Key Points
Obesity is not associated with MGUS or LC-MGUS.
High body mass index during midlife is associated with increased risk of progressing from MGUS and LC-MGUS to MM and other LP diseases.
Abstract
All multiple myeloma (MM) cases are preceded by the premalignant state monoclonal gammopathy of undetermined significance (MGUS). Results from previous studies show a positive association between obesity and MM; however, the association between obesity and MGUS is controversial. The aims were to determine (1) if obesity is associated with an increased risk of MGUS and light-chain MGUS (LC-MGUS) and (2) whether obesity is associated with a higher risk of progression to MM and other lymphoproliferative (LP) diseases. Data from the population-based Age, Gene/Environment Susceptibility–Reykjavik Study (N = 5764) were used. We performed serum protein electrophoresis and serum free light-chain assay on all subjects to identify MGUS and LC-MGUS cases. We included 11 different measures on current and previous obesity in our analysis. Logistic regression and Cox proportional-hazard regression were used to analyze the associations. A total of 300 (5.2%) MGUS and 275 (4.8%) LC-MGUS cases were identified. During a median follow-up of 8 years, 18 had progressed to MM and 11 to other LP diseases. We found no association between the 11 obesity markers and MGUS or LC-MGUS (odds ratios 0.81 to 1.15 for all 11 variables in both conditions). Interestingly, we found that high midlife body mass index increased risk of progression to MM and other LP diseases (hazard ratio, 2.66; 95% confidence interval, 1.17-6.05). To conclude, obesity was not associated with MGUS. However, we found overweight/obesity to be a risk factor for progression from MGUS to MM and other LP diseases, suggesting that obesity plays a role in the transformation of MGUS to MM.
Introduction
Overweight and obesity have consistently been recognized as risk factors for many common cancers.1-4 The World Health Organization (WHO) recently concluded that there is now sufficient evidence for the effect of weight on 13 types of cancers, including multiple myeloma (MM).5,6 MM is a chronic B-cell disorder characterized by a monoclonal proliferation of plasma cells in the bone marrow.7 All cases of MM are preceded by the premalignant state monoclonal gammopathy of undetermined significance (MGUS),8,9 which is an asymptomatic condition characterized by the presence of an M-protein in serum without evidence of MM or other lymphoproliferative (LP) diseases.10 The prevalence of MGUS increases with age and is reported to be found in ∼5% of individuals older than 70 years.11 The average risk of progression from MGUS to MM is estimated to be 1% per year.12,13 Light-chain MGUS (LC-MGUS) has recently been described as a precursor condition to light-chain MM.14,15 LC-MGUS is defined as an abnormal free light-chain (FLC) ratio with no expression of heavy chains, along with an increased concentration of the light chain involved, and its prevalence has been estimated to be 0.7% to 0.8%.14,15
The etiology of MGUS and LC-MGUS is to a large extent unknown. However, researchers have found higher risk of MGUS among males,11 blacks,16,17 those with family history of MGUS and related diseases,18 and those with prior personal and family history of immune-related conditions.19,20 Obesity has been identified as a risk factor for MM, with6,21,22 2 meta-analyses finding a 10% to 20% increased risk of MM for a 5 kg/m2 increase in body mass index (BMI).21,22 However, BMI is a limited measurement as it does not provide information on fat distribution, which is of importance as many of the complications associated with obesity have been shown to be closely related to abdominal obesity.23,24 A recent pooled analysis of 20 prospective studies found that waist circumference is a risk factor for MM mortality and that BMI in early adulthood plays an important role later in life regarding MM mortality, particularly in women.25 In addition, a recent cross-sectional study (n = 72) found that patients recently diagnosed with MM had higher abdominal fat compared with patients with MGUS, indicating that this parameter might serve as a biomarker for progression from MGUS to MM.26
The biological mechanisms through which obesity might influence MM etiology are not yet established. Suggested mechanisms are effects of interleukin-6 (IL-6), insulin-like growth factor 1, and adiponectin. IL-6 is directly associated with obesity, ∼15% to 35% of total IL-6 is produced by adipose tissue, and IL-6 is a growth factor in MM.27 Obesity can lead to elevated levels of bioavailable insulin-like growth factor 1, which may increase MM cell proliferation and inhibit apoptosis.28 Additionally, levels of adiponectin are lower in obese individuals,29 and a recent study showed that higher levels of adiponectin lowered the risk of MM30 as well as progression from MGUS to MM.31
To date, only 2 studies on the association between obesity and MGUS have been conducted. A study from the United States based on 60 cases found a twofold increased risk of MGUS in obese (BMI >30 kg/m2) vs nonobese women.32 Another screening study from the United States, based on 365 cases of MGUS identified through the National Health and Nutritional Examination Survey, did not find an association.33 Both studies relied only on BMI as a marker for obesity. However, obesity is an outcome of many different factors, both physiological and behavioral, and could therefore be a proxy for these factors that might independently have an effect on MGUS risk. The association between obesity and LC-MGUS has, to our knowledge, not been studied.
