4 risk groups. Patients without ACAs formed the standard-risk (SR) group, patients with isolated 3q26.2 rearrangement, −7/7q−, or i(17q) and patients with HR complex ACAs formed the HR group, patients with +8, +Ph, or other single ACAs formed the intermediate-1 (Int-1) risk group, and patients with other complex ACAs formed the intermediate-2 (Int-2) risk group (Figure 2C-F).
By risk group, the median duration from ACA emergence to the onset of BP was unreached for Int-1 and Int-2, and 20.6 months for HR group when group 2 patients were excluded (Figure 2C), or unreached for Int-1, 19.2 months for Int-2, and 1.9 months for HR group when group 2 patients were included (Figure 2D). The 5-year cumulative probability of blastic transformation from ACA emergence in Int-1, Int-2, and HR groups was 18.1%, 30.0%, and 66.8%, respectively, when group 2 patients were excluded (Figure 2C), or 31.9%, 53.4%, and 80.7%, respectively, when group 2 patients were included (Figure 2D).
By risk group, the median duration from initial CML diagnosis to the onset of BP was unreached for SR, Int-1, and Int-2, and 58.2 months for HR group when group 2 patients were excluded (Figure 2E), or unreached for SR and Int-1, 83.7 months for Int-2, and 27.0 months for the HR group when group 2 patients were included (Figure 2F). The 5-year cumulative probability of blastic transformation from initial CML diagnosis in SR, Int-1, Int-2, and HR groups was 6.8%, 14.4%, 18.8%, and 51.6%, respectively, when group 2 patients were excluded (Figure 2E), or 9.8%, 28.0%, 41.7%, and 67.4%, respectively, when group 2 patients were included (Figure 2F).
ACA-dependent patient survival
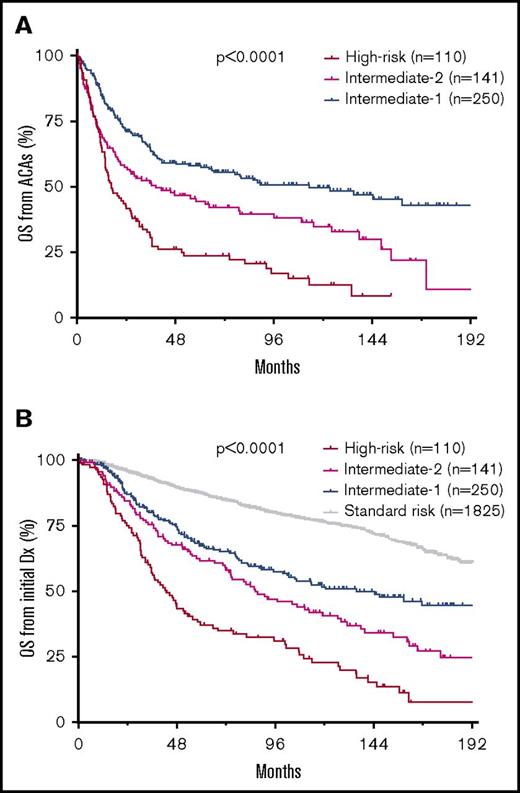
Patient OS correlated well with the risk of blastic transformation by ACA type or by risk group, from ACA emergence or from initial CML diagnosis (Figure 3; supplemental Figure 2). When all patients were included, the median OS from initial CML diagnosis in SR, Int-1, Int-2, and HR groups was unreached, 135.5, 86.2, and 42.7 months, respectively, and 8-year OS was 79.7%, 57.6%, 47.0%, and 31.2%, respectively (Figure 3B).
ACA-dependent patient survival in CML. (A) OS from emergence of ACAs by risk group in the entire cohort. (B) OS from initial diagnosis of CML by risk group in the entire cohort.
ACA-dependent patient survival in CML. (A) OS from emergence of ACAs by risk group in the entire cohort. (B) OS from initial diagnosis of CML by risk group in the entire cohort.
