Key Points
HNE inhibition of the TrxR/Trx system induces p38 MAPK activation–dependent TF decryption by increasing PS levels in the outer leaflet.
HNE-induced mitochondrial ROS generation also contributes to TF decryption, independent of TrxR/Trx and p38 MAPK activation.
Abstract
Many pathophysiologic agents transform cryptic tissue factor (TF) on cells to prothrombotic TF, and one such stimulus is 4-hydroxy-2-nonenal (HNE), the most abundant aldehyde produced by the oxidation of ω-6 polyunsaturated fatty acids. HNE was shown to induce reactive oxygen species (ROS) generation and p38 MAPK activation, but the link between them and their role in TF decryption are unclear. The present study was carried out to elucidate potential mechanisms involved in HNE-induced TF decryption in monocytic cells. The data presented herein show that mitochondria are the primary source for HNE-induced ROS generation. The inhibition of mitochondrial electron transport chain complex III and V blocked HNE-induced ROS generation, but not p38 MAPK activation. These inhibitors reduced phosphatidylserine (PS) externalization and TF decryption significantly, but not completely. HNE treatment inhibited the activities of thioredoxin reductase (TrxR) and thioredoxin (Trx), independent of ROS. Inhibition of the TrxR/Trx system by HNE or pharmacological inhibitors induced p38 MAPK activation, PS externalization, and TF decryption. Additional studies revealed that the inhibition of TrxR/Trx led to activation of apoptosis signal-regulating kinase (ASK-1) and mitogen-activated protein kinase kinase 3/6. Inhibition of ASK-1 expression by small interfering RNA or its activity by pharmacological inhibitors diminished HNE-induced TF decryption. Overall, our data suggest that HNE induces TF decryption by 2 distinctive pathways. One is ROS dependent but independent of p38 MAPK activation, and the other is via TrxR/Trx and is p38 MAPK activation dependent. However, both mechanisms result in the enhancement of PS at the outer leaflet that is responsible for TF decryption.
Introduction
Oxidative stress has been shown to play a crucial role in many disease processes, particularly diseases associated with coagulopathy, such as atherosclerosis, sepsis, and diabetes.1-3 It had been shown that reactive oxygen species (ROS) and lipid peroxidation products generated during oxidative stress could enhance tissue factor (TF) expression and procoagulant activity.4-6 Our recent studies7 show that 4-hydroxy-2-nonenal (HNE), the most abundant and stable unsaturated aldehyde generated from the oxidation of ω-6 polyunsaturated fatty acids,8 increased TF activity on the cell surface of monocytic cells without increasing TF antigen levels. These studies indicated that HNE-induced ROS generation and p38 MAPK activation and led to the externalization of phosphatidylserine (PS). However, at present, the link between HNE-induced ROS generation and p38 MAPK activation and their roles in the externalization of PS and TF activation are unknown.
ROS have the potential to oxidize and unfold proteins,9 contributing to the damaging effects of oxidative stress. ROS transiently modulate protein activity by protein modification, most commonly via the formation of disulfide bonds.10-12 Recent studies suggest that thiol exchange pathways involving protein disulfide isomerase (PDI) regulate TF activation via reversible disulfide bond formation.13-16 Wang et al17 showed that thioredoxin (Trx) knockdown caused a significant increase in cell surface TF procoagulant activity in MDA-MB-231 cells, indicating that the thioredoxin reductase (TrxR)/Trx system negatively regulates TF coagulant activity. Rothmeier et al18 reported that adenosine triphosphate (ATP)-induced TF activation and TF+ microvesicle (MV) release in mouse macrophages is also dependent on the TrxR/Trx system. Although the mechanism by which the TrxR/Trx system regulates cell surface TF activity is unclear, it had been suggested that PDI-mediated disulfide bond formation in TF may be responsible for TF activation.17,18 HNE was shown to inhibit Trx either directly by forming a covalent adduct with Trx or indirectly through the inhibition of TrxR activity.19 These observations raise the possibility that HNE-induced inhibition of TrxR/Trx-dependent thiol exchange pathways may be responsible for TF activation in monocytes and macrophages. Alternatively, inhibition of the Trx/TrxR system may lead to dissociation of Trx and the oxidant-sensitive kinase, apoptosis signal-regulating kinase 1 (ASK-1), leading to ASK-1 activation.20,21 Activation of ASK-1 promotes the activation of the downstream MKK3/6 (mitogen-activated protein kinase kinase 3/6), the kinase that activates p38 MAPK,22,23 and resulting p38 MAPK activation-dependent PS externalization.7
The present study was carried out to elucidate potential mechanisms involved in HNE-induced TF decryption. Understanding mechanistic details of HNE-induced TF decryption will not only provide molecular insights into how oxidative stress modulates TF activity but also have therapeutic implications, because the knowledge gained from the study could be used to develop strategies to suppress TF activation. The data presented herein show that HNE induces TF decryption via 2 separate and distinctive pathways. One is ROS dependent but independent of p38 MAPK activation, and the second is TrxR1/Trx and p38 MAPK activation dependent. Both mechanisms result in the externalization of PS at the cell surface that contributes to TF decryption.
Material and methods
Reagents
HNE was purchased from Cayman Chemical (Ann Arbor, MI). Recombinant human factor VIIa was provided by the late Walter Kisiel (University of New Mexico, Albuquerque, NM). Purified human factor X (FX) and Xa and prothrombin were purchased from Enzyme Research Laboratories (South Bend, IN). Purified human α-thrombin and factor Va were purchased from Hematologic Technologies (Essex Junction, VT). Annexin V was obtained from eBioscience (Thermo Fisher Scientific, Waltham, MA). Antibodies against phospho-p38 MAPK, phospho-MKK3/6, phospho-ASK-1 (Thr 845), total p38 MAPK, total MKK3, and total ASK-1 were obtained from Cell Signaling Technology (Danvers, MA). Cell culture medium RPMI 1640, Dulbecco’s modified Eagle medium, and fetal bovine serum were from Gibco (Invitrogen, Carlsbad, CA). Fluorescence-conjugated secondary antibodies, Alexa Fluor 488 (AF488)-conjugated annexin V, and H2DCFDA (2′,7′-dichlorodihydrofluorescein diacetate) were from Life Technologies (Grand Island, NY). NBD-PS (1-oleoyl-2-sn-glycero-3-phosphoserine) was obtained from Avanti Polar Lipids (Alabaster, AL).
Cell culture
THP-1 cells were obtained from ATCC (Rockville, MD) and cultured in RPMI medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. The cells were cultured in T-75 flasks for propagation. For experiments, THP-1 cells were cultured in suspension in 5- or 15-mL culture tubes.
Cell viability test
THP-1 cells treated with control vehicle or HNE with or without inhibitors, at the concentrations and time durations employed in our experiments, were evaluated for cell viability using the trypan blue exclusion method. Only experimental conditions that showed no significant cell death (<5%) were chosen for the investigation.
Generation of ASK-1 siRNA lentivirus
The iLenti small interfering RNA (siRNA) expression system (Applied Biological Materials Richmond, BC, Canada) was used to generate ASK-1 siRNA lentiviruses. Briefly, 293T cells (LV010) cultured to 80% confluence in 35-mm culture dishes were cotransfected with 1 of the 4 MAP3K5-set siRNA/short hairpin RNA/RNA interference expression vectors and second-generation lentivirus packaging (2 µg each) using 8 µL Lentifectin transfection reagent as per the vendor’s suggested protocol. The transfected cells were incubated for 24 hours at 37°C, and the medium was collected. After collecting the medium, 2 mL complete culture medium was added to cells and cultured for 48 hours, and the supernatant medium was collected for additional virus collection. The viral titer was analyzed by transducing CHO-K1 cells with different volumes of the lentivirus and counting the number of cells expressing GFP by fluorescence microscopy. For the stable knockdown of ASK-1 in THP-1 cells, polybrene (10 µg/mL) was added to the viral stock, and THP-1 cells were infected with the lentivirus. After 24 hours, the medium was replaced with fresh medium containing 1 µg/mL puromycin and cultured for 1 week to select stable transfectants. ASK-1 expression in THP-1 cells transfected with each of the expression plasmids was assessed by immunoblotting, and the stable transfectant that showed maximal ASK-1 knockdown was chosen for experiments.