The aim of this study was to analyze the association between 11 different obesity assessment methods and MGUS and LC-MGUS using data from a large population-based cohort, and, furthermore, to analyze if obesity is a risk factor for progression from MGUS or LC-MGUS to MM and other LP diseases.
Methods
Study population
This study is based on participants from the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-RS), which is a continuation of the population-based Reykjavik Study.34 The Reykjavik Study began recruiting in 1967 a sample of more than 30 000 residents of Reykjavik born in 1907 to 1935. In 2002, the AGES-RS started recruiting 5764 of the surviving members. A detailed description of the study and data collection has been published previously.34
All participants in the AGES-RS signed an informed consent form, and the study was approved by the Icelandic National Bioethics Committee (VSN-00-063-V35), the Icelandic Data Protection Authority, and the Institutional Review Board of the National Institute on Aging in the USA.
Material and measures
A 0.5-mL aliquot from the serum collected at AGES-RS study baseline (2002-2006) was obtained for each study subject. Each sample tube was labeled only with the participant’s coded ID number and the specimen collection year. All specimens were shipped on dry ice to the Multiple Myeloma Research Laboratory at the National Cancer Institute, where protein assays were performed. To identify MGUS cases in the AGES-RS cohort, we performed conventional agarose-gel serum protein electrophoresis (SPEP) for all subjects (Helena Laboratories, Beaumont, TX). Samples with an equivocal or definite M-protein present on SPEP were subjected to serum protein immunofixation for confirmation and typing of the M-protein.11 Serum FLC assay (FREELITE; The Binding Site Ltd., Birmingham, UK) was performed on all samples.35 All testing and interpretations were done by 2 individuals (R.C. and D.B.) blinded to all demographics and other details pertaining to the samples being tested. The results were merged with the data from the original AGES-RS cohort.
MGUS cases were defined as having 1 or several M-protein bands on SPEP and an M-protein concentration <30 g/L.36 The criteria for LC-MGUS were having no M-protein band visible on SPEP and a pathological FLC ratio (<0.26 or >1.65) on FLC analysis in combination with an increased concentration of the light chain concerned (f-κ >19.4 mg/L, f-λ >26.3 mg/L).37
We included 11 different measures on obesity in our analysis. Baseline measures were weight (kg), BMI (kg/m2), percent body fat (%), fat (kg), total body fat (cm2), visceral fat (cm2), subcutaneous fat (cm2), and 2 versions of abdominal circumference (cm). We also included self-reported lifetime maximum weight and measured midlife BMI (obtained from the Reykjavik Study data). Weight was measured using a digital scale, and height was measured using a stadiometer. Both measures were performed multiple times, and mean numbers found. Percent body fat and fat were calculated from bioelectric impedance (Xitron Hydra ECF/ICF Bio-Impedance Spectrum Analyzer), a commonly used method for estimating body composition. Computed tomography (CT) imaging of the abdomen at the level of the L4/L5 vertebrae was performed to calculate total body fat, visceral fat, subcutaneous fat, and abdominal circumference.34 Abdominal circumference was also conventionally measured38 multiple times, and a mean number found. Midlife BMI was calculated from weight and height measured at enrollment into the Reykjavik Study. The mean age of participants at Reykjavik Study entry was 53.3 years for women and 52.1 years for men.39
A total of 5764 persons were enrolled in AGES-RS. A total of 39 (0.7%) were excluded from analysis in this study, 21 subjects because of previous LP diseases, 16 because of missing blood sample available for analysis, 1 subject had a missing consent form, and 1 subject had an M-protein concentration above the limit for MGUS and therefore fulfilling the criteria for smoldering MM. One subject was excluded from the progression analysis only because of zero follow-up days. Cases of MM and other LP diseases were found by cross-linking the AGES-RS data with the Icelandic Cancer Registry. The Icelandic Cancer Registry is in accordance with internationally accepted standards as it has high diagnostic accuracy and completeness (99%).40 End of follow-up was March 2014.
