Key Points
VWF A2-domain intracellular proteolysis within ECs is enhanced upon disrupting calcium binding (eg, in VWD type 2A mutants).
VWF string cleavage on ECs is calcium independent and is strongly dependent on platelet binding.
Abstract
von Willebrand factor (VWF) and the metalloprotease a disintegrin and metalloprotease with thrombospondin type 1 motif 13 (ADAMTS13) are present both within endothelial cells (ECs) and in peripheral blood. Calcium concentrations are lower in intracellular compartments (80-400 μM) compared with the extracellular milieu (∼1.25 mM). Because low calcium favors VWF A2-domain proteolysis by ADAMTS13, the dependence of proteolysis rates on calcium was assayed both within ECs and in blood. Confocal microscopy studies demonstrate partial perinuclear colocalization of VWF with ADAMTS13 in human umbilical vein ECs (HUVECs). Consequently, low levels (5%-10%) of VWF cleavage products were detected in HUVEC lysates and also culture-supernatant following EC stimulation. This proteolysis occurred before disulfide bond formation. Compared with wild-type VWF A2-domain, calcium-binding mutants including the common von Willebrand disease (VWD) type 2A R1597W mutant were expressed in an open conformation in ECs and were highly susceptible to intracellular proteolysis. Fluorescence resonance energy transfer measurements demonstrate strong calcium-dependent VWF-A2 conformation changes at concentrations <500 μM, with unfolding rates being fourfold higher for monomeric VWF A2-domain compared with multimeric, full-length VWF. Under shear, physiological levels of ADAMTS13 did not cleave VWF strings on HUVECs, unless platelets were attached to stretch these strings under flow. Further, VWF-platelet string cleavage under shear proceeded with equal efficiency in the absence and presence of calcium at shear stress ≥1 dyn/cm2. Overall, low calcium levels may promote intracellular VWF proteolysis particularly during VWD type 2A disease. Calcium has a negligible effect on VWF-platelet string proteolysis under physiologically relevant fluid shear.
Introduction
von Willebrand factor (VWF) is a linear biopolymer that participates in blood coagulation and thrombosis.1 It is secreted into blood by endothelial cells (ECs) and megakaryocytes/platelets. The function of VWF is profoundly affected by its size, with larger multimer proteins being more biologically active. Under physiological conditions, VWF size is partly regulated by the constitutively active blood metalloprotease, a disintegrin and metalloprotease with thrombospondin type 1 motif 13 (ADAMTS13).2-4 This protease is secreted into blood by hepatic stellate cells3,5,6 and is also synthesized by ECs7-9 and platelets.10,11 ADAMTS13 cleaves the Tyr1605-Met1606 scissile bond located within the A2-domain of VWF (VWF-A2) that is exposed upon the application of fluid/hydrodynamic shear stress.12
There is considerable interest in understanding the role of cations in regulating VWF-A2 proteolysis. In this regard, VWF-A2 contains a calcium-binding site near the C terminus of its α3β4 loop.13,14 Here, calcium coordinates with main-chain carbonyls of Arg1597 and Ala1600 of VWF-A2, the side-chain carboxyls of Asp1498 and Asp1596, the side-chain carbonyl of Asn1602, and water. Isothermal calorimetry demonstrates an A2-domain-Ca2+ binding dissociation constant (KD) of 3.8 ± 1.0 μM.15 Calcium protects the proteolysis of VWF because VWF-A2 cleavage by ADAMTS13 in the presence of urea is higher in the absence of Ca2+ compared with 2.5 mM Ca2+.13 In optical tweezer studies, calcium marginally increased the force required for VWF-A2 unfolding from 5 to 10 pN in the absence of calcium to ∼14 pN with calcium.14 This divalent ion also promoted refolding, with the unstressed refolding constant increasing four- to fivefold from 0.14/s in the absence of Ca2+ to 0.65/s in the presence of 5 mM Ca2+.15 Although calcium plays an important role in these biophysical, single-molecule force spectroscopy measurements, the relevance of this to VWF size regulation under physiological hydrodynamic shear flow remains unknown.
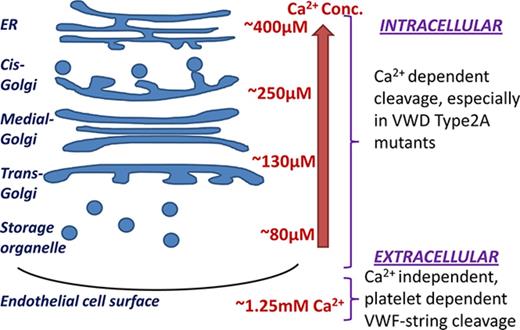
During its synthesis and maturation, VWF is exposed to a range of calcium concentrations, from low levels (100-500 μM) in intracellular compartments to 1.2 to 1.3 mM in the extracellular milieu.16,17 In this regard, intracellular [Ca2+] varies as: endoplasmic reticulum (ER, ∼400 µM) > cis-Golgi apparatus (∼250 µM) > trans-Golgi (∼130 µM) > secretory vesicles (∼80 µM).18 Whether low calcium in endothelial compartments promotes ultra-large VWF proteolysis by ADAMTS13 remains unknown, and this concept was tested here in the context of von Willebrand disease (VWD) type-2A mutants that have calcium-binding deficiency.