TF and prothrombinase activity assays
THP-1 cells (2 × 105/mL), resuspended in RPMI medium containing 2% serum, were treated with a control vehicle or HNE as specified in “Results” and the figure legends. Where cells were treated with inhibitors, inhibitors were added 1 hour before HNE treatment unless otherwise stated. Cells were kept at 37°C in the CO2 incubator during the entire duration of treatment. After treatment, cells were washed once with buffer A (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 0.15 M NaCl, 4 mM KCl, and 11 mM glucose, pH 7.5) before use in the assays. TF procoagulant activity was measured using an FX activation assay as described earlier,24 whereas prothrombinase activity was measured using a thrombin generation assay.25
ROS generation
THP-1 cells (5 × 105/mL), resuspended in serum-free medium, were incubated with MitoTracker Red FM (200 nM) for 30 minutes at 37°C. The cells were then loaded with 5 µM H2DCFDA (2′,7′-dichlorodihydrofluorescein diacetate) for 5 minutes at 37°C. After 5 minutes, cells were washed once and resuspended in serum-free medium. The cells were treated with a control vehicle or HNE (20 µM) for 20 minutes at 37°C. After treatment, the cells were washed and resuspended in 10 µL HEPES buffer (pH 7.5), and the cell suspension was loaded onto the glass slides for fluorescence microscopy. The fluorescence of cells was viewed using laser wavelength settings of 488 ± 10 nm for excitation and 525 ± 10 nm for emission for H2DCFDA and 580 ± 10 nm for excitation and 640 ± 10 nm for emission for MitoTracker Red, and digital images of the fluorescence were captured using the LSM 510 Meta confocal system (Zeiss) equipped with an Axio Observer Z1 microscope (Carl Zeiss, Jena, Germany). Differential interference contrast images of the same fields were also obtained. The percent of cells emitting fluorescence was determined by counting the total number of cells (from differential interference contrast images) and the number of fluorescent cells from multiple fields. To quantify the fluorescence intensity, a region of interest was made encircling individual cells and measuring the fluorescence intensity within the region of interest using ZEN 2009 software (Carl Zeiss). At least 50 cells from ≥3 fields were used for quantification.
Annexin V staining
THP-1 cells were fixed with 4% paraformaldehyde, and the fixed cells were then labeled with AF488-tagged annexin V (1:20) as described in the manufacturer’s protocol. The staining was evaluated using the LSM 510 Meta confocal microscope system.
TrxR and Trx activity assays
THP-1 cells were treated with varying concentrations of HNE (0 to 80 µM) for a fixed period or treated with 20 µM HNE for varying times. After treatment, the cells were washed twice with 1X phosphate-buffered saline (PBS) and lysed in the assay buffer provided with TrxR activity assay kit (BioVision, Milpitas, CA). TrxR activity was assayed based on the reduction of 5, 5′-dithiobis (2-nitrobenzoic) acid with NADPH to 5-thio-2-nitrobenzoic acid that generates a strong yellow color, which was measured with a spectrophotometer at λmax = 412 nm as per the manufacturer’s protocol (BioVision). Trx activity was measured based on the reduction of insulin disulfides by reduced Trx with TrxR in a fluorescence-based assay using a kit for assay of Trx (IMCO; Cayman Chemical Company, Ann Arbor, MI).
Immunoprecipitation and western blotting
THP-1 cells, treated with control vehicle or inhibitors ± HNE, were washed once with ice-cold PBS and then lysed in immunoprecipitation lysis buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.5, containing 1 mM Na2EDTA, 1 mM EGTA, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na2VO4, and 1 µg/mL leupeptin) supplemented with the protease and phosphatase inhibitors. Protein concentration in cell lysates was determined using the BCA protein assay kit (Bio-Rad). For immunoprecipitation, cell lysates were incubated with the primary antibody overnight at 4°C, followed by incubation with protein G covalently attached to magnetic beads (Pierce, Thermo Fisher) for an additional 2 hours at 4°C. The antigen–antibody complex was then separated using a magnetic separator, washed 5 times with ice-cold lysis buffer, and eluted with low-pH glycine buffer or sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer. The samples were subjected to western blot analysis using standard procedures.
Thiol labeling of cell surface TF
THP-1 cells (5 × 106) were treated with either a control vehicle or 20 µM HNE for 4 hours. As a positive control, cells were treated with HgCl2 (100 µM for 5 minutes). After treatment, cells were washed once with 1X PBS and then resuspended in 1 mL of PBS containing 100 µM of 3-(N-maleimido-propionyl)biocytin (MPB). After 30 minutes of incubation at room temperature, cells were washed 3 times, and the unreacted MPB was quenched with 200 µM reduced glutathione for 30 minutes. After quenching, cells were washed 3 times with 1X PBS and lysed in immunoprecipitation lysis buffer. Cell lysates were incubated overnight with a mixture of TF monoclonal antibody at 4°C, followed by protein G coupled to magnetic beads for additional 2 hours at 4°C. The bound protein was eluted with 100 mM glycine (pH 2.3), and the eluate was subjected to western blot analysis and probed with streptavidin-peroxidase or rabbit anti-TF antibody.
Measurement of transbilayer movement of PS
To measure the effect of HNE on PS transport from the outer leaflet to the inner leaflet, THP-1 cells (1 × 106 cells/mL) were first treated with HNE (20 µM) for 4 hours at room temperature. At the end of incubation, cells were chilled on ice in the calcium-containing buffer for 5 minutes. Then, NBD-PS (2 µM) was added to the ±HNE-treated cells and allowed to incubate for 10 minutes at 4°C. At the end of the 10-minute period, cells were washed twice with ice-cold buffer, suspended in the same buffer that was prewarmed to 37°C, and allowed to stand at 37°C. At varying time intervals, aliquots of cells were removed, and fluorescence intensity of the cells was measured in the presence and absence of the cell-impermeant reducing agent dithionite (25 µM), which quenches the NBD-PS exposed on the cell surface. A BioTek Cytation 5 Multi-Mode Reader (Winooski, VT) was used for fluorescence measurements. The percentage of NBD-PS internalized was determined as the percentile of dithionite-resistant NBD-PS fluorescence from the total NBD-PS fluorescence as described earlier.26
Measurement of ATP levels
THP-1 cells (2 × 105 ) were treated with a control vehicle or electron transport chain inhibitors with or without HNE. Following the treatment, the cells were washed once with ice-cold phosphate-buffered saline and lysed in ATP detection sample buffer provided in the ATP detection assay kit (Cayman Chemicals). The level of ATP was detected based on the conversion of ATP and luciferin to oxyluciferin and light by firefly luciferase. The amount of light generated was recorded with a Cytation 5 Multi-Mode Reader, and the values were extrapolated from a standard curve generated using known concentrations of ATP to determine total cellular ATP levels.
Data analysis
All experiments were performed at least 3 times in duplicate. The values shown are mean ± standard error of the mean. The Student t test was used to determine statistical significance between the groups.
Results
The mitochondrial electron transport chain plays a crucial role in HNE-induced ROS generation and TF decryption
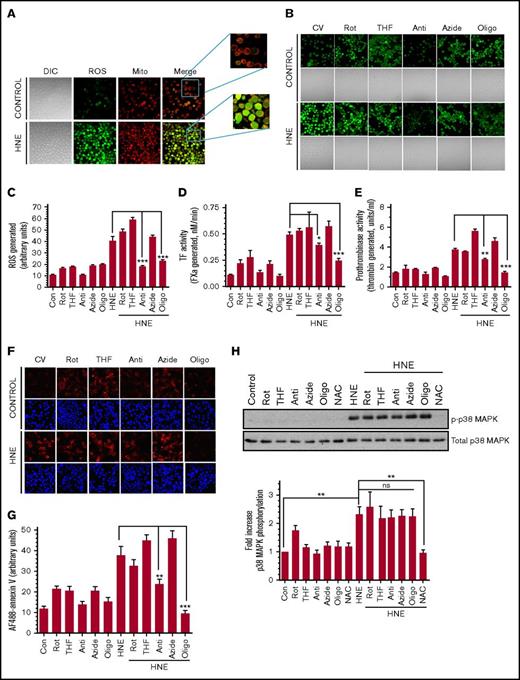
Our recent studies showed that HNE treatment generated ROS in monocytic cells, but the source of ROS generation was not investigated.7 To investigate whether mitochondria are the major source of HNE-induced ROS generation, live THP-1 cells were loaded with the mitochondrial-specific stain MitoTracker Red FM, as well as H2DCFDA, a ROS indicator, before stimulation with HNE. In cells treated with a control vehicle, very little ROS was found in either the mitochondria or cytosol. Upon HNE treatment, almost all mitochondria showed a detectable ROS signal, and there was a good overlap between the staining of mitochondria and ROS (Figure 1A; supplemental Figure 1), suggesting that mitochondria are the source of HNE-induced ROS generation. The levels of ROS generated in THP-1 cells in response to HNE was heterogeneous. At the time of analysis, ROS were not limited to mitochondria and were present throughout the cell. This could reflect the diffusion of ROS from mitochondria to other compartments or ROS-induced ROS generation.27,28 To delineate the role of the mitochondrial electron transport chain in HNE-induced ROS generation and TF decryption, cells were pretreated with specific inhibitors of electron transport chain complexes. As shown in Figure 1B, the inhibitors of complex I, II, and IV (ie, rotenone, THF, and sodium azide, respectively) did not inhibit HNE-induced ROS generation. In contrast, the inhibitors of complex III and complex V (antimycin and oligomycin, respectively) inhibited HNE-induced ROS generation. Quantification of ROS fluorescence staining showed a fourfold increase in ROS generation in HNE-treated cells, and antimycin and oligomycin significantly reduced HNE-induced ROS generation (Figure 1C). Antimycin and oligomycin, but not other inhibitors, significantly inhibited the HNE-induced increase in factor VIIa activation of FX (Figure 1D). The addition of anti-TF immunoglobulin G attenuated the increased activation of FX in HNE-treated cells as well as basal FX activation in control cells (supplemental Figure 2). As expected from our earlier investigation,29 HNE treatment did not alter TF antigen levels (supplemental Figure 3). These data confirm that HNE-induced increase in FX activation in THP-1 cells stems from TF decryption. Next, we investigated whether HNE-induced, ROS-mediated TF decryption stems from an increased exposure of PS at the cell surface. As shown in Figure 1E, antimycin and oligomycin markedly attenuated HNE-induced prothrombinase activity. Reduced annexin V staining of HNE-stimulated THP-1 cells that were pretreated with oligomycin or antimycin compared with a control vehicle further support the observation that HNE-induced PS externalization is mediated by mitochondrial complex III– and complex V–mediated ROS generation (Figure 1F-G). Although both antimycin and oligomycin inhibited HNE-induced PS exposure and TF decryption, the extent of their effect on HNE-induced ROS production did not correlate with their effect on HNE-induced PS exposure and TF decryption. This could be due to differences in their ability to inhibit ATP production (supplemental Figure 4). Because the flippase that maintains PS in the inner leaflet of the membrane requires ATP,30 a decrease in ATP may result in PS externalization. This could partially negate the result of their inhibitory effect on ROS-dependent TF decryption.