Statistical analysis
Logistic regression was used to estimate the association between MGUS and LC-MGUS and the 11 obesity markers, and the results are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). Analyses were performed for both MGUS and LC-MGUS separately and together. Adjustment was made for age and sex in a multivariable analysis. Adjusting for height in multivariable models where height is not taken into account in the measurement, such as weight, waist circumference, and CT measures, did not affect the estimates and was therefore not included in the analysis. To test whether history of obesity had an effect on risk of MGUS/LC-MGUS, individuals were grouped in accordance to their midlife BMI and BMI at AGES-RS entry. Individuals with low midlife BMI and low BMI at AGES-RS entry were the referent group for groups with an increase in BMI, decrease in BMI, and constantly high BMI between these 2 time points. Cox proportional-hazard regression was used to test whether obesity was a risk factor for progression to MM or other LP diseases. Analyses were performed in R version 3.1.2.41
Results
A total of 5725 subjects were included in our analysis, with a mean age of 77 years (range 66-98). In the sample, 3306 (58%) individuals were women and 2419 were men. MGUS was identified in 300 (5.2%) subjects and LC-MGUS in 275 (4.8%) subjects (Table 1). Of the 300 MGUS patients, the Ig isotype was immunoglobulin G (IgG) in 159 (53%) patients, IgA in 27 (9%), IgM in 81 (27%), IgD in 1 (0.3%), and biclonal in 32 (10.7%). During a median follow-up of 8 years, a total of 18 individuals progressed to MM (17 of these from MGUS and 1 from LC-MGUS), and 11 individuals progressed to other LP diseases such as Hodgkin and non-Hodgkin lymphoma, Waldenström’s macroglobulinemia, leukemia, chronic lymphocytic leukemia, and acute lymphocytic leukemia (2 of these from LC-MGUS).
Characteristics of the study participants
| Characteristics . | Without MGUS, . | MGUS, . | LC-MGUS, . | MM, . | MM and other LP diseases, . |
|---|---|---|---|---|---|
| n = 5150 (93.9%) . | n = 300 (5.2%) . | n = 275 (4.8%) . | n = 18 (3.1%)* . | n = 29 (5.1%)* . | |
| Sex, n (%) | |||||
| Female | 3046 (59.1) | 141 (47.0) | 119 (43.3) | 10 (55.6) | 14 (48.3) |
| Male | 2104 (40.9) | 159 (53.0) | 156 (56.7) | 8 (44.4) | 15 (51.7) |
| Mean age (range), y | 76.8 (66-98) | 78.3 (67-93) | 79.4 (66-97) | 77.8 (69-87) | 77.4 (68-87) |
| BMI, mean (SD), kg/m2 | 27.0 (4.5) | 26.7 (4.1) | 27.0 (4.5) | 27.1 (2.4) | 26.5 (2.8) |
| BMI, % (n) | |||||
| <25.0 | 1716 (34.0) | 101 (34.1) | 93 (34.2) | 5 (27.8) | 8 (27.6) |
| 25.0-29.9 | 2222 (43.6) | 146 (49.3) | 121 (44.4) | 10 (55.6) | 18 (62.1) |
| ≥30.0 | 1154 (22.7) | 49 (16.6) | 58 (21.3) | 3 (16.7) | 3 (10.3) |
| BMI midlife, mean (SD), kg/m2 | 25.2 (3.6) | 25.5 (3.7) | 25.6 (3.8) | 26.2 (2.7) | 26.2 (3.2) |
| BMI midlife, n (%) | |||||
| <25 | 2686 (52.4) | 139 (46.7) | 128 (46.7) | 5 (27.8) | 8 (27.6) |
| ≥25 | 2442 (47.6) | 159 (53.3) | 146 (53.3) | 13 (72.2) | 21 (72.4) |
| Weight, mean (SD), kg | 75.3 (1.7) | 75.4 (14.2) | 76.4 (15.6) | 77.2 (13.5) | 76.8 (13.7) |
| Max weight, mean (SD), kg | 80.7 (15.5) | 82.4 (15.2) | 83.1 (16.1) | 85.4 (13.7) | 85.7 (13.8) |