Overall, this study examines physiological intra- and extracellular conditions in which calcium may regulate VWF-A2 proteolysis. It reveals prominent VWF-A2 domain conformation changes under conditions resembling the intracellular milieu (Ca2+ <500 μM). Minor cleavage of normal VWF by ADAMTS13 is noted in human umbilical vein ECs (HUVECs), with cleavage being enhanced in calcium-binding mutants including a common VWD type 2A variant, R1597W. Platelets were noted to be major regulators of VWF-string cleavage on ECs, with changes in extracellular calcium having a negligible role at fluid shear stress >1 dyn/cm2. Overall, calcium may influence VWF proteolysis within ECs and regulate VWF multimer distribution in selected VWD type 2A disease mutants.
Methods
Basic methods
HEK293Ts were maintained in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum. HUVECs from Lonza (Allendale, NJ) were maintained in EGM-2 media. Immunocytochemistry followed standard protocols (supplemental Methods). All chemicals were from Sigma unless otherwise noted.
Recombinant proteins
All VWF constructs are described in the supplemental Methods and Results. These were expressed in mammalian HEK293T cells and HUVECs. The backbone vectors for multimeric VWF expression pCDNAΔ3-VWF19 and fluorescence resonance energy transfer (FRET)20 were described previously. All recombinant constructs contain the VWF signal peptide. HEK cell expression used the calcium-phosphate method.21 HUVECs used either the Targefect-HUVEC transfection reagent (Targeting Systems, Santee, CA) or lentivirus.21 ADAMTS13 was produced using a vector kindly provided by Kenji Soejima (Kaketsuken, Japan22 ). ADAMTS13 unit activity was calculated based on the World Health Organization 1st International Standard.23
Western blots
Cell lysates were prepared in the presence or absence of 10 mM EDTA following 3 protocols: (1) Heating cells to 95°C in Laemmli sample buffer containing 5% β-mercaptoethanol; (2) detergent lysis by incubation with 100 mM Trizma-Maleate, 2% Triton X-100 pH 7.2 buffer on ice for 1 hour; or (3) freeze-thaw using 3 rapid cycles in 10 mM Tris, 150 mM NaCl, pH 7.4 buffer. Clear lysate was obtained in all cases by subjecting samples to shear (10 passages through a 25-g syringe needle) and centrifugation at 14 000g. In other runs, culture supernatant was collected from HUVEC monolayers after stimulation for 2 hours at 37°C with 25 µM histamine, 50 ng/mL tumor necrosis factor-α (TNF-α; R&D Systems), 100 ng/mL interleukin-8 (IL-8; R&D) or 10 µM calcium ionophore A23187, either in the presence or absence of 10 mM EDTA. In instances where VWF was immunoprecipitated, 10 to 50 µg of HUVEC lysate or supernatant was incubated with 1 µg of either anti-human VWF-A2 monoclonal antibody (mAb) 210909 (R&D) or anti-D′D3 mAb DD3.124 overnight at 4°C before adding 20 µL protein-A/G agarose beads. Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis was performed under standard reducing conditions, using either a 4% to 20% gradient gel or 4% stacking-6% resolving gel. Following transfer, the nitrocellulose membrane was probed with 0.02 to 0.2µg/mL: (1) rabbit anti-human VWF polyclonal antibody (polyclonal antibody [pAb]; Dako), (2) THE mouse anti-c-Myc tag mAb (Genscript), or (3) anti-tetra His mAb (Qiagen). Membranes were then incubated with appropriate horseradish peroxidase –coupled secondary Ab and developed using Clarity ECL substrate (Bio-Rad).
FRET
HUVECs were transduced to express a panel of FRET probes. HUVECs were imaged using a Zeiss LSM 710 confocal microscope with a 40×/1.4 Plan-Apochromat DIC objective and QUASAR PMT detectors. Data were acquired using: (1) 405-nm laser excitation and emission at either 435 to 510 nm (Cerulean signal) or 554 to 735 nm (FRET signal); and (2) 520-nm excitation with 554- to 735-nm emission (Venus signal). Following data acquisition, Venus signal in a defined region of interest was photobleached using the Zeiss Intune laser at 520 nm. The %FRET then quantified the change/increase in Cerulean fluorescence along the length of individual cells according to: %FRET = 100×(postbleaching – prebleaching)/prebleaching.25
A Fortessa flow cytometer (BD Biosciences) was used to measure FRET signal in HUVECs and transfected HEK293Ts. Here, the 405 violet laser measured Cerulean and FRET signal using 450/50 and 525/50 emission filters, respectively. Venus signal was measured using 488-nm excitation and emission at 515/20 nm. FRET ratio quantified emission intensity in the Cerulean/FRET channels for individual cells. High FRET ratio corresponds to an open configuration with low donor-acceptor energy transfer.