HNE-induced ROS generation in mitochondria and its role in TF decryption. (A) THP-1 cells were labeled with the mitochondrial staining dye MitoTracker (200 nM) for 30 minutes and then loaded with 5 µM H2DCFDA for 5 minutes at 37°C. Cells were then washed and treated with 20 µM HNE for 20 min at 37°C. After HNE treatment, cells were washed and analyzed for their fluorescence by confocal microscopy. (B) THP-1 cells were treated with specific electron transport chain inhibitors for 1 hour and then loaded with 5 µM H2DCFDA for 5 minutes at 37°C. Cells were treated with HNE for 20 minutes, and ROS generation was analyzed by the fluorescence of H2DCFDA that results from its oxidation by ROS by confocal microscopy. (C) ROS generation was quantified by measuring the fluorescence intensity of the cells as described in “Materials and methods.” The inhibitors used were 2.5 µM rotenone (Rot), 0.5 mM 2-thenoyltrifluoroacetone (THF), 10 µM antimycin (Anti), 10 mM sodium azide (Azide), and 5 µM oligomycin (Oligo). (D-E) THP-1 cells were treated with electron transport chain inhibitors for 1 hour in concentrations indicated in Figure 1B and then 20 µM HNE for 4 hours. Following HNE treatment, cell surface TF (D) and prothrombinase (E) activity was analyzed. (F) THP-1 cells were treated with electron transport chain inhibitors and HNE as described in panels D-E, and PS exposure on the cell surface was analyzed by labeling the fixed cells with AF488-annexin V. 4′-6-Diamidino-2-phenylindole was used to stain nuclei. (G) Quantification of annexin V bound to THP-1 cells (from the fluorescence staining shown in panel F) treated with HNE with or without electron transport chain inhibitors. (H) To investigate the effect of electron transport chain inhibitors on HNE-induced p38 MAPK activation, THP-1 cells were treated with the inhibitors as described above and then treated with HNE for 15 minutes. Cell lysates were subjected to western blot analysis to probe for p38 MAPK phosphorylation and total MAPK. Intensities of phosphorylated and total p38 MAPK bands on the blots were quantified to obtain fold increase in p38 MAPK phosphorylation. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). Images in panels A-B,F were obtained at 63× magnification (oil immersion). Con, control (no treatments); CV, control vehicle; DIC, differential interference contrast; Mito, mitochondria.
HNE-induced ROS generation in mitochondria and its role in TF decryption. (A) THP-1 cells were labeled with the mitochondrial staining dye MitoTracker (200 nM) for 30 minutes and then loaded with 5 µM H2DCFDA for 5 minutes at 37°C. Cells were then washed and treated with 20 µM HNE for 20 min at 37°C. After HNE treatment, cells were washed and analyzed for their fluorescence by confocal microscopy. (B) THP-1 cells were treated with specific electron transport chain inhibitors for 1 hour and then loaded with 5 µM H2DCFDA for 5 minutes at 37°C. Cells were treated with HNE for 20 minutes, and ROS generation was analyzed by the fluorescence of H2DCFDA that results from its oxidation by ROS by confocal microscopy. (C) ROS generation was quantified by measuring the fluorescence intensity of the cells as described in “Materials and methods.” The inhibitors used were 2.5 µM rotenone (Rot), 0.5 mM 2-thenoyltrifluoroacetone (THF), 10 µM antimycin (Anti), 10 mM sodium azide (Azide), and 5 µM oligomycin (Oligo). (D-E) THP-1 cells were treated with electron transport chain inhibitors for 1 hour in concentrations indicated in Figure 1B and then 20 µM HNE for 4 hours. Following HNE treatment, cell surface TF (D) and prothrombinase (E) activity was analyzed. (F) THP-1 cells were treated with electron transport chain inhibitors and HNE as described in panels D-E, and PS exposure on the cell surface was analyzed by labeling the fixed cells with AF488-annexin V. 4′-6-Diamidino-2-phenylindole was used to stain nuclei. (G) Quantification of annexin V bound to THP-1 cells (from the fluorescence staining shown in panel F) treated with HNE with or without electron transport chain inhibitors. (H) To investigate the effect of electron transport chain inhibitors on HNE-induced p38 MAPK activation, THP-1 cells were treated with the inhibitors as described above and then treated with HNE for 15 minutes. Cell lysates were subjected to western blot analysis to probe for p38 MAPK phosphorylation and total MAPK. Intensities of phosphorylated and total p38 MAPK bands on the blots were quantified to obtain fold increase in p38 MAPK phosphorylation. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). Images in panels A-B,F were obtained at 63× magnification (oil immersion). Con, control (no treatments); CV, control vehicle; DIC, differential interference contrast; Mito, mitochondria.
In additional studies, we investigated the effect of antimycin or oligomycin on p38 MAPK activation, because our earlier study7 indicated that HNE-induced TF decryption was dependent on p38 MAPK activation. Interestingly, antimycin and oligomycin had no significant effect on HNE-induced p38 MAPK activation (Figure 1H). As shown in the earlier study, N-acetyl cysteine (NAC), a general antioxidant, completely abrogated HNE-induced p38 MAPK activation (Figure 1H). The above data suggest that HNE-induced ROS generation contributes to TF decryption independent of p38 MAPK activation. Inhibition of p38 MAPK activation in THP-1 cells in which HNE-induced ROS generation was blocked by oligomycin further reduced HNE-induced TF decryption (supplemental Figure 5).
HNE inhibition of TrxR and its role in TF decryption
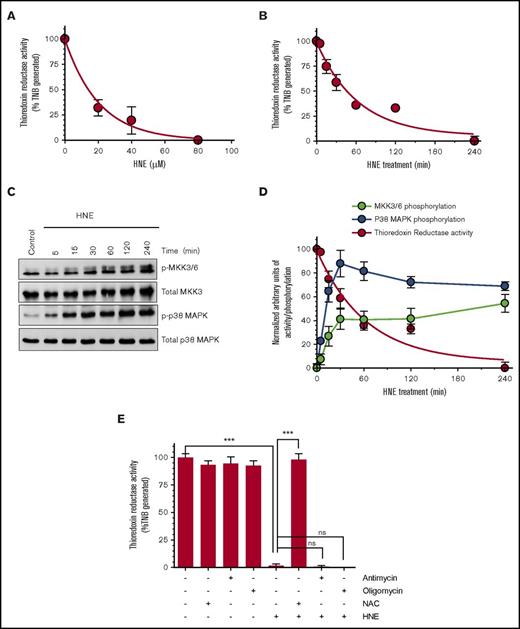
Recent studies by Wang et al17 showed that TrxR/Trx negatively regulates TF coagulant activity in MDA-231 breast cancer cells. HNE was shown to irreversibly inhibit TrxR and Trx activity via modification of their catalytic active residues.19 To determine whether HNE inhibition of TrxR or Trx is responsible for HNE-induced TF decryption, we first examined the effect of HNE treatment on TrxR activity under our experimental conditions. As shown in Figure 2A-B, HNE inhibited TrxR activity in a dose- and time-dependent manner. Comparison of the time course of HNE-mediated TrxR inhibition and HNE-induced activation of MKK3/6 and p38 MAPK (Figure 2C) showed an inverse correlation with TrxR activity (Figure 2D). Antimycin and oligomycin, the inhibitors of mitochondrial respiratory chain complexes that prevent HNE-induced ROS generation, had no significant effect on HNE-mediated TrxR inhibition (Figure 2E). In contrast, antioxidant NAC completely prevented HNE-mediated inhibition of TrxR activity. These data suggest that the HNE-induced mitochondrial ROS generation is not involved in HNE inhibition of TrxR activity.