| Percent body fat (BIA), mean (SD), % | 29.0 (7.9) | 26.8 (8.4) | 26.9 (7.7) | 28.7 (7.6) | 26.8 (7.9) |
| Fat (BIA), mean (SD), kg | 22.0 (7.9) | 20.5 (7.9) | 20.7 (7.4) | 22.7 (6.7) | 20.6 (7.1) |
| Total body fat (CT), mean (SD), cm2 | 494.3 (167.0) | 482.5 (166.5) | 495.6 (173.7) | 509.0 (130.0) | 478.5 (151.5) |
| Visceral fat (CT), mean (SD), cm2 | 171.9 (79.5) | 174.8 (85.1) | 187.5 (86.9) | 178.6 (91.4) | 174.1 (92.4) |
| Subcutaneous fat (CT), mean (SD), cm2 | 257.3 (112.1) | 241.0 (108.4) | 238.6 (108.4) | 261.1 (99.8) | 237.1 (100.5) |
| Waist circumference (CT), mean (SD), cm | 125.8 (14.0) | 125.7 (13.4) | 126.5 (14.2) | 127.8 (11.8) | 125.4 (13.3) |
| Waist circumference, mean (SD), cm | 100.8 (12.1) | 100.8 (11.2) | 101.6 (11.5) | 103.8 (9.2) | 101.5 (9.9) |
| Characteristics . | Without MGUS, . | MGUS, . | LC-MGUS, . | MM, . | MM and other LP diseases, . |
|---|---|---|---|---|---|
| n = 5150 (93.9%) . | n = 300 (5.2%) . | n = 275 (4.8%) . | n = 18 (3.1%)* . | n = 29 (5.1%)* . | |
| Sex, n (%) | |||||
| Female | 3046 (59.1) | 141 (47.0) | 119 (43.3) | 10 (55.6) | 14 (48.3) |
| Male | 2104 (40.9) | 159 (53.0) | 156 (56.7) | 8 (44.4) | 15 (51.7) |
| Mean age (range), y | 76.8 (66-98) | 78.3 (67-93) | 79.4 (66-97) | 77.8 (69-87) | 77.4 (68-87) |
| BMI, mean (SD), kg/m2 | 27.0 (4.5) | 26.7 (4.1) | 27.0 (4.5) | 27.1 (2.4) | 26.5 (2.8) |
| BMI, % (n) | |||||
| <25.0 | 1716 (34.0) | 101 (34.1) | 93 (34.2) | 5 (27.8) | 8 (27.6) |
| 25.0-29.9 | 2222 (43.6) | 146 (49.3) | 121 (44.4) | 10 (55.6) | 18 (62.1) |
| ≥30.0 | 1154 (22.7) | 49 (16.6) | 58 (21.3) | 3 (16.7) | 3 (10.3) |
| BMI midlife, mean (SD), kg/m2 | 25.2 (3.6) | 25.5 (3.7) | 25.6 (3.8) | 26.2 (2.7) | 26.2 (3.2) |
| BMI midlife, n (%) | |||||
| <25 | 2686 (52.4) | 139 (46.7) | 128 (46.7) | 5 (27.8) | 8 (27.6) |
| ≥25 | 2442 (47.6) | 159 (53.3) | 146 (53.3) | 13 (72.2) | 21 (72.4) |
| Weight, mean (SD), kg | 75.3 (1.7) | 75.4 (14.2) | 76.4 (15.6) | 77.2 (13.5) | 76.8 (13.7) |
| Max weight, mean (SD), kg | 80.7 (15.5) | 82.4 (15.2) | 83.1 (16.1) | 85.4 (13.7) | 85.7 (13.8) |
| Percent body fat (BIA), mean (SD), % | 29.0 (7.9) | 26.8 (8.4) | 26.9 (7.7) | 28.7 (7.6) | 26.8 (7.9) |
| Fat (BIA), mean (SD), kg | 22.0 (7.9) | 20.5 (7.9) | 20.7 (7.4) | 22.7 (6.7) | 20.6 (7.1) |
| Total body fat (CT), mean (SD), cm2 | 494.3 (167.0) | 482.5 (166.5) | 495.6 (173.7) | 509.0 (130.0) | 478.5 (151.5) |
| Visceral fat (CT), mean (SD), cm2 | 171.9 (79.5) | 174.8 (85.1) | 187.5 (86.9) | 178.6 (91.4) | 174.1 (92.4) |
| Subcutaneous fat (CT), mean (SD), cm2 | 257.3 (112.1) | 241.0 (108.4) | 238.6 (108.4) | 261.1 (99.8) | 237.1 (100.5) |
| Waist circumference (CT), mean (SD), cm | 125.8 (14.0) | 125.7 (13.4) | 126.5 (14.2) | 127.8 (11.8) | 125.4 (13.3) |
| Waist circumference, mean (SD), cm | 100.8 (12.1) | 100.8 (11.2) | 101.6 (11.5) | 103.8 (9.2) | 101.5 (9.9) |
Missing values are 65 for BMI, 25 for midlife BMI, 57 for weight, 479 for maximum weight, 1666 for percent body fat and fat, 449 for the CT variables (total body fat, visceral fat, subcutaneous fat, and CT waist circumference), and 53 for waist circumference.
BIA, bioelectrical impedance analysis; SD, standard deviation.
Proportion of cases that progressed from MGUS or LC-MGUS.