In some cases, FRET was measured in suspension using recombinant VWF-A2 FRET and multimeric VWF (mVWF) FRET proteins resuspended in 50 mM Tris (pH 7.5), following urea concentration perturbations as described in “Results.” The BioTek Synergy-4 reader (Winooski, VT) used for these measurements used standard 96-well black plates and the following fluorescence filter pairs for Venus (excitation:emission::485/20:528/20 nm), Cerulean (420/50:485/20 nm), and FRET (420/50:528/20 nm). In some instances, full emission spectrum was also measured using a Hitachi fluorometer (model F-2500, Japan). FRET ratio measured the emission intensity ratio in the Cerulean/FRET channels, similar to the cytometer studies. VWF-A2 FRET and mVWF FRET folding and unfolding rate constants were quantified by fitting FRET time-course data to a 2-step model (supplemental Material).
String formation studies
All human subject protocols were approved by the State University of New York-Buffalo institutional review board in accordance with the Declaration of Helsinki. Platelet rich-plasma (PRP) was obtained by centrifugation of normal human blood drawn from healthy adult volunteers in 1:9 sodium citrate containing 2 µM prostaglandin-E1.26 To label cells, 0.4 nM 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (Invitrogen) was added to a portion of the PRP for 10 minutes at room temperature. To obtain washed platelets, both labeled and unlabeled PRP were pelleted and resuspended at 109 platelets/mL in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) buffer (30 mM HEPES, 110 mM NaCl, 10 mM KCl, 2 mM MgCl2, 10 mM glucose, 0.35% human serum albumin, pH 7.3).
Confluent HUVEC monolayers in 60-mm dishes were stimulated with 100 µM histamine for 5 minutes. These were mounted onto a custom vacuum-sealed microfluidic flow cell (100 μm height × 400 μm width × 1 cm length) and placed on the stage of a motorized Zeiss AxioObserver Z1 microscope with a 40× objective.21 A variety of experimental protocols were then performed (see “Results”). In each case, images were captured at 2- to 60-second intervals in either bright-field green fluorescence or alternating bright-field plus green fluorescence channels using an sCMOS camera (PCO.edge, Germany). VWF string length quantifies the distance between the first and last platelet attached to a single protein bundle stretched in the direction of flow.
Statistics
Data are presented as mean ± standard deviation for N ≥ 3. Student two-tailed t test was used for dual comparisons. Analysis of variance followed by the Tukey test was used for multiple comparisons. P < .05 was considered to be statistically significant.
Results
Intracellular cleavage of VWF in HUVECs by ADAMTS13
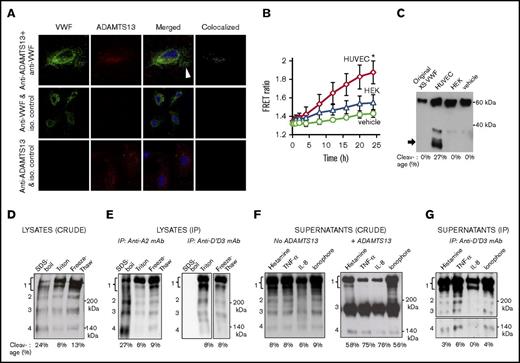
ADAMTS13 expression and activity in HUVECs was quantified (Figure 1). Using confocal microscopy, a partial overlap between ADAMTS13 (red) and VWF (green) was observed in the perinuclear region of HUVECs (Figure 1A, top). The calculated Pearson and Mander coefficients for 6 independent fields of view were 0.54 ± 0.12 and 0.64 ± 0.05, respectively. No signal overlap was observed in the peripheral region in the Weibel-Palade bodies (white arrow, top). Thus, contact between ADAMTS13 and VWF occurs during protein maturation. Signal colocalization was absent when isotype control antibodies were applied in place of either anti-ADAMTS13 (second row) or anti-VWF (bottom row) Abs. A panel of additional images showing the perinuclear colocalization of VWF with ADAMTS13 is presented in supplemental Figure 1. ADAMTS13 activity in HUVECs was measured by incubating the XS-VWF FRET20 substrate with cell lysate. Here, a FRET ratio increase indicating substrate proteolysis was observed for HUVEC lysates only, and not in controls with vehicle or HEK293T lysate (Figure 1B). Western blot results are consistent with fluorescence measurements, and they confirm ADAMTS13 activity in HUVECs (Figure 1C).