HNE inhibition of TrxR activity inversely correlates with MKK3/6 and p38 MAPK activation. (A-B) THP-1 cells were treated with varying concentrations of HNE for 1 hour (A) or with 20 µM HNE for varying time periods (B). After treatment, cells were washed, resuspended in the assay buffer, and sonicated to lyse cells. Equal amounts of protein were taken to measure TrxR activity using the TrxR activity assay kit. (C) Cell lysates of THP-1 cells treated with 20 µM HNE for varying time periods were probed to analyze the phosphorylation status of MKK3/6 and p38 MAPK by western blot analysis. (D) The phosphorylation status of MKK3/6 and p38 MAPK upon HNE stimulation was quantified and plotted against HNE-induced inhibition of TrxR activity at identical time periods. (E) THP-1 cells were treated with the antioxidant NAC (3 mM) or the electron chain transport inhibitors antimycin (Anti; 10 µM) or oligomycin (Oligo; 5 µM) for 1 hour prior to HNE treatment for 4 hours. TrxR activity was analyzed as described above. ***P < .001. ns, not significant; TNB, 5-thio-2-nitrobenzoic acid.
HNE inhibition of TrxR activity inversely correlates with MKK3/6 and p38 MAPK activation. (A-B) THP-1 cells were treated with varying concentrations of HNE for 1 hour (A) or with 20 µM HNE for varying time periods (B). After treatment, cells were washed, resuspended in the assay buffer, and sonicated to lyse cells. Equal amounts of protein were taken to measure TrxR activity using the TrxR activity assay kit. (C) Cell lysates of THP-1 cells treated with 20 µM HNE for varying time periods were probed to analyze the phosphorylation status of MKK3/6 and p38 MAPK by western blot analysis. (D) The phosphorylation status of MKK3/6 and p38 MAPK upon HNE stimulation was quantified and plotted against HNE-induced inhibition of TrxR activity at identical time periods. (E) THP-1 cells were treated with the antioxidant NAC (3 mM) or the electron chain transport inhibitors antimycin (Anti; 10 µM) or oligomycin (Oligo; 5 µM) for 1 hour prior to HNE treatment for 4 hours. TrxR activity was analyzed as described above. ***P < .001. ns, not significant; TNB, 5-thio-2-nitrobenzoic acid.
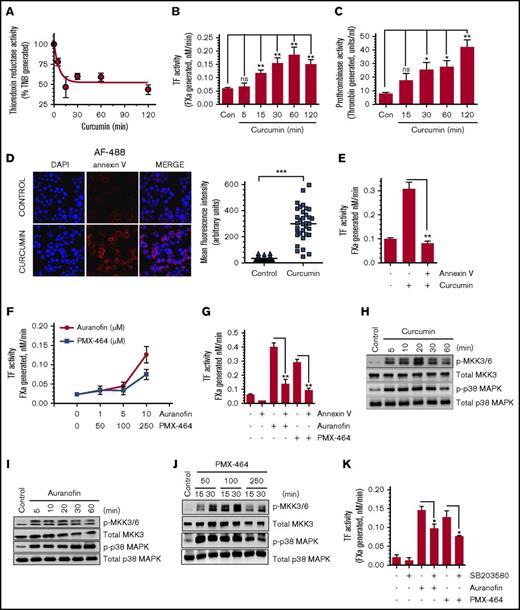
To further strengthen the data that HNE inhibition of TrxR is responsible for TF activation, TrxR activity was inhibited by treating THP-1 cells with known pharmacological inhibitors, and their effect on TF activation was analyzed. Curcumin was shown to irreversibly inhibit TrxR activity by alkylating both the Cys496/Sec497 residues in the catalytically active site of the enzyme.31 Curcumin (25 µM) treatment markedly reduced TrxR activity in THP-1 cells (Figure 3A). The same concentration of curcumin treatment led to a fourfold increase in cell surface TF activity (Figure 3B). Western blot analysis of TF antigen showed no increase in TF antigen level in THP-1 cells treated with curcumin, ruling out the possibility that curcumin induces de novo synthesis of TF protein (data not shown). Next, to determine whether the inhibition of TrxR activity by curcumin results in an increased PS exposure at the cell surface that could contribute to TF decryption, we analyzed the PS exposure in control and curcumin-treated THP-1 cells by measuring cell surface prothrombinase activity and staining intact cells with AF488-annexin V. Curcumin treatment significantly enhanced cell surface prothrombinase activity (Figure 3C) and increased AF488-annexin V binding to cells (Figure 3D). Blockade of cell surface PS by annexin V fully attenuated curcumin-induced the increase in TF activity at the cell surface (Figure 3E). Overall, these data indicate that inhibition of TrxR activity results in PS externalization, which contributes to TF activation. Because curcumin is a broad-spectrum inhibitor that could affect many cellular functions other than inhibition of TrxR/Trx, additional studies were performed with the specific inhibitors auranofin and PMX-464, which inhibit TrxR and the Trx/TrxR system, respectively. Both auranofin and PMX-464 treatment significantly enhanced cell surface TF activity (Figure 3F). Blockade of cell surface PS by annexin V attenuated auranofin- and PMX-464–induced TF decryption, further supporting the hypothesis that inhibition of the Trx/TrxR system enhances TF decryption in a PS-dependent manner (Figure 3G).
Pharmacological inhibition of TrxR enhances cell surface TF activity in THP-1 cells. (A) THP-1 cells were treated with curcumin (25 µM) for varying time periods, and TrxR activity was measured. (B-C) THP-1 cells were treated with curcumin (25 µM) for varying time periods. Cell surface TF (B) and prothrombinase (C) activity was analyzed. (D) THP-1 cells were treated with curcumin (25 µM) for 2 hours and then fixed with 4% paraformaldehyde. After fixation, cells were washed and stained with AF488-annexin V and analyzed using confocal fluorescence microscopy, and the intensity of fluorescence was quantified. Images were obtained at 63× magnification (oil immersion). (E) THP-1 cells were treated with curcumin (25 µM) for 2 hours. After removing curcumin and washing cells once, cells were incubated with annexin V (400 nM) for 30 minutes to block cell surface PS before measuring TF activity. (F) THP-1 cells were treated with specific inhibitors of TrxR (auranofin or PMX-464) at varying concentrations for 2 hours, and cell surface TF activity was measured. (G) THP-1 cells were treated with 10 µM auranofin or 250 µM PMX-464 for 2 hours. Thereafter, a control vehicle or annexin V (400 nM) was added to cells for 30 minutes before measuring cell surface TF activity. THP-1 cells were treated with 25 µM curcumin (H), 10 µM auranofin (I), or 3 different doses of PMX-464 (50, 100, or 250 µM) (J) for a time period between 0 and 60 minutes, and the activation of MKK3/6 and p38 MAPK was analyzed by western blot analysis. (K) THP-1 cells were pretreated with the p38 MAPK activation inhibitor SB203580 (20 µM) for 1 hour, followed by PMX-464 (250 µM) or auranofin (10 µM) for 2 hours, and then cell surface TF activity was measured. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). DAPI, 4′-6-diamidino-2-phenylindole.