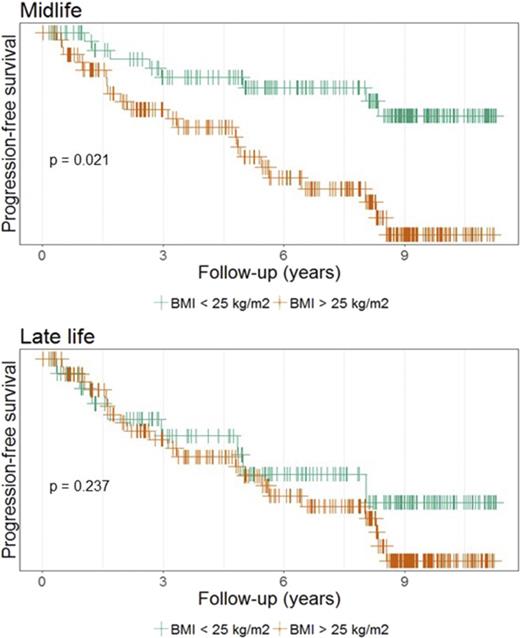
No association was found between the 11 obesity markers and MGUS or LC-MGUS (Table 2). Additionally, BMI history was not associated with MGUS and LC-MGUS when joint effects of midlife BMI and BMI at study entry were examined, either when MGUS and LC-MGUS were analyzed together (Table 3) or separately (data not shown). Finally, based on a small number of cases, a nonsignificant increased risk of progression (hazard ratio [HR], 2.64; 95% CI, 0.93-7.48) from MGUS/LC-MGUS to MM was found with high midlife BMI (>25 kg/m2). When other LP cases were combined with MM cases, the risk was statistically significantly increased (HR, 2.66; 95% CI, 1.17-6.05) (Table 4). There was no difference in distribution of known risk factors for MGUS progression (isotype, M-protein concentration, or FLC ratio) between individuals with low (<25 kg/m2) and high (≥25 kg/m2) BMI (data not shown).
ORs and 95% CIs for association between obesity and MGUS and LC-MGUS
| . | MGUS . | LC-MGUS . | ||||
|---|---|---|---|---|---|---|
| n . | OR . | 95% CI . | n . | OR . | 95% CI . | |
| Baseline BMI | ||||||
| <25 | 101 | Ref. | Ref. | 93 | Ref. | Ref. |
| 25-29.9 | 146 | 1.14 | 0.88-1.49 | 121 | 1.07 | 0.81-1.41 |
| ≥30 | 49 | 0.81 | 0.57-1.16 | 58 | 1.14 | 0.81-1.61 |
| Midlife BMI | ||||||
| <25 | 139 | Ref. | Ref. | 128 | Ref. | Ref. |
| ≥25 | 159 | 1.15 | 0.90-1.45 | 146 | 1.10 | 0.86-1.41 |
| Weight | 296 | 0.97 | 0.91-1.05 | 272 | 1.04 | 0.96-1.12 |
| Max weight | 266 | 1.03 | 0.96-1.11 | 240 | 1.02 | 0.94-1.10 |
| Percent body fat (BIA) | 192 | 0.95 | 0.80-1.13 | 174 | 1.15 | 0.96-1.39 |
| Fat (BIA) | 192 | 0.94 | 0.76-1.16 | 174 | 1.10 | 0.88-1.36 |
| Total body fat (CT) | 269 | 1.00 | 0.99-1.00 | 242 | 1.00 | 1.00-1.01 |
| Visceral fat (CT) | 269 | 1.00 | 0.98-1.01 | 242 | 1.01 | 1.00-1.02 |
| Subcutaneous fat (CT) | 269 | 1.00 | 0.99-1.01 | 242 | 1.00 | 0.99-1.01 |
| Waist circumference (CT) | 269 | 1.00 | 0.94-1.06 | 242 | 1.05 | 0.99-1.13 |
| Waist circumference | 296 | 0.97 | 0.89-1.07 | 272 | 1.02 | 0.93-1.12 |
| . | MGUS . | LC-MGUS . | ||||
|---|---|---|---|---|---|---|
| n . | OR . | 95% CI . | n . | OR . | 95% CI . | |
| Baseline BMI | ||||||
| <25 | 101 | Ref. | Ref. | 93 | Ref. | Ref. |
| 25-29.9 | 146 | 1.14 | 0.88-1.49 | 121 | 1.07 | 0.81-1.41 |
| ≥30 | 49 | 0.81 | 0.57-1.16 | 58 | 1.14 | 0.81-1.61 |
| Midlife BMI | ||||||
| <25 | 139 | Ref. | Ref. | 128 | Ref. | Ref. |
| ≥25 | 159 | 1.15 | 0.90-1.45 | 146 | 1.10 | 0.86-1.41 |
| Weight | 296 | 0.97 | 0.91-1.05 | 272 | 1.04 | 0.96-1.12 |
| Max weight | 266 | 1.03 | 0.96-1.11 | 240 | 1.02 | 0.94-1.10 |
| Percent body fat (BIA) | 192 | 0.95 | 0.80-1.13 | 174 | 1.15 | 0.96-1.39 |
| Fat (BIA) | 192 | 0.94 | 0.76-1.16 | 174 | 1.10 | 0.88-1.36 |
| Total body fat (CT) | 269 | 1.00 | 0.99-1.00 | 242 | 1.00 | 1.00-1.01 |
| Visceral fat (CT) | 269 | 1.00 | 0.98-1.01 | 242 | 1.01 | 1.00-1.02 |
| Subcutaneous fat (CT) | 269 | 1.00 | 0.99-1.01 | 242 | 1.00 | 0.99-1.01 |
| Waist circumference (CT) | 269 | 1.00 | 0.94-1.06 | 242 | 1.05 | 0.99-1.13 |
| Waist circumference | 296 | 0.97 | 0.89-1.07 | 272 | 1.02 | 0.93-1.12 |
Results were obtained with multinomial logistic regression. ORs for continuous variables are per SD increase.