ADAMTS13 activity in ECs. (A) Confocal image of HUVECs stained with anti-VWF pAb (green, column 1), anti-ADAMTS13 mAb (red, column 2), merged image with 4′,6-diamidino-2-phenylindole (column 3) and green + red colocalization signal (column 4). Top row shows HUVECs stained with both Abs. Second and third rows represent controls with either isotype control anti-l-selectin mAb DREG56 (in place of anti-ADAMTS13, middle) or anti-myosin smooth muscle pAb (in place of anti-VWF, bottom). (B) 2 mg/mL HUVEC or HEK293T cell lysates prepared using detergent lysis was added to 1 μM XS-VWF FRET substrate. Final Triton-X concentration (vehicle control) was 0.2% in all samples. FRET ratio (indicating XS-VWF FRET cleavage) was measured over time. *P < .05 with respect to all other treatments. (C) Western blot showing XS-VWF FRET cleavage at 24 hours in the presence of HUVEC lysate, but not HEK293T lysate or vehicle control. (D) Western blot of crude HUVEC lysates prepared using 3 methods in the presence of 10 mM EDTA: heat denaturation/SDS-boil, detergent lysis/triton, and freeze-thaw. Four bands are noted: band 1 mass corresponds to mature VWF and its glycoforms (230-250 kDa), band 2 (∼200 kDa) origin is unknown, and bands 3 and 4 masses correspond to C-terminal (176 kDa) and N-terminal (140 kDa) cleavage fragments. No bands were present below 140 kDa (data not shown). (E) VWF immunoprecipitated from HUVEC lysates, prepared using different methods, using either anti VWF-A2 mAb 210909 (left gel) or anti-D′D3 mAb DD3.1 (right gel). The same 4 bands are observed. (F) Western blot of HUVEC supernatants collected 2 hours after stimulation with 25 µM histamine, 50 ng/mL TNF-α, 100 ng/mL IL-8, or 10 µM calcium ionophore (left gel) show all 4 bands. Overnight addition of 2 U/mL ADAMTS13 and 1.6M urea in right gel, results in amplification of 176 kDa band. (G) VWF from samples in panel C (left gel, no ADAMTS13) was immunoprecipitated using anti-D′D3 mAb DD3.1 before western blotting. VWF cleavage bands at 176 and 140 kDa are present in all panels. The bottom portion of the panel G gel, containing the 140-kDa fragment, was developed for a longer time. In all cases, % cleavage = cleaved VWF/total VWF = 100× intensity of 176 KDa band/(sum of intensity of 176 KDa + 250 KDa bands). SDS-polyacrylamide gel electrophoresis was performed under reducing conditions and all blots were probed with anti-VWF pAb (Dako). Functionally active ADAMTS13 activity and perinuclear colocalization of VWF and ADAMTS13 was observed in HUVECs.
ADAMTS13 activity in ECs. (A) Confocal image of HUVECs stained with anti-VWF pAb (green, column 1), anti-ADAMTS13 mAb (red, column 2), merged image with 4′,6-diamidino-2-phenylindole (column 3) and green + red colocalization signal (column 4). Top row shows HUVECs stained with both Abs. Second and third rows represent controls with either isotype control anti-l-selectin mAb DREG56 (in place of anti-ADAMTS13, middle) or anti-myosin smooth muscle pAb (in place of anti-VWF, bottom). (B) 2 mg/mL HUVEC or HEK293T cell lysates prepared using detergent lysis was added to 1 μM XS-VWF FRET substrate. Final Triton-X concentration (vehicle control) was 0.2% in all samples. FRET ratio (indicating XS-VWF FRET cleavage) was measured over time. *P < .05 with respect to all other treatments. (C) Western blot showing XS-VWF FRET cleavage at 24 hours in the presence of HUVEC lysate, but not HEK293T lysate or vehicle control. (D) Western blot of crude HUVEC lysates prepared using 3 methods in the presence of 10 mM EDTA: heat denaturation/SDS-boil, detergent lysis/triton, and freeze-thaw. Four bands are noted: band 1 mass corresponds to mature VWF and its glycoforms (230-250 kDa), band 2 (∼200 kDa) origin is unknown, and bands 3 and 4 masses correspond to C-terminal (176 kDa) and N-terminal (140 kDa) cleavage fragments. No bands were present below 140 kDa (data not shown). (E) VWF immunoprecipitated from HUVEC lysates, prepared using different methods, using either anti VWF-A2 mAb 210909 (left gel) or anti-D′D3 mAb DD3.1 (right gel). The same 4 bands are observed. (F) Western blot of HUVEC supernatants collected 2 hours after stimulation with 25 µM histamine, 50 ng/mL TNF-α, 100 ng/mL IL-8, or 10 µM calcium ionophore (left gel) show all 4 bands. Overnight addition of 2 U/mL ADAMTS13 and 1.6M urea in right gel, results in amplification of 176 kDa band. (G) VWF from samples in panel C (left gel, no ADAMTS13) was immunoprecipitated using anti-D′D3 mAb DD3.1 before western blotting. VWF cleavage bands at 176 and 140 kDa are present in all panels. The bottom portion of the panel G gel, containing the 140-kDa fragment, was developed for a longer time. In all cases, % cleavage = cleaved VWF/total VWF = 100× intensity of 176 KDa band/(sum of intensity of 176 KDa + 250 KDa bands). SDS-polyacrylamide gel electrophoresis was performed under reducing conditions and all blots were probed with anti-VWF pAb (Dako). Functionally active ADAMTS13 activity and perinuclear colocalization of VWF and ADAMTS13 was observed in HUVECs.