Pharmacological inhibition of TrxR enhances cell surface TF activity in THP-1 cells. (A) THP-1 cells were treated with curcumin (25 µM) for varying time periods, and TrxR activity was measured. (B-C) THP-1 cells were treated with curcumin (25 µM) for varying time periods. Cell surface TF (B) and prothrombinase (C) activity was analyzed. (D) THP-1 cells were treated with curcumin (25 µM) for 2 hours and then fixed with 4% paraformaldehyde. After fixation, cells were washed and stained with AF488-annexin V and analyzed using confocal fluorescence microscopy, and the intensity of fluorescence was quantified. Images were obtained at 63× magnification (oil immersion). (E) THP-1 cells were treated with curcumin (25 µM) for 2 hours. After removing curcumin and washing cells once, cells were incubated with annexin V (400 nM) for 30 minutes to block cell surface PS before measuring TF activity. (F) THP-1 cells were treated with specific inhibitors of TrxR (auranofin or PMX-464) at varying concentrations for 2 hours, and cell surface TF activity was measured. (G) THP-1 cells were treated with 10 µM auranofin or 250 µM PMX-464 for 2 hours. Thereafter, a control vehicle or annexin V (400 nM) was added to cells for 30 minutes before measuring cell surface TF activity. THP-1 cells were treated with 25 µM curcumin (H), 10 µM auranofin (I), or 3 different doses of PMX-464 (50, 100, or 250 µM) (J) for a time period between 0 and 60 minutes, and the activation of MKK3/6 and p38 MAPK was analyzed by western blot analysis. (K) THP-1 cells were pretreated with the p38 MAPK activation inhibitor SB203580 (20 µM) for 1 hour, followed by PMX-464 (250 µM) or auranofin (10 µM) for 2 hours, and then cell surface TF activity was measured. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). DAPI, 4′-6-diamidino-2-phenylindole.
Next, we examined the effect of Trx/TrxR inhibition by its pharmacological inhibitors on activation of MKK3/6 and p38 MAPK. As shown in Figure 3H-J, all inhibitors of the Trx/TrxR system (ie, curcumin, auranofin, and PMX-4) induced MKK3/6 and p38 MAPK activation in THP-1 cells. Pretreatment of THP-1 cells with the specific inhibitor of p38 MAPK (SB203580), which inhibited p38 MAPK activation by ∼70% in our cell system (supplemental Figure 6), significantly impeded TF decryption induced by TrxR-specific inhibitors (Figure 3K), indicating that the TrxR/Trx system regulates TF activity through activation of the p38 MAPK pathway. Additional experiments revealed that the inhibition of p38 MAPK activation with SB203580 reduced levels of cell surface PS as measured in the prothrombinase activity assay (supplemental Figure 7), indicating that blockade of p38 MAPK activation blocks TF decryption by decreasing levels of cell surface PS.
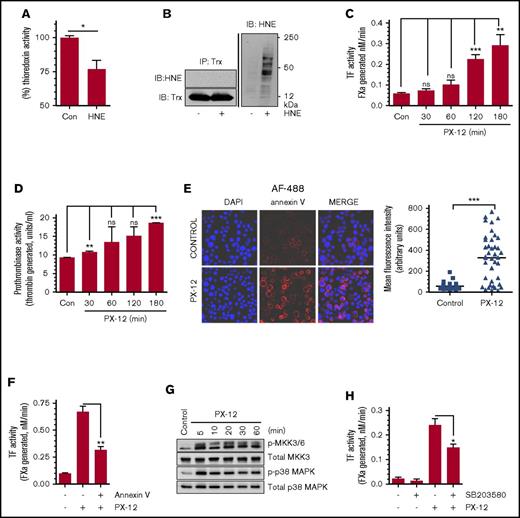
HNE inhibition of Trx and its role in TF decryption
Trx is the cellular substrate of TrxR that relays TrxR-induced thiol-dependent thiol-disulfide exchange reactions to the downstream molecules, altering their activation status. Therefore, we next investigated the effect of HNE on Trx activity and its role in HNE-induced TF decryption. HNE treatment attenuated Trx activity in THP-1 cells significantly, but not completely (Figure 4A). Inhibition of Trx activity could stem from either an adduct formation between the active site cysteine residue of Trx and HNE or the inhibition of TrxR. We were unable to detect an adduct formation between HNE and Trx from the analysis of immunoprecipitate of Trx from HNE-treated cells under our experimental conditions and methods (Figure 4B). To investigate the involvement of Trx in regulating TF decryption, we examined the effect of PX-12, a specific inhibitor of Trx, on TF activity. As shown in Figure 4C, inhibition of Trx by PX-12 increased cell surface TF activity in a time-dependent manner. Next, to determine whether the increased TF activity following the inhibition of Trx was due to PS externalization, we analyzed the PS exposure in THP-1 cells treated with a Trx inhibitor. Inhibition of Trx by PX-12 resulted in a significant increase in cell surface prothrombinase activity (Figure 4D) and annexin V binding to cells (Figure 4E). Blockade of the plasma membrane PS by annexin V markedly reduced PX-12–induced TF decryption, suggesting that PX-12–induced TF decryption was dependent on PS externalization (Figure 4F). PX-12–induced inhibition of Trx, as well as HNE treatment, enhanced the activation of both MKK 3/6 and p38 MAPK (Figure 4G). Pretreatment of THP-1 cells with the p38 MAPK inhibitor SB203580 significantly reduced PX-12–induced TF decryption (Figure 4H). Overall, these data provide strong support to the hypothesis that HNE induces TF decryption by inhibiting the TrxR/Trx system, which ultimately leads to p38 MAPK activation-dependent PS externalization.
Inhibition of Trx contributes to p38 MAPK activation– and PS-dependent TF decryption. (A) THP-1 cells were treated with 20 µM HNE for 2 hours at 37°C and then washed and lysed in assay buffer. Equal amounts of protein were used to measure Trx activity using a Trx activity assay kit. (B) THP-1 cells were treated with HNE as described above. Cells were lysed, and equal amounts of protein were pulled down using anti-Trx antibody. Immunoprecipitates were probed with either anti-HNE antibody or anti-Trx antibody. Total cell lysates were probed with anti-HNE antibodies (right). (C-D) THP-1 cells were treated with PX-12 (40 µM), a specific inhibitor of Trx, for varying time intervals, and cell surface TF activity (C) and prothrombinase activity (D) were determined. (E) THP-1 cells were treated with PX-12 (40 µM) for 3 hours, fixed, and stained with 4′-6-diamidino-2-phenylindole (DAPI) and AF488-annexin V; the fluorescence intensity of the staining was then quantified. Images were obtained at 63× magnification (oil immersion). (F) THP-1 cells were treated with PX-12 for 3 hours, followed by annexin V (400 nM) for 30 min. Cell surface TF activity was measured with an FX activation assay. (G) THP-1 cells were treated with PX-12 (40 µM) for varying time periods, and the phosphorylation of MKK 3/6 and p38 MAPK were analyzed by western blot analysis. (H) THP-1 cells were pretreated with SB203580 (20 µM) for 1 hour, followed by PX-12 (40 µM) for 3 hours. At the end of PX-12 treatment, cell surface TF activity was measured in an FX activation assay. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). IB, immunoblot; IP, immunoprecipitation.
Inhibition of Trx contributes to p38 MAPK activation– and PS-dependent TF decryption. (A) THP-1 cells were treated with 20 µM HNE for 2 hours at 37°C and then washed and lysed in assay buffer. Equal amounts of protein were used to measure Trx activity using a Trx activity assay kit. (B) THP-1 cells were treated with HNE as described above. Cells were lysed, and equal amounts of protein were pulled down using anti-Trx antibody. Immunoprecipitates were probed with either anti-HNE antibody or anti-Trx antibody. Total cell lysates were probed with anti-HNE antibodies (right). (C-D) THP-1 cells were treated with PX-12 (40 µM), a specific inhibitor of Trx, for varying time intervals, and cell surface TF activity (C) and prothrombinase activity (D) were determined. (E) THP-1 cells were treated with PX-12 (40 µM) for 3 hours, fixed, and stained with 4′-6-diamidino-2-phenylindole (DAPI) and AF488-annexin V; the fluorescence intensity of the staining was then quantified. Images were obtained at 63× magnification (oil immersion). (F) THP-1 cells were treated with PX-12 for 3 hours, followed by annexin V (400 nM) for 30 min. Cell surface TF activity was measured with an FX activation assay. (G) THP-1 cells were treated with PX-12 (40 µM) for varying time periods, and the phosphorylation of MKK 3/6 and p38 MAPK were analyzed by western blot analysis. (H) THP-1 cells were pretreated with SB203580 (20 µM) for 1 hour, followed by PX-12 (40 µM) for 3 hours. At the end of PX-12 treatment, cell surface TF activity was measured in an FX activation assay. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). IB, immunoblot; IP, immunoprecipitation.