Ref., reference group.
Joint effect of midlife BMI and baseline BMI on MGUS/LC-MGUS
| Joint BMI categories . | MGUS/LC-MGUS, n (%) . | OR . | 95% CI . |
|---|---|---|---|
| Low midlife BMI, low current BMI | 149 (27.8) | Ref. | Ref. |
| Low midlife BMI, medium current BMI | 99 (21.0) | 0.99 | 0.98-1.01 |
| Low midlife BMI, high current BMI | 16 (3.8) | 1.00 | 0.97-1.04 |
| High midlife BMI, low current BMI | 45 (5.9) | 1.03 | 1.00-1.04 |
| High midlife BMI, medium current BMI | 166 (22.6) | 1.02 | 1.00-1.04 |
| High midlife BMI, high current BMI | 91 (19.0) | 0.99 | 0.98-1.01 |
| Joint BMI categories . | MGUS/LC-MGUS, n (%) . | OR . | 95% CI . |
|---|---|---|---|
| Low midlife BMI, low current BMI | 149 (27.8) | Ref. | Ref. |
| Low midlife BMI, medium current BMI | 99 (21.0) | 0.99 | 0.98-1.01 |
| Low midlife BMI, high current BMI | 16 (3.8) | 1.00 | 0.97-1.04 |
| High midlife BMI, low current BMI | 45 (5.9) | 1.03 | 1.00-1.04 |
| High midlife BMI, medium current BMI | 166 (22.6) | 1.02 | 1.00-1.04 |
| High midlife BMI, high current BMI | 91 (19.0) | 0.99 | 0.98-1.01 |
Results were obtained with logistic regression. Low midlife BMI, <25 kg/m2; high midlife BMI, ≥25 kg/m2; low current BMI, <25 kg/m2; medium current BMI, 25-29.9 kg/m2; high current BMI, ≥30 kg/m2.
HRs and 95% CIs for obesity and risk of progression from MGUS to MM and other LP diseases
| . | MM . | MM and other LP diseases . | ||||
|---|---|---|---|---|---|---|
| n . | HR . | 95% CI . | n . | HR . | 95% CI . | |
| Baseline BMI | ||||||
| <25.0 | 5 | Ref. | Ref. | 8 | Ref. | Ref. |
| ≥25.0 | 13 | 1.33 | 0.47-3.75 | 21 | 1.30 | 0.57-2.94 |
| Midlife BMI | ||||||
| <25.0 | 5 | Ref. | Ref. | 8 | Ref. | Ref. |
| ≥25.0 | 13 | 2.64 | 0.93-7.48 | 21 | 2.66 | 1.17-6.05 |
| Weight | 18 | 1.10 | 0.84-1.44 | 29 | 1.04 | 0.84-1.29 |
| Max weight | 18 | 1.16 | 0.88-1.54 | 29 | 1.15 | 0.93-1.43 |
| Percent body fat (BIA) | 10 | 1.54 | 0.35-6.86 | 19 | 0.96 | 0.88-1.05 |
| Fat (BIA) | 10 | 1.92 | 0.81-4.57 | 19 | 0.79 | 0.29-2.13 |
| Total body fat (CT) | 17 | 1.00 | 0.98-1.02 | 28 | 1.00 | 0.98-1.01 |
| Visceral fat (CT) | 17 | 1.00 | 0.96-1.05 | 28 | 0.99 | 0.95-1.03 |
| Subcutaneous fat (CT) | 17 | 1.01 | 0.97-1.04 | 28 | 0.99 | 0.96-1.02 |
| Waist circumference (CT) | 17 | 1.01 | 0.97-1.04 | 28 | 0.96 | 0.79-1.16 |
| Waist circumference | 18 | 1.16 | 0.82-1.64 | 29 | 1.00 | 0.75-1.33 |
| . | MM . | MM and other LP diseases . | ||||
|---|---|---|---|---|---|---|
| n . | HR . | 95% CI . | n . | HR . | 95% CI . | |
| Baseline BMI | ||||||
| <25.0 | 5 | Ref. | Ref. | 8 | Ref. | Ref. |
| ≥25.0 | 13 | 1.33 | 0.47-3.75 | 21 | 1.30 | 0.57-2.94 |
| Midlife BMI | ||||||
| <25.0 | 5 | Ref. | Ref. | 8 | Ref. | Ref. |
| ≥25.0 | 13 | 2.64 | 0.93-7.48 | 21 | 2.66 | 1.17-6.05 |
| Weight | 18 | 1.10 | 0.84-1.44 | 29 | 1.04 | 0.84-1.29 |
| Max weight | 18 | 1.16 | 0.88-1.54 | 29 | 1.15 | 0.93-1.43 |
| Percent body fat (BIA) | 10 | 1.54 | 0.35-6.86 | 19 | 0.96 | 0.88-1.05 |
| Fat (BIA) | 10 | 1.92 | 0.81-4.57 | 19 | 0.79 | 0.29-2.13 |
| Total body fat (CT) | 17 | 1.00 | 0.98-1.02 | 28 | 1.00 | 0.98-1.01 |
| Visceral fat (CT) | 17 | 1.00 | 0.96-1.05 | 28 | 0.99 | 0.95-1.03 |
| Subcutaneous fat (CT) | 17 | 1.01 | 0.97-1.04 | 28 | 0.99 | 0.96-1.02 |
| Waist circumference (CT) | 17 | 1.01 | 0.97-1.04 | 28 | 0.96 | 0.79-1.16 |
| Waist circumference | 18 | 1.16 | 0.82-1.64 | 29 | 1.00 | 0.75-1.33 |
Results were obtained with Cox proportional hazard regression. HRs for continuous variables are per SD increase.
Sensitivity analysis