ACA type–dependent myeloid and lymphoid blastic transformation
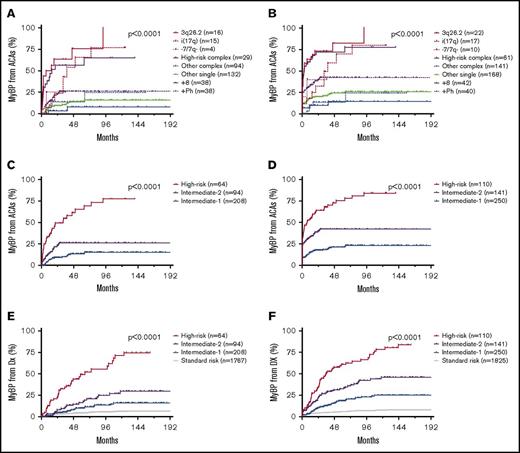
Given the different frequencies of MyBP and LyBP associated with each ACA, we then studied the impact of ACAs on the development of MyBP or LyBP separately. As shown in Figure 4, all ACAs carried a variable degree of risk of myeloid blastic transformation. Patients with isolated 3q26.2 rearrangement, −7/7q−, or i(17q) and patients with HR complex ACAs had a significantly higher risk than patients with other ACAs, individually (Figure 4A-B) or by risk group (Figure 4C-F), whether group 2 patients were excluded (Figure 4A,C,E) or included (Figure 4B,D,F).
ACA-dependent myeloid blastic transformation of CML. (A-B) Time course of myeloid blastic transformation from emergence of ACAs by ACA type. Patients with ACAs detected at blastic transformation were excluded in panel A but included in panel B. (C-D) Time course of myeloid blastic transformation from emergence of ACAs by risk group. Patients with ACAs detected at blastic transformation were excluded in panel C but included in panel D. (E-F) Time course of myeloid blastic transformation from initial diagnosis of CML by risk group. Patients with ACAs detected at blastic transformation and those who had BP at initial diagnosis were excluded in panel E but included in panel F.
ACA-dependent myeloid blastic transformation of CML. (A-B) Time course of myeloid blastic transformation from emergence of ACAs by ACA type. Patients with ACAs detected at blastic transformation were excluded in panel A but included in panel B. (C-D) Time course of myeloid blastic transformation from emergence of ACAs by risk group. Patients with ACAs detected at blastic transformation were excluded in panel C but included in panel D. (E-F) Time course of myeloid blastic transformation from initial diagnosis of CML by risk group. Patients with ACAs detected at blastic transformation and those who had BP at initial diagnosis were excluded in panel E but included in panel F.
Despite the impact of all ACAs on myeloid blastic transformation, surprisingly however, only −7/7q− carried a significant risk of lymphoid blastic transformation (Figure 5 and data not shown). Patients with all other ACAs did not significantly differ from each other or from patients without ACAs in frequency of or latency to lymphoid blastic transformation, individually or by risk group, with group 2 patients excluded or included. This correlated with the finding that only −7/7q− was associated with a significantly higher incidence of lymphoid blastic transformation (Figure 1).
ACA-dependent lymphoid blastic transformation of CML. Time course of lymphoid blastic transformation from emergence of ACAs by ACA type. Patients with ACAs detected at blastic transformation were excluded in panel A but included in panel B.
ACA-dependent lymphoid blastic transformation of CML. Time course of lymphoid blastic transformation from emergence of ACAs by ACA type. Patients with ACAs detected at blastic transformation were excluded in panel A but included in panel B.
Impact of ACAs at initial diagnosis and blast count on blastic transformation
We then analyzed the impact of ACAs detected at initial diagnosis and ACAs acquired during therapy separately. A total of 164 patients had ACAs at initial diagnosis and 337 patients acquired ACAs later during therapy before or at blastic transformation. Karyotype remained predictive of risk of blastic transformation regardless of the emerging time, initially (Figure 6A-B) or during therapy (Figure 6C-D), whether group 2 patients were excluded (Figure 6A,C) or included (Figure 6B,D), particularly, the HR ACAs. Similarly, ACAs was predictive of OS regardless of the emerging time (supplemental Figure 3).
Impact of ACAs at initial diagnosis and ACAs acquired during therapy on blastic transformation. (A-B) Impact of ACAs at initial diagnosis on blastic transformation of CML by risk group. Patients who had BP at initial diagnosis were excluded in panel A but included in panel B. (C-D) Impact of ACAs acquired during therapy on blastic transformation of CML from emergence of ACAs. Patients with ACAs detected at blastic transformation were excluded in panel C but included in panel D.