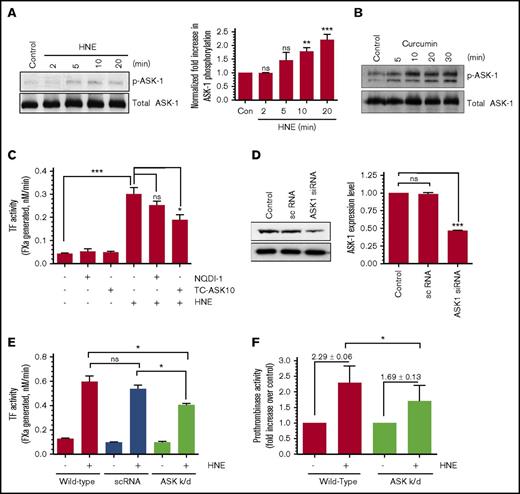
Activation of ASK-1 links HNE inhibition of the TrxR/Trx system to p38 MAPK activation and TF decryption
The reduced form of Trx binds ASK-1 and inhibits the activity of ASK-1.20 Oxidation or inhibition/inactivation of Trx disrupts the Trx–ASK-1 complex, and dissociation of the Trx–ASK-1 complex leads to activation of ASK-1.22,32 Activation of ASK-1 was shown to promote the activation of downstream MKK3/6, which leads to p38 MAPK activation. Because our data clearly show that HNE inhibits the TrxR/Trx system, we investigated whether HNE treatment results in activation of ASK-1 as well as the role of ASK-1 activation in HNE-induced TF decryption. THP-1 cells were treated with HNE for varying time periods, and ASK-1 activation was evaluated by monitoring its phosphorylation. HNE treatment weakly but clearly induced the activation of ASK-1 in THP-1 cells (Figure 5A). Curcumin, which inhibits the TrxR/Trx system, also induced the activation of ASK-1 (Figure 5B). These data indicate that inhibition of TrxR/Trx leads to ASK-1 activation in THP-1 cells. Inhibition of ASK-1 activity by its pharmacologic inhibitor, TC-ASK10, significantly attenuated HNE-induced TF decryption in THP-1 cells (Figure 5C). Another ASK-1 inhibitor, NQD1, also decreased HNE-induced TF decryption, but this decrease was not statistically significant. In additional studies, we knocked down ASK-1 expression by infecting THP-1 cells with lentivirus encoding ASK-1 siRNA. After multiple trials, we were successful in knocking down ASK-1 significantly, but not completely (Figure 5D). Even with the partial knockdown, ASK-1 suppression was found to significantly block HNE-induced PS externalization and TF decryption (Figure 5E-F), indicating that HNE activation of ASK-1 plays a role in HNE-induced PS externalization and TF decryption. Under our experimental conditions, inhibition of ASK-1 activity or expression did not affect cell viability (∼93% to 95% of cells were viable in both control and ASK-1–tampered cells). The partial inhibition of ASK-1 did not permit us to obtain conclusive data on the effect of HNE-induced ASK-1 activation on p38 MAPK activation, because the p38 MAPK activation assay was not as robust as the TF and prothrombinase activity assays.
Inhibition of ASK-1 partly attenuates HNE-induced TF activation. (A-B) THP-1 cells were treated with 20 µM HNE (A) or 25 µM curcumin (B) for varying time periods. Phosphorylation of ASK-1 analyzed by western blot analysis using antibodies against phosphorylated ASK-1 (Thr 845). ASK-1 phosphorylation levels in HNE-treated cells were quantified by densitometry of phosphorylated ASK-1 protein band on western blots (A, right). (C) THP-1 cells were pretreated with the ASK-1 inhibitors NQD1 (10 µM) or TC-ASK 10 (12.5 µM) overnight and stimulated with HNE (20 µM) for 4 hours, and cell surface TF activity was measured. (D) ASK-1 levels in untransfected THP-1 cells or THP-1 cells stably transfected with a scrambled (sc) siRNA or ASK-1 siRNA lentivirus. Western blot analysis (left) and quantification of ASK-1 band intensity by densitometry (right). (E) THP-1 cells transfected with a control or ASK-1 siRNA lentivirus were treated with HNE (20 µM) for 4 hours, and cell surface TF activity was measured in an FX activation assay. (F) THP-1 cells (wild-type or transfected with ASK-1 siRNA lentivirus) were treated with HNE (20 µM) for 4 hours, and cell surface prothrombinase activity was measured using a prothrombin activation assay. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). k/d, knocked-down.
Inhibition of ASK-1 partly attenuates HNE-induced TF activation. (A-B) THP-1 cells were treated with 20 µM HNE (A) or 25 µM curcumin (B) for varying time periods. Phosphorylation of ASK-1 analyzed by western blot analysis using antibodies against phosphorylated ASK-1 (Thr 845). ASK-1 phosphorylation levels in HNE-treated cells were quantified by densitometry of phosphorylated ASK-1 protein band on western blots (A, right). (C) THP-1 cells were pretreated with the ASK-1 inhibitors NQD1 (10 µM) or TC-ASK 10 (12.5 µM) overnight and stimulated with HNE (20 µM) for 4 hours, and cell surface TF activity was measured. (D) ASK-1 levels in untransfected THP-1 cells or THP-1 cells stably transfected with a scrambled (sc) siRNA or ASK-1 siRNA lentivirus. Western blot analysis (left) and quantification of ASK-1 band intensity by densitometry (right). (E) THP-1 cells transfected with a control or ASK-1 siRNA lentivirus were treated with HNE (20 µM) for 4 hours, and cell surface TF activity was measured in an FX activation assay. (F) THP-1 cells (wild-type or transfected with ASK-1 siRNA lentivirus) were treated with HNE (20 µM) for 4 hours, and cell surface prothrombinase activity was measured using a prothrombin activation assay. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). k/d, knocked-down.
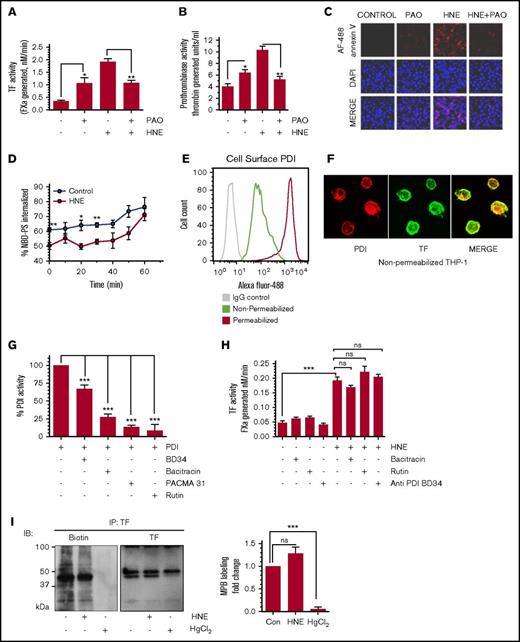
Role of thiol-disulfide exchange reactions in HNE-induced TF decryption
Earlier studies from others indicated that thiol-disulfide exchange processes catalyzed by PDI might play a role in ATP- and antithymocyte globulin–induced TF activation in monocytic cells.16,33 To determine the importance of thiol-disulfide exchange reactions in HNE-induced TF decryption, free thiol groups in THP-1 cells were blocked by treating the cells with phenylarsine oxide (PAO) prior to HNE treatment. Blocking free thiols with PAO in itself resulted in increased basal TF activity. However, PAO treatment attenuated the HNE-induced TF activity increase (Figure 6A), suggesting that HNE-induced TF decryption may involve thiol exchange reactions. PAO did not affect HNE-induced p38 MAPK activation (supplemental Figure 8). Next, we investigated the effect of PAO on PS externalization to the outer leaflet by analyzing cell surface prothrombinase activity (Figure 6B) and annexin V binding to the cell surface (Figure 6C). Although PAO treatment increased cell surface PS levels in control cells to some extent, PAO treatment markedly inhibited the HNE-induced increase in PS levels in the outer leaflet (Figure 6B-C). Earlier studies showed that activity of the aminophospholipid translocase flippase can be altered by sulfhydryl-modifying agents11 and thereby affect PS asymmetry at the cell membrane. To investigate whether HNE impairs flippase activity, we analyzed internalization of NBD-PS from the outer leaflet in control and HNE-treated cells. As shown in Figure 6D, HNE treatment significantly delayed the internalization of NBD-PS, suggesting an inhibitory effect of HNE on flippase activity.