Given the unusually high prevalence of LC-MGUS in our cohort (4.8%), with a high prevalence of κ cases (96%), we performed an additional sensitivity analysis. As the distribution of log-transformed κ and λ values resembled the normal distribution, we evaluated the effect of using the 97.5th percentile as a cutoff for normal values for the involved light-chain. Using a definition of LC-MGUS as a pathological FLC ratio of <0.26 and >1.65, in combination with an increased concentration of >40.0 mg/L of the light-chain involved, resulted in 52 LC-MGUS cases (0.9%), of which 41 were κ and 11 were λ. Results from the sensitivity analysis confirmed previous findings (data not shown).
Discussion
In this large population-based screening study on 5725 subjects, we found that obesity is not associated with MGUS or LC-MGUS. This was true independent of various obesity assessment techniques, including BIA, CT scans, and other anthropometric measurements such as height, weight, and waist circumference. Additionally, we did not find history of obesity or weight change to have an effect on MGUS/LC-MGUS. Interestingly, we found that high BMI, measured at midlife, was associated with an increased risk of progression to MM and other LP diseases.
We found no association between any of the 11 obesity measures and MGUS or LC-MGUS. Previously, only 2 studies have examined the association between MGUS and obesity, with conflicting results.32,33 Our results are in line with the population-based National Health and Nutritional Examination Survey study (N = 12 482; MGUS cases, 365) that found that BMI did not have an effect on MGUS risk,33 whereas the Southern Community study (N = 1996; MGUS cases, 60) found a twofold increased risk of MGUS in obese (BMI >30 kg/m2) vs nonobese women.32 Differences in the nature of the cohorts such as the cohort size, and age, sex, and race distribution, as well as differences in recruitment of participants, might explain the discrepancy between the studies. It has been suggested that the increased risk of MM with increasing BMI22,23 is explained by an increased risk of MGUS, which in turn puts individuals at risk for developing MM.32 Our findings do not confirm this. LC-MGUS is a newly defined disorder, and factors that influence the development of the condition and its progression are largely unknown. This is the first study to date to examine the association with obesity.
Our results suggest that high BMI during midlife is associated with an increased risk of progression from MGUS/LC-MGUS to MM and other LP diseases later in life. This was not explained by known risk factors for progression. Previous studies have indicated that obesity might have a role in the etiology of MM.21,22,25,26 Recent pooled analysis of 20 prospective studies found that waist circumference is a risk factor for MM mortality and that BMI in early adulthood plays an important role in myelomagenesis.25 Additionally, on the basis of the available data, WHO recently concluded that there now is sufficient evidence behind the association between body fatness and MM.6 To date, only 1 study has examined the role of obesity in the progression of MGUS to MM. A study on a cohort of US veterans within the Veterans Health Administration system found an increased risk of MM to be associated with both overweight (HR, 1.55; 95% CI, 1.16-2.06) and obesity (HR, 1.98; 95% CI, 1.47-2.68) at MGUS diagnosis.42 We thus speculate that the observed risk of MM in individuals with obesity is not because of increased risk of MGUS, but rather that they have similar MGUS prevalence but a higher risk of progression.