Impact of ACAs at initial diagnosis and ACAs acquired during therapy on blastic transformation. (A-B) Impact of ACAs at initial diagnosis on blastic transformation of CML by risk group. Patients who had BP at initial diagnosis were excluded in panel A but included in panel B. (C-D) Impact of ACAs acquired during therapy on blastic transformation of CML from emergence of ACAs. Patients with ACAs detected at blastic transformation were excluded in panel C but included in panel D.
Rea et al previously showed that CML patients with ACAs and concurrent hematological AP (HEM-AP) had less favorable outcome.34 In our cohort, 33 of 366 patients from groups 1 and 4 had increased BM blast counts in the range of HEM-AP. Overall, HEM-AP correlated with a higher risk of blastic transformation (supplemental Figure 4A). By ACA risk group, HEM-AP increased the risk of blastic transformation in HR and Int-2 groups (supplemental Figure 4B). Increased blast count seemed to have less impact on the risk of blastic transformation in the Int-1 group. Regardless, ACAs remained predictive of risk of blastic transformation in the absence of increased blast counts.
Predominant role of interval 2 in determining patient outcome
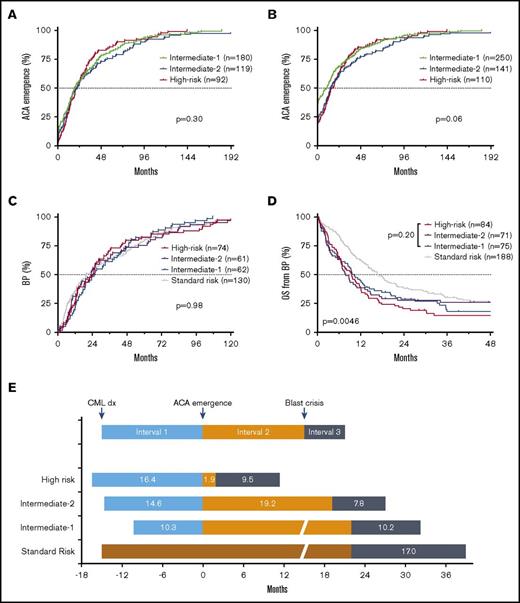
Patient survival is determined by the duration from initial CML diagnosis to ACA emergence (interval 1), duration from ACA emergence to the onset of BP (interval 2), and survival after the onset of BP (interval 3) (Figure 7). We then investigated the durations of intervals 1 and 3 in different risk groups.
Impact of 3 intervals on patient survival. (A-B) Time course of ACA emergence (interval 1) by risk group. Patients with ACAs at initial diagnosis were excluded in panel A but included in panel B. (C) Time course of blastic transformation from initial diagnosis (intervals 1 + 2) in patients who developed BP. Patients with BP at initial diagnosis were not included. (D) Patient survival after onset of BP (interval 3) by risk group. (E) Schematic representation of patient survival by risk groups. The OS is determined by time intervals from initial CML diagnosis to ACA emergence (interval 1), from ACA emergence to blastic transformation (interval 2), and survival after onset of blastic transformation (interval 3).
Impact of 3 intervals on patient survival. (A-B) Time course of ACA emergence (interval 1) by risk group. Patients with ACAs at initial diagnosis were excluded in panel A but included in panel B. (C) Time course of blastic transformation from initial diagnosis (intervals 1 + 2) in patients who developed BP. Patients with BP at initial diagnosis were not included. (D) Patient survival after onset of BP (interval 3) by risk group. (E) Schematic representation of patient survival by risk groups. The OS is determined by time intervals from initial CML diagnosis to ACA emergence (interval 1), from ACA emergence to blastic transformation (interval 2), and survival after onset of blastic transformation (interval 3).
The median durations of interval 1 were similar among Int-1, Int-2, and HR groups (Figure 7A-B). The median duration of interval 1 was 13.2 months in the entire cohort. In those who developed BP during therapy, the duration from initial CML diagnosis to the onset of BP among 4 risk groups was similar (median, 23.4 months; Figure 7C). Approximately 64.5%, 74.0%, and 80.1% of BP patients developed BP within the first 3, 4, and 5 years after initial diagnosis, respectively, when patients with BP at initial diagnosis were excluded, or 72.2%, 79.7%, and 84.4%, respectively, when patients with BP at initial diagnosis were included.
Similarly, the median durations of interval 3 were similar among Int-1, Int-2, and HR groups: 9.3 months for these 3 ACA groups combined and 17.0 months for the SR group (Figure 7D-E). These results indicate that the predominant determinant of patient outcome lies in the duration of interval 2, which is in turn highly ACA type–dependent (Figure 7E).