To determine if ADAMTS13 in HUVECs can intracellularly cleave native VWF, cell lysates were analyzed by western blotting. Here, the intact VWF glycoforms appear at 230 to 250 kDa (band 1, Figure 1D). Faint bands with molecular mass corresponding to the 176-kDa C-terminal (band 3) and 140 kDa N-terminal (band 4) ADAMTS13 cleavage products were also detected in HUVEC-lysates prepared using 3 different cell-lysis protocols in the presence of 10 mM EDTA. To verify the observation, VWF was immunoprecipitated from HUVEC lysates using 2 different mAbs against the VWF-A2 and D′D3 domains before western blotting (Figure 1E). Here, also, the same pattern of bands was observed. Because the anti-D′D3 mAb binds native but not denatured VWF,24 it did not immunoprecipitate VWF from SDS-boil lysates (Figure 1E, first lane of right gel).
In addition to cell lysates, cleaved VWF products, including the 176- and 140-kDa bands, were also observed in HUVEC culture supernatants collected following histamine, TNF-α, IL-8, and calcium ionophore stimulation27 (Figure 1F, left). As expected, the intensity of the 176-kDa band increased upon overnight incubation with recombinant ADAMTS13 and urea (Figure 1F, right). Immunoprecipitation of VWF from the supernatants using the anti-D′D3 mAb also resulted in the 176- and 140-kDa bands (Figure 1G). Thus, VWF may be partially proteolyzed by ADAMTS13 in cells under physiological conditions. Based on densitometry analysis of the 176-kDa cleavage band relative to the 250-kDa band under different conditions, ∼5% to 10% VWF proteolysis may occur before secretion.
VWF cleavage occurs before disulfide bonding in HUVECs
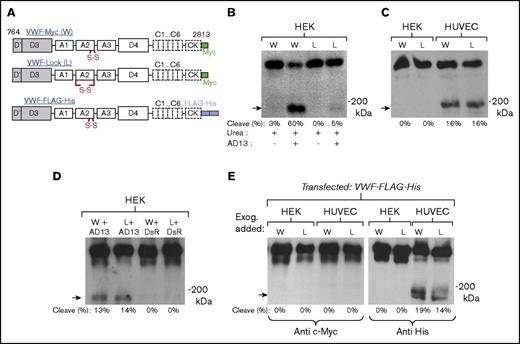
Three multimeric VWF constructs were developed to follow VWF proteolysis in HUVECs (Figure 2). This includes 2 wild-type VWF constructs with c-Myc (VWF-Myc) or His-tag (VWF-FLAG-His), both containing vicinal cysteines, and the VWF-Lock protein where the N- and C terminus of VWF-A2 are disulfide bridged/locked using N1493C/C1669G mutations (Figure 2A). Both wild-type and VWF-Lock were produced with comparable multimer distributions in HEK cells (supplemental Figure 2). Similar to the results of Baldauf et al,28 wild-type VWF but not VWF-Lock was readily cleaved by ADAMTS13 in the presence of urea (Figure 2B). Unlike HEK293T cells, however, both wild-type VWF and VWF-Lock were proteolytically digested to an equal extent when expressed in HUVECs (Figure 2C; supplemental Table 1). Thus, VWF proteolysis may occur before A2-domain cysteine disulfide bond formation which is catalyzed by ER-resident protein disulfide isomerases.29
Intracellular cleavage of recombinant VWF expressed in HUVECs. (A) Schematic of Myc-tagged proteins VWF-Myc/wild-type (W), VWF-Lock (L), and VWF-FLAG-His. (B) 2 µg/mL recombinant VWF-Myc and VWF-Lock from HEK293Ts was incubated with 1.6 M urea with or without 4 U/mL recombinant ADAMTS13 (AD13) for 4 hours. Anti c-Myc mAb detected the ∼176 kDa C-terminal fragment in the case of VWF-Myc but not VWF-Lock (arrow). (C) HUVECs and HEK293Ts were transfected with VWF-Myc and VWF-Lock for 72 hours. Culture supernatant was concentrated 20-fold before western blotting with anti-c-Myc mAb. VWF-Myc and VWF-Lock were equally cleaved in HUVECs only. (D) VWF-Myc or VWF-Lock expressed in HEK293Ts either upon cotransfection with ADAMTS13 (lanes 1, 2) or control plasmid pLKO.1-PGK-DsRed (DsR, lanes 3, 4). Equal cleavage of VWF-Myc and VWF-Lock is observed upon ADAMTS13 cotransfection. (E) Both HUVECs and HEK293Ts were transiently transfected with VWF-FLAG-His. Transfection media were replaced the next day, and recombinant c-Myc tagged VWF-Myc or VWF-Lock protein produced in HEK293Ts was added to culture media to monitor extracellular cleavage in culture medium. Western blot of culture supernatant collected at 48 hours shows that only VWF-His produced in HUVECs was cleaved. Exogenously added c-Myc tagged proteins in culture medium remained intact.