Although our results are limited by the small number of cases and should be interpreted with caution, the consistently high risk estimate both when MM was analyzed with and without other LP diseases in our main analysis and sensitivity analysis, and a recent study on the role of obesity in MGUS progression,42 strengthens the notion that obesity plays a role in myelomagenesis and is possibly the first identified modifiable risk factor for progression of MGUS to MM. We did not find body weight or body composition at study baseline in MGUS/LC-MGUS cases to be risk factors for progression to MM and other LP diseases. The induction time for MM and other LP diseases is long, and we do not have information on how long each individual has met the criteria for MGUS/LC-MGUS before developing MM or other LP diseases. A US-based screening study on a clinical cohort concluded that when first clinically recognized, MGUS has likely been present in an undetected state for a median duration of >10 years,43 and the interval from diagnosis of MGUS to diagnosis of MM or related diseases ranges from 1 to 32 years (median, 10.4 years) according to a follow-up study on 241 MGUS patients at Mayo Clinic.44 Based on these results and the high mean age of the population under study (77 years), it is possible that body weight and composition at time of MGUS/LC-MGUS diagnosis is not a reliable indicator for MM progression, as earlier life physique might be.
A major strength of this study is the design as this is a population-based screened cohort study with high internal and external validity. Another major strength is the use of various and precise measures to assess obesity. BMI is a limited measure as it does not give information on fat distribution and does not distinguish between lean and fat mass; however, studies have shown that BMI is highly correlated with other more precise measures of body composition and fat distribution and has been shown to be at least approximately equivalent in the ability to predict diseases.45 We found in our data that the obesity measures were highly correlated (r = 0.37-0.95), but the lack of an acceptable gold standard for measuring body fatness makes it difficult to use only 1 measure. Using multiple markers for late-life body fatness deals with the limitations that pertain to each measure and strengthens our findings. An additional strength is the consistency in the findings throughout the assessment methods.
Some limitations need to be kept in mind when interpreting the results. Although this is a screened study, a selection bias might be present. The mean age is high (77 years), and therefore, the cohort might represent a selection of the population that is healthier than the general population. Additionally, Iceland has an exclusively white population, and in view of previous findings regarding MGUS variance across ethnic groups,18,38 our results may not be applicable to other races. As we do not have bone marrow samples from our participants, we cannot therefore truly distinguish what could be considered as smoldering MM. Missing data highly influence the number of cases in our BIA models, mainly in our progression analysis; these results should therefore be interpreted with caution. A low number of cases that progressed could mean that we had insufficient statistical power, which might affect our results.
In conclusion, in this Icelandic population-based cohort study, obesity is not associated with MGUS or LC-MGUS. However, based on 29 cases that progressed to MM or other LP diseases later in life, our study found midlife obesity to be a risk factor for progression among individuals diagnosed with MGUS/LC-MGUS. This study provides evidence that obesity might be the first modifiable risk factor for MGUS/LC-MGUS progression, but more studies, both large-scale population-based studies and clinical trials, are needed for better understanding of the etiology of MGUS/LC-MGUS and MM.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute on Aging (contract N01-AG012100), the National Institute on Aging Intramural Research Program, a National Eye Institute Intramural Research Program Award (ZIAEY000401), and an award from the National Institute on Deafness and Other Communication Disorders, Division of Scientific Programs (IAA Y2-DC_1004-02); Hjartavernd (the Icelandic Heart Association); Althingi (the Icelandic Parliament); the University of Iceland Research Fund; the Icelandic Centre for Research (RANNIS); the Landspitali University Hospital Research Fund; the Karolinska Instituted Foundations; the Marie Curie CIG; and the Memorial Sloan Kettering Core Grant (P30 CA008748) from the National Cancer Institute, National Institutes of Health.
Authorship
Contribution: S.Y.K., E.K.L., O.L., and M.T. designed the study; R.C. and D.B. performed laboratory analysis; M.T. and S.H.L. performed the statistical analysis; M.T. and S.Y.K. wrote the manuscript; and all authors were involved in the interpretation of the results and the preparation of the final manuscript.
Conflict-of-interest disclosure: S.M. reported serving as a principal investigator for clinical trials with research funding from Juno Therapeutics and Takeda Oncology. The remaining authors declare no competing financial interests.
Correspondence: Sigurdur Y. Kristinsson, Faculty of Medicine, University of Iceland, Reykjavik, Iceland; e-mail: sigyngvi@hi.is.