Discussion
Karyotype-based prediction of blastic transformation in the TKI era
Here, we demonstrated the crucial role of ACAs in determining the risk of blastic transformation in CML patients treated with TKIs. Based on the risk of blastic transformation, patients are stratified into 4 different cytogenetic groups. The emergence of 3q26.2 rearrangement, −7/7q−, or i(17q), whether as an isolated single ACA or as a component of complex ACAs, predicts a rapid blastic transformation and dismal survival, and thus patients with these ACAs belong to the HR group. Patients with other complex ACAs without a HR component form the Int-2 group, and patients with +8, +Ph, or other single ACAs form the Int-1 group. Patients without ACAs form the SR group. Additionally, the impact of ACAs on blastic transformation is lineage-specific: all ACAs carry a variable degree of risk of progression to MyBP, whereas only −7/7q− is associated with a significant risk of progression to LyBP. Given that ACAs detected at diagnosis of BP (group 2) could emerge before or simultaneously with the onset of BP, inclusion of these patients would overestimate the risk of BP in patients who have not developed BP. However, exclusion of these patients would underestimate the risk of BP in patients who have not acquired but may acquire ACAs later.
“Other single” and “other complex” ACAs are quite heterogeneous. Most are numerical abnormalities involving loss or gain of an entire chromosome or unbalanced alterations lacking an obvious pattern. Other than 3q26.2 rearrangement, the reciprocal translocations typical for de novo acute myeloid leukemia are rare in our cohort. These “other” reciprocal translocations include inv(16)(p13q22) (n = 8), t(9;11)(p21;q23) (n = 3), t(8;21)(q22;q22) (n = 2), and t(15;17)(q24;q21) (n = 2). A previous small case series showed that CML patients with 11q23 rearrangement have a higher risk of blastic transformation and a poor survival.35
Latencies and blastic transformation in TKI vs pre-TKI eras
In this study, the durations of both interval 1 and interval 3 in patients treated with TKIs are similar among different ACA risk groups. In the pre-TKI era, most CML patients treated with busulfan or hydroxyurea underwent blastic transformation within 2 to 4 years regardless of ACAs, and the median survival after the onset of BP was only 3 to 4 months.10 It is reasonable to speculate that the durations of both interval 1 and interval 3 were not significantly different among different risk groups in the pre-TKI era. Our study results support that TKI therapy improves patient outcome through prolonging the duration of interval 2. However, the impact of TKIs on the duration of interval 2 is highly ACA type–dependent. TKI therapy significantly mitigates the risk of blastic transformation associated with SR or Int-1–risk ACAs, but not the risk associated with HR ACAs: 3q26.2 rearrangement, −7/7q− or i(17q). The pre-BP disease course in HR patients treated with TKIs highly resembles that observed in CML patients treated in the pre-TKI era. Interestingly, in patients who develop BP, the duration before BP is strikingly similar regardless of the presence or absence of ACAs or the type of ACAs (Figure 7).
AP concept revisited
In the 2016 revision to the World Health Organization (WHO) classification, a variety of parameters are included as criteria for defining AP of CML.18 These parameters include clinical findings (counts of leukocytes, basophils, blasts and platelets, and splenomegaly), cytogenetic data (major-route ACAs, complex karyotype, and abnormalities of 3q26.2 at diagnosis; any ACAs acquired during therapy), and response criteria (ABL1 mutation and treatment resistance). Some of these parameters are included based on their impact in CML patients treated in the pre-TKI era.2,36 With the evolution of therapy, the prognostic value of these factors may change. Indeed, it has been shown that TKI therapy has minimized the impact of most clinical parameters, including counts of leukocytes, basophils, and platelets as well as spleen size.37
ACAs are observed in ∼5% of patients with CP, 30% with AP, and 70% to 80% with BP. Apparently, the emergence of ACAs indicates clonal evolution and disease progression. However, it seems quite possible that some of these chromosomal alterations may be just bystanders of genomic instability of CML whereas others could function as drivers of leukemic progression. This is reflected in the markedly heterogeneous impact of different ACAs in blastic transformation and patient outcome reported in this study and in earlier studies.21,32,38
The changing impact of clinical parameters due to changing therapy and the heterogeneity of ACAs suggests that the concept of AP needs to be revisited. CML is constantly evolving at the molecular level even in “CP.” Identification of new risk factors, such as ABL1 mutation, has resulted in some patients previously considered in CP being reclassified in AP using the WHO provisional criteria. Although the “lumping” approach for AP has some value, it does not fully reflect the evolving nature of the disease or the markedly different risk associated with different ACAs, and thus does not reflect the actual risk in each patient. Our stratification of patients into 4 different cytogenetic-risk groups provides a valuable alternative to the current CP vs AP binary classification. By analogy, in myelodysplastic syndrome, the current International Prognostic Scoring System for predicting survival and evolution to acute leukemia stratifies patients into 4 risk groups based on BM blast count, chromosomal alterations, and cytopenia.