Effect of thiol blocker PAO and PDI inhibitors on HNE-induced PS exposure and TF activation. (A-B) THP-1 cells were treated with PAO (10 µM) for 15 minutes. After washing cells in serum-free medium, cells were treated with HNE (20 µM) for 4 hours, and cell surface TF activity (A) or prothrombinase (B) activity was measured. (C) THP-1 cells were treated with PAO and HNE as described for panels A-B, and fixed cells were stained with 4′-6-diamidino-2-phenylindole (DAPI) and AF488-annexin V. (D) THP-1 cells were treated with HNE (20 µM) for 4 hours; after washing with buffer A, cells were chilled on ice in buffer A containing Ca2+ for 5 minutes. Cells were then incubated with NBD-PS (2 µM) for 10 minutes on ice and washed twice with ice-cold buffer A. Cells were resuspended in buffer A containing Ca2+ prewarmed to 37°C, and fluorescence was read for a time course of 0 to 60 minutes with or without sodium dithionite to determine % NBD-PS internalized (see “Materials and methods”). (E) THP-1 cells, intact or permeabilized, were labeled with anti-PDI antibody and subjected to flow cytometry. Green, intact cells stained with the anti-PDI antibody; red, permeabilized cells stained with anti-PDI antibody; gray, intact cells stained with control immunoglobulin G. (F) Nonpermeabilized THP-1 cells were labeled with either anti-PDI antibody (red) or anti-TF antibody (green) and subjected to confocal microscopy. (G) Recombinant PDI was treated with various PDI inhibitors or anti-PDI inhibitory antibody for 1 hour at the concentrations indicated in the figure. PDI activity was measured using a fluorescence-based insulin reduction assay. (H) THP-1 cells were pretreated with PDI inhibitors or the inhibitory antibody for 1 hour, followed by HNE (20 µM) for 4 hours. Cell surface TF activity was measured in an FX activation assay. (I) THP-1 cells were treated with either HNE (20 µM) for 4 hours or HgCl2 (100 µM) for 5 minutes. After treatment, cells were washed and labeled with MPB (100 µM) for 30 minutes, and excess, unbound MPB was removed and quenched with reduced glutathione (200 µM) for 30 minutes. Cells were then lysed, and TF was pulled down using anti-TF antibodies. The sample was then run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed for MPB and TF using streptavidin and anti-TF antibodies, respectively. Quantification of thiol-labeled TF protein band by densitometry (right). The band intensity measured in control vehicle-treated cells was taken as 1. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). Images in panels C,F were obtained at 63× magnification (oil immersion). IB, immunoblot; IgG, immunoglobulin G.
Effect of thiol blocker PAO and PDI inhibitors on HNE-induced PS exposure and TF activation. (A-B) THP-1 cells were treated with PAO (10 µM) for 15 minutes. After washing cells in serum-free medium, cells were treated with HNE (20 µM) for 4 hours, and cell surface TF activity (A) or prothrombinase (B) activity was measured. (C) THP-1 cells were treated with PAO and HNE as described for panels A-B, and fixed cells were stained with 4′-6-diamidino-2-phenylindole (DAPI) and AF488-annexin V. (D) THP-1 cells were treated with HNE (20 µM) for 4 hours; after washing with buffer A, cells were chilled on ice in buffer A containing Ca2+ for 5 minutes. Cells were then incubated with NBD-PS (2 µM) for 10 minutes on ice and washed twice with ice-cold buffer A. Cells were resuspended in buffer A containing Ca2+ prewarmed to 37°C, and fluorescence was read for a time course of 0 to 60 minutes with or without sodium dithionite to determine % NBD-PS internalized (see “Materials and methods”). (E) THP-1 cells, intact or permeabilized, were labeled with anti-PDI antibody and subjected to flow cytometry. Green, intact cells stained with the anti-PDI antibody; red, permeabilized cells stained with anti-PDI antibody; gray, intact cells stained with control immunoglobulin G. (F) Nonpermeabilized THP-1 cells were labeled with either anti-PDI antibody (red) or anti-TF antibody (green) and subjected to confocal microscopy. (G) Recombinant PDI was treated with various PDI inhibitors or anti-PDI inhibitory antibody for 1 hour at the concentrations indicated in the figure. PDI activity was measured using a fluorescence-based insulin reduction assay. (H) THP-1 cells were pretreated with PDI inhibitors or the inhibitory antibody for 1 hour, followed by HNE (20 µM) for 4 hours. Cell surface TF activity was measured in an FX activation assay. (I) THP-1 cells were treated with either HNE (20 µM) for 4 hours or HgCl2 (100 µM) for 5 minutes. After treatment, cells were washed and labeled with MPB (100 µM) for 30 minutes, and excess, unbound MPB was removed and quenched with reduced glutathione (200 µM) for 30 minutes. Cells were then lysed, and TF was pulled down using anti-TF antibodies. The sample was then run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed for MPB and TF using streptavidin and anti-TF antibodies, respectively. Quantification of thiol-labeled TF protein band by densitometry (right). The band intensity measured in control vehicle-treated cells was taken as 1. *P < .05; **P < .01; ***P < .001; ns, not significant (compared with the values obtained in their respective controls or as indicated in the figures). Images in panels C,F were obtained at 63× magnification (oil immersion). IB, immunoblot; IgG, immunoglobulin G.
Next, to determine the potential involvement of PDI in HNE-induced TF activation, we first analyzed the presence of PDI on the surface of THP-1 cells. Both flow cytometry (Figure 6E) and confocal microscopy (Figure 6F) data showed the presence of PDI on the cell surface of THP-1 cells. To investigate the role of PDI in HNE-induced TF decryption, we first inhibited PDI activity in THP-1 cells using a number of specific inhibitors prior to HNE treatment. All of the inhibitors tested significantly inhibited PDI activity (Figure 6G). However, none of the PDI inhibitors had a significant effect on HNE-induced TF activation (Figure 6H). These observations suggest that although thiol groups are important in regulating HNE-induced TF activation, PDI was not involved in HNE-induced TF decryption.
Finally, to investigate whether HNE-induced thiol exchange pathways alter disulfide bonding in TF, THP-1 cells were treated with a control vehicle or HNE, and cell surface thiol groups were labeled with MPB. TF in THP-1 cells contained free thiols, but HNE treatment did not affect the status of free thiols in TF (Figure 6I). In a control experiment, treatment of THP-1 cells with HgCl2 prior to MPB labeling depleted free thiol markedly (Figure 6I). Overall, the above cumulative data suggest that thiol-disulfide exchange reactions play a role in HNE-induced TF decryption by regulating the exposure of PS at the cell surface, but not through PDI-mediated disulfide bond switching in TF.
Discussion
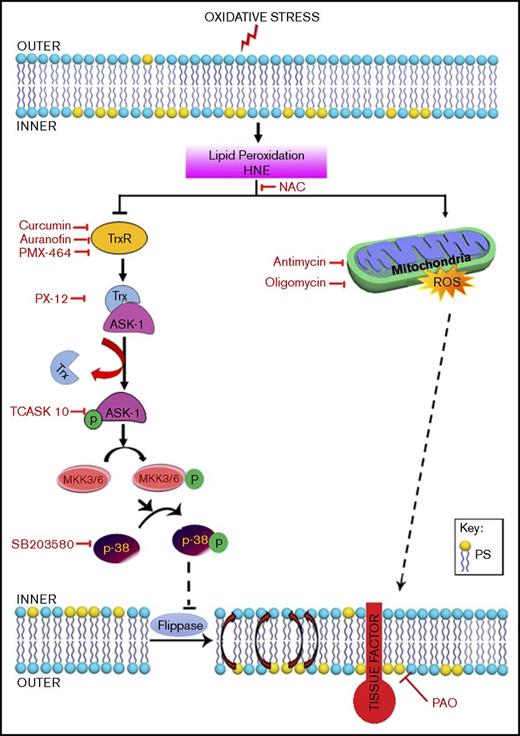
HNE is a major α, β-unsaturated aldehyde produced during oxidative stress–induced peroxidation of ω-6 fatty acid. HNE forms Michael adducts with various biological macromolecules containing nucleophilic thiol and amino groups and alters cell signaling.34 Elevated HNE formation is implicated in various oxidative stress–related diseases, such as cardiovascular diseases, neurodegenerative diseases, metabolic syndrome, and cancer.1,2 The high oxidative capacity of the myocardium serves as a constant source of HNE, which can migrate to distant sites from its origin.2 The present study extends our earlier observation that HNE enhances TF activity through decryption and provides novel mechanistic insights into HNE-induced TF decryption. The data presented herein show that HNE induces TF decryption in monocytic cells via 2 distinct and independent pathways. One pathway is dependent on the TrxR/Trx system and p38 MAPK signaling, whereas the other is independent of p38 MAPK but dependent on ROS generation through the mitochondria. However, both pathways lead to elevation of PS levels on the cell surface, the critical determinant in TF decryption.
The THP-1 cell system is an appropriate model system to investigate mechanistic details of HNE-induced TF decryption in monocytes and macrophages because our earlier studies7 showed that both human macrophages and THP-1 cells respond to HNE to a similar extent in a similar dose- and time-dependent manner. Our earlier studies showed that HNE treatment generated ROS and activated p38 MAPK in THP-1 cells and the blockade of p38 MAPK activation inhibited HNE-induced PS exposure and TF decryption.7 However, the source of ROS generation and the link between ROS generation and p38 MAPK activation were not examined. Our present data show that HNE treatment induces ROS generation through the mitochondria. HNE-induced ROS generation was inhibited by the mitochondrial electron transport chain inhibitors antimycin and oligomycin, which block electron flow at complex III and prevent protons from entering the matrix through ATP synthase, respectively. An earlier study suggested that because the conversion of superoxides to hydrogen peroxide might require the presence of protons in the mitochondrial matrix, ROS generation is therefore sensitive to oligomycin.13 Both antimycin and oligomycin inhibited TF activation significantly, but not completely. More importantly, these inhibitors did not affect HNE-induced p38 MAPK activation, indicating that HNE-induced ROS-dependent TF decryption is not mediated through ROS activation of p38 MAPK.