Intracellular cleavage of recombinant VWF expressed in HUVECs. (A) Schematic of Myc-tagged proteins VWF-Myc/wild-type (W), VWF-Lock (L), and VWF-FLAG-His. (B) 2 µg/mL recombinant VWF-Myc and VWF-Lock from HEK293Ts was incubated with 1.6 M urea with or without 4 U/mL recombinant ADAMTS13 (AD13) for 4 hours. Anti c-Myc mAb detected the ∼176 kDa C-terminal fragment in the case of VWF-Myc but not VWF-Lock (arrow). (C) HUVECs and HEK293Ts were transfected with VWF-Myc and VWF-Lock for 72 hours. Culture supernatant was concentrated 20-fold before western blotting with anti-c-Myc mAb. VWF-Myc and VWF-Lock were equally cleaved in HUVECs only. (D) VWF-Myc or VWF-Lock expressed in HEK293Ts either upon cotransfection with ADAMTS13 (lanes 1, 2) or control plasmid pLKO.1-PGK-DsRed (DsR, lanes 3, 4). Equal cleavage of VWF-Myc and VWF-Lock is observed upon ADAMTS13 cotransfection. (E) Both HUVECs and HEK293Ts were transiently transfected with VWF-FLAG-His. Transfection media were replaced the next day, and recombinant c-Myc tagged VWF-Myc or VWF-Lock protein produced in HEK293Ts was added to culture media to monitor extracellular cleavage in culture medium. Western blot of culture supernatant collected at 48 hours shows that only VWF-His produced in HUVECs was cleaved. Exogenously added c-Myc tagged proteins in culture medium remained intact.
Consistent with the notion that VWF proteolysis may occur intracellularly, the expression of either VWF-Myc or VWF-Lock in HEK293Ts led to equal cleavage upon cotransfection with ADAMTS13 encoding plasmid, but not the control DsRed vector (Figure 2D). In addition to c-Myc proteins, similar observations were also made using His-tagged VWF-FLAG-His (Figure 3E). Here, HEK293Ts and HUVECs transfected with VWF-FLAG-His were cultured for 48 hours in media supplemented with either exogenous recombinant uncleaved VWF-Myc or VWF-Lock produced using HEK293Ts. Here, only VWF-FLAG-His produced in HUVECs, but not the proteins added to culture supernatant, was digested. Because the VWF-Myc proteins in culture remained intact (left gel), the data confirm that VWF-FLAG-His proteolysis occurs within cells. ADAMTS13 activity in culture supernatant is insufficient to cleave the secreted proteins. Overall, the equal cleavage of VWF-Myc and VWF-Lock in HUVECs, under a variety of conditions, suggests that ADAMTS13-mediated proteolysis may occur in the early ER before disulfide bonding.
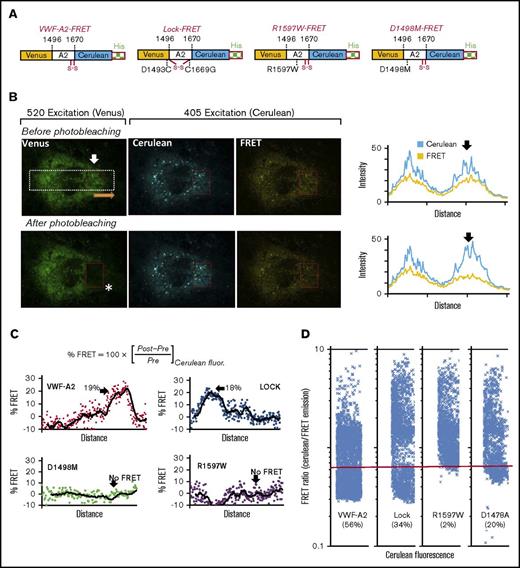
Calcium-binding deficiency causes VWF-A2 to be unfolded within HUVECs. (A) Schematic of 4 VWF-A2-FRET proteins, with Venus/YFP and Cerulean/CFP flanking the A2-domain: wild-type VWF-A2, A2-Lock (D1493C, C1669G), R1597W, and D1498M. (B) Wild-type VWF-A2-FRET expressed in HUVECs. Venus signal was measured in first column (excitation = 520 nm; emission = 554-735 nm). Cerulean was excited at 405 nm, and its emission was measured at wavelengths that either do not overlap (em = 437-510 nm, second/Cerulean channel) or that do overlap with Venus (em = 554-735 nm, third/FRET channel). A white rectangular region was marked and fluorescence along its length was measured, in the direction of the orange arrow both for the Cerulean and FRET channels (data in fourth column). Following data capture, Venus was photobleached using a 520-nm laser in the region indicated by the red box (white asterisk). This photobleaching increased Cerulean and depressed FRET signal as shown in the rightmost panel. (C) %FRET was calculated along the length of the white region of interest based on the change in Cerulean signal pre- and postbleaching. This was calculated for all 4 constructs expressed in HUVECs. In each case, arrow indicated the region where photobleaching was performed. A total of 18% to 19% FRET was measured for wild-type and Lock proteins. Calcium-binding mutants (D1498M, R1597W) did not exhibit FRET, suggesting that the proteins are unfolded. (D) HUVECs expressing each of the 4 constructs were analyzed using flow cytometry. FRET ratio (= emitted light in Cerulean/FRET channels) was measured. As seen, the FRET ratio was lower for wild-type VWF-A2 and Lock, compared with the 2 calcium-binding mutants. Thus, R1597W and D1478A remain unfolded in HUVECs.