Genomic instability and prevention of blastic transformation
Currently, BP-CML remains a major therapeutic challenge. With limited treatment options available and dismal outcome of BP patients, the best approach to treat BP might be to prevent the onset of BP. Thus, predicting the onset of BP becomes essential. Based on ACAs, we are able to predict the risk of blastic transformation and identify a group of HR patients. We believe the HR patients may benefit from timely alternative treatments, such as allo-HSCT, before BP develops. A recent study suggested the potential survival benefit of early elective transplantation when both disease risk and transplantation risk are taken into consideration.39
The emergence of ACAs results from the genomic instability in CML cells. Enhanced DNA damage from accumulation of reactive oxygen species followed by compromised DNA repair due to long-standing BCR-ABL1 expression leads to genomic instability.40 To prevent blastic transformation, another question is whether the emergence of ACAs can be prevented as raised by Hehlmann et al.41 Although reducing BCR-ABL1 transcript level with TKI therapy greatly decreases the probability of developing ACAs and thus blastic transformation, a deep molecular response is only observed in a limited percentage of CML patients. In vitro studies have shown the effect of antioxidants in decreasing mutagenesis and frequency of imatinib resistance.42,43 Further studies are required to assess the value of clinical use of antioxidants in CML patients.
Lineage specificity of blastic transformation
It is not fully understood why some patients develop MyBP, whereas others develop LyBP. In a large comprehensive review, Johansson et al found that −7 had a higher frequency in LyBP, whereas i(17q) had a higher frequency in MyBP in the pre-TKI era.1 Our previous study confirmed the validity of their conclusion in CML patients treated in the TKI era.23 In the current study, we found that all ACAs carry a variable degree of risk of developing MyBP whereas only −7/7q− carry a significant risk in promoting LyBP. The exact mechanism explaining such difference remains to be uncovered. A small population of abnormal B lymphoblasts is present in a significant minority of patients at initial diagnosis of CP by flow cytometry, and the early detection of this population of B cells is associated with early progression to LyBP.44 The cytogenetic profile acquired before the onset of BP mainly reflects that of the myeloid population. However, it is the small cytogenetically undetectable lymphoblast population that contributes to the later lymphoid blastic transformation. Alternatively, the development of LyBP or MyBP may result from genetic changes at different levels: chromosomal or molecular. Consistent with this, p16/ARF homozygous deletion and Rb mutation/deletion is very common in LyBP whereas p53 mutation common in MyBP.45
In summary, chromosomal alterations predict the risk of blastic transformation in CML patients treated with TKIs. Patients can be stratified into 4 different cytogenetic risk groups based on ACA type–dependent risk of blastic transformation. This risk stratification correlates well with the patient survival. By prolonging the duration of interval 2, TKI therapy mitigates the risk of blastic transformation associated with low-risk ACAs or no ACAs but does not alter the natural course of CML associated with HR ACAs: 3q26.2 rearrangement, −7/7q− and i(17q). Thus, we identify a group of patients who are at a HR of rapid disease progression. These patients may benefit from early allo-HSCT to prevent blastic transformation.
The full-text version of this article contains a data supplement.
Acknowledgment
The authors thank Kathryn Hale for her assistance in language editing.
Authorship
Contribution: Z.G. and S.H. designed the study and drafted the manuscript; Z.G., Z.C., L.Z., Y.L., S.B., and S.H. participated in data collection and analysis; and L.J.M., J.E.C., P.L., R.N.M., J.L.J., T.J.M., W.W., and H.M.K. contributed pathology materials and patient information.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shimin Hu, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0072, Houston, TX 77030; e-mail: shu1@mdanderson.org.