The TrxR/Trx system is the major regulator of intracellular protein thiol redox balance, and inhibition of the TrxR/Trx system disrupts a number of redox-sensitive functions in cells.10,35 It has been shown that inhibition of TrxR promotes the activation of ASK-1 by dissociating ASK-1 from the Trx–ASK-1 complex, which in turn promotes the activation of MKK3/6, leading to p38 MAPK activation.20,22 Reactive aldehydes, such as HNE, are strong electrophiles that react readily with nucleophiles.34 In biological systems, they appear to have the highest reactivity with thiols, typically through Michael addition.34 Although we found no evidence for the formation of HNE-Trx adducts in our study, our data clearly show that HNE inhibits the activity of both TrxR and Trx. Consistent with earlier data showing that inhibition of Trx induces ASK-1 activation,22,32 HNE inhibition of Trx resulted in activation of ASK-1 and the downstream signaling molecules MKK3/6 and p38 MAPK. Inhibition of TrxR/Trx by specific pharmacological inhibitors also led to activation of ASK-1, MKK3/6, and p38 MAPK. As with HNE, treatment of THP-1 cells with these inhibitors also led to an increase in PS levels at the cell surface and enhanced cell surface TF activity. Taken together, our data indicate that HNE inhibition of TrxR/Trx leads to increased PS on the cell surface via ASK-1 and p38 MAPK signaling and that the increased PS is responsible for HNE-induced TF decryption (Figure 7).
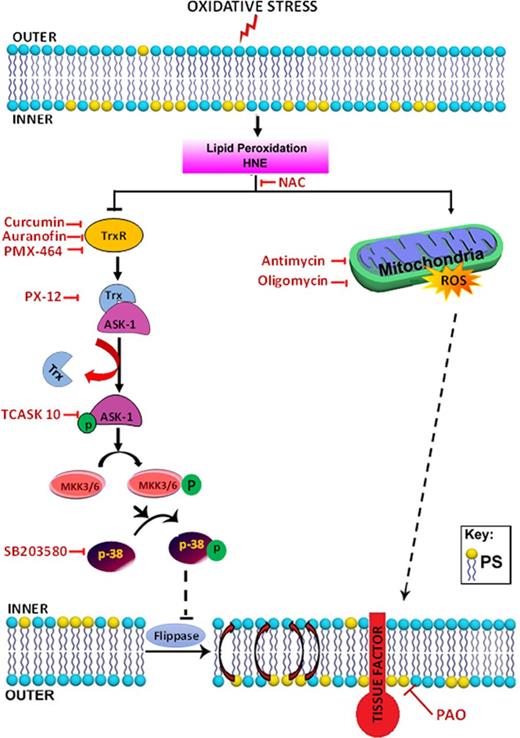
Schematic representation of the proposed signaling mechanisms involved in HNE-induced TF decryption. Oxidative stress causes lipid peroxidation and produces reactive aldehydes, such as HNE. HNE induces ROS generation in mitochondria, and ROS increase PS levels in the outer leaflet. Increased PS levels at the outer leaflet contribute to TF decryption. At present, the mechanism by which ROS increase PS levels is unknown. HNE, in addition to generating ROS, also inactivates the TrxR/Trx system by inhibiting its activity or forming adducts. Inhibition of Trx leads to dissociation of the Trx–ASK-1 complex, leading to ASK-1 activation. ASK-1 activation promotes the activation of MKK3/MKK6 and the upstream activation of p38 MAPK. p38 MAPK activation leads to inhibition of flippase activity and a resultant increase in PS levels at the outer leaflet, which contributes to TF activation. HNE-induced TF decryption can be inhibited using specific inhibitors that target different steps in the HNE-induced signaling pathway.
Schematic representation of the proposed signaling mechanisms involved in HNE-induced TF decryption. Oxidative stress causes lipid peroxidation and produces reactive aldehydes, such as HNE. HNE induces ROS generation in mitochondria, and ROS increase PS levels in the outer leaflet. Increased PS levels at the outer leaflet contribute to TF decryption. At present, the mechanism by which ROS increase PS levels is unknown. HNE, in addition to generating ROS, also inactivates the TrxR/Trx system by inhibiting its activity or forming adducts. Inhibition of Trx leads to dissociation of the Trx–ASK-1 complex, leading to ASK-1 activation. ASK-1 activation promotes the activation of MKK3/MKK6 and the upstream activation of p38 MAPK. p38 MAPK activation leads to inhibition of flippase activity and a resultant increase in PS levels at the outer leaflet, which contributes to TF activation. HNE-induced TF decryption can be inhibited using specific inhibitors that target different steps in the HNE-induced signaling pathway.
A recent study by Wang et al17 showed that the TrxR/Trx system functions as a negative regulator of cell surface TF activity, as Trx knockdown resulted in a significant increase in cell surface TF procoagulant activity in cancer cells. Using purified recombinant soluble TF and Trx, the study showed that the TrxR/Trx redox system reduces the Cys186-Cys209 disulfide bond in TF (Trx forms mixed disulfide bonds with TF) and abrogated TF procoagulant activity. Based on this, Wang et al17 suggested that TrxR/Trx may regulate TF activity at the cell surface through regulation of Cys redox states in the TF extracellular domain. It is also possible that TrxR may also modulate redox states in TF via PDI, because PDI is one of the potential substrates of TrxR.36 However, we found no evidence in our studies that HNE treatment affects the status of thiols in TF. Our data showed that although a fraction of TF on THP-1 cells exists in the reduced thiol form, HNE treatment did not alter levels of TF thiols. Therefore, it is unlikely that HNE-induced TrxR/Trx-mediated TF decryption involves modification in Cys disulfide bonding in TF.
Recently, Rothmeier et al18 reported that ATP-induced TF decryption and TF+ MV release results from uncoupling of the TrxR/Trx system. Our data are in broad agreement with the concept that the TrxR/Trx system plays an important role in TF encryption/decryption, but the role of the TrxR/Trx system in HNE-induced TF activation appears to differ from that in ATP-induced TF activation and TF+ MV release. It is interesting to note that HNE treatment, unlike ATP, does not increase TF+ MV release in monocytic THP-1 cells.7 Thiol blocking and PDI have been suggested to regulate ATP-induced TF decryption in macrophages. Blockade of the thiol groups with PAO significantly attenuated HNE-induced TF decryption, indicating the involvement of thiol exchange pathways in HNE-induced TF decryption. However, our data clearly show that the effect of the thiol blocker was not due to its influence on PDI-mediated thiol modifications, because inhibition of PDI with various inhibitors did not affect HNE-induced TF activation. Earlier data suggested that sulfhydryl-modifying agents modulate cell-surface PS dynamics by regulating the aminophospholipid translocases.37-39 Therefore, it is possible that a direct modification of aminophospholipid translocases by PAO may be responsible for blocking HNE-induced PS externalization and TF decryption. Overall, our data are in agreement with earlier reports that show thiol pathways play a major role in the regulation of PS dynamics in the plasma membrane.39-41
In summary, our present study delineates potential mechanisms by which oxidative stress transforms cryptic TF into procoagulant-active TF on the cell surface of monocytes and macrophages. Our data show that oxidative stress can activate TF via at least 2 different nonoverlapping pathways: a ROS-dependent pathway and a TrxR/Trx-dependent pathway. The TrxR/Trx-dependent pathway appears to modulate TF decryption by a novel pathway involving the activation of ASK-1, MKK3/6, and p38 MAPK activation. Both pathways modulate TF decryption by modulating PS externalization. Identification of the key players involved in TF activation would be helpful in developing therapeutic strategies to attenuate thrombotic complications.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R01-HL124055 (L.V.M.R.) and American Heart Association grant 15GRNT22620004 (U.R.P.).
Authorship
Contribution: S.A.A. performed experiments, summarized the data, and wrote the initial draft of the manuscript; U.R.P. contributed to experimental design, generated lentiviral siRNA constructs, reviewed data, and edited the manuscript; L.V.M.R. conceived and designed the study, analyzed the data, and wrote the manuscript; and all authors contributed to the preparation of the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, Department of Cellular and Molecular Biology, The University of Texas Health Science Center at Tyler, 11937 US Highway 271, Tyler, TX 75708; e-mail: vijay.rao@uthct.edu.