Calcium-binding deficiency causes VWF-A2 to be unfolded within HUVECs. (A) Schematic of 4 VWF-A2-FRET proteins, with Venus/YFP and Cerulean/CFP flanking the A2-domain: wild-type VWF-A2, A2-Lock (D1493C, C1669G), R1597W, and D1498M. (B) Wild-type VWF-A2-FRET expressed in HUVECs. Venus signal was measured in first column (excitation = 520 nm; emission = 554-735 nm). Cerulean was excited at 405 nm, and its emission was measured at wavelengths that either do not overlap (em = 437-510 nm, second/Cerulean channel) or that do overlap with Venus (em = 554-735 nm, third/FRET channel). A white rectangular region was marked and fluorescence along its length was measured, in the direction of the orange arrow both for the Cerulean and FRET channels (data in fourth column). Following data capture, Venus was photobleached using a 520-nm laser in the region indicated by the red box (white asterisk). This photobleaching increased Cerulean and depressed FRET signal as shown in the rightmost panel. (C) %FRET was calculated along the length of the white region of interest based on the change in Cerulean signal pre- and postbleaching. This was calculated for all 4 constructs expressed in HUVECs. In each case, arrow indicated the region where photobleaching was performed. A total of 18% to 19% FRET was measured for wild-type and Lock proteins. Calcium-binding mutants (D1498M, R1597W) did not exhibit FRET, suggesting that the proteins are unfolded. (D) HUVECs expressing each of the 4 constructs were analyzed using flow cytometry. FRET ratio (= emitted light in Cerulean/FRET channels) was measured. As seen, the FRET ratio was lower for wild-type VWF-A2 and Lock, compared with the 2 calcium-binding mutants. Thus, R1597W and D1478A remain unfolded in HUVECs.
Prominent conformation change and proteolysis of VWD type2A mutant
Free calcium levels in the ER/Golgi are low (80-500 μM) compared with the extracellular milieu (∼1.25 mM). Thus, intracellular proteolysis may be prominent for VWF constructs that have impaired calcium binding, for example the common VWD type-2A R1597W mutant.30,31 To test this hypothesis, a series of FRET constructs were expressed in which monomeric YFP (Venus) and CFP (Cerulean) flank the full VWF-A2 domain (Figure 3). Besides wild-type (VWF-A2 FRET) and Lock (Lock-FRET) proteins, 2 calcium-binding mutants R1597W-FRET and D1498M-FRET were also developed (Figure 3A). These molecules were expressed in HUVECs using lentivirus. Confocal microscopy measured VWF-A2 conformation in the ECs (Figure 3B). The measurements include endogenous Venus and Cerulean fluorescence, and also emission in the FRET channel where Cerulean emission overlaps with Venus absorbance wavelengths. Following initial image acquisition, Venus was photobleached in a portion of the cell. The %FRET quantified the increase in Cerulean signal in this region following photobleaching, treating the nonbleached region as control (Figure 3C; supplemental Figure 3). Here, %FRET was high (15%-20%) for wild-type VWF-A2 FRET and Lock-FRET proteins suggesting that these molecules are well-folded or in a closed state in HUVECs. All cells with calcium-binding mutations (D1498M-FRET and R1597W-FRET) exhibited low %FRET. Thus, these molecules are in an unfolded or open state.
Microscopy observations were consistent with flow cytometer measurements of FRET ratio (Figure 3D; supplemental Figure 4). Here, a majority (56%) of the cells expressing VWF-A2 FRET had FRET ratio < 0.66 confirming the well-folded nature of this protein. The calcium-binding mutants either did not exhibit significant FRET (R1597W) or exhibited lower FRET values (D1478A) compared with either the wild-type or Lock constructs. FRET ratio was heterogeneous in the cell population, reflecting biological variability possibly from the HUVEC growth status. Similar observations were made with HEK293Ts transfected to express the 4 constructs (supplemental Figure 5). Thus, disruption of calcium binding in addition to proteolysis is sufficient to alter A2-domain conformation.
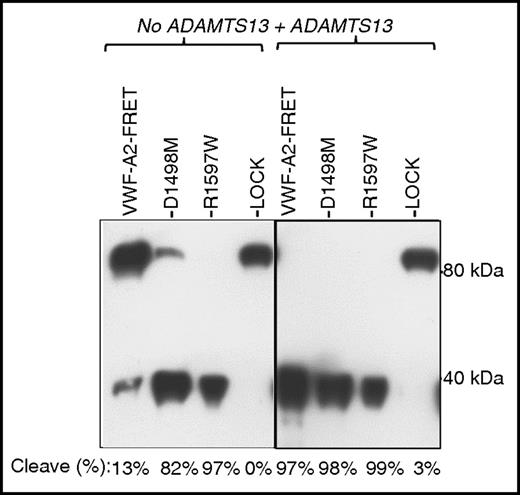
The analysis of FRET proteins secreted from HUVECs by western blotting revealed that a small portion of VWF-A2 FRET was cleaved inside cells (Figure 4). Proteolysis was much more prominent for the calcium mutants, with D1478A and R1597W being almost completely cleaved. Thus, calcium mutants in HUVECs may exist in an open conformation that makes them more susceptible to intracellular proteolysis in the relatively low calcium milieu of the ER.
Intracellular cleavage of VWF calcium mutants in HUVECs. VWF-A2 FRET proteins were collected and concentrated from HUVEC culture supernatant. These proteins were either incubated in buffer with 1.6M urea alone or buffer with urea and 8 U/mL ADAMTS13 at 37°C for 4 hours. Monoclonal anti-VWF-A2-C-domain antibody (MAB2764, R&D Systems) was used for probing the western blot. Results show that R1597W is completely cleaved and D1498M is mostly cleaved, even in the absence of exogenous ADAMTS13 addition.
Intracellular cleavage of VWF calcium mutants in HUVECs. VWF-A2 FRET proteins were collected and concentrated from HUVEC culture supernatant. These proteins were either incubated in buffer with 1.6M urea alone or buffer with urea and 8 U/mL ADAMTS13 at 37°C for 4 hours. Monoclonal anti-VWF-A2-C-domain antibody (MAB2764, R&D Systems) was used for probing the western blot. Results show that R1597W is completely cleaved and D1498M is mostly cleaved, even in the absence of exogenous ADAMTS13 addition.
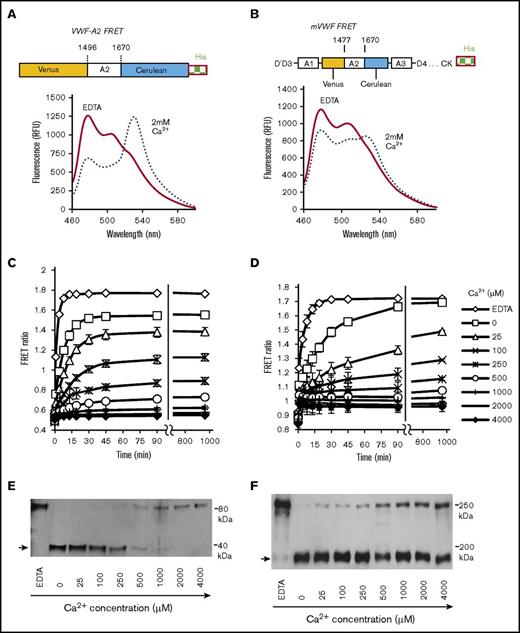
VWF-A2 undergoes calcium-dependent conformation change under conditions resembling ER/Golgi
To characterize calcium-dependent VWF-A2 conformation regulation in detail, in addition to single-domain VWF FRET, a multimeric VWF FRET protein with Venus and Cerulean flanking VWF-A2 (mVWF FRET, Figure 5B) was also produced in HEK293Ts. In fluorimeter assays, the FRET ratio of both VWF FRET and mVWF FRET increased upon calcium depletion from ∼0.5 to 0.8 in the presence of 2 mM calcium to ∼1.75 in the presence of 10 mM EDTA (Figure 5A-D). This was due to the coordinated increase in Cerulean-channel and reduction in FRET-channel emission upon calcium depletion. Urea was used here because calcium perturbation alone is insufficient for protein conformation change in solution (supplemental Figure 6). Further, in controls, neither urea nor calcium alone affects the absolute fluorescence of Venus or Cerulean (supplemental Figure 7).
VWF-A2 FRET calcium dependence. (A-B) Schematic of VWF-A2 FRET and mVWF FRET proteins. Emission spectra of these FRET proteins were collected in the milieu of either 10 mM EDTA (0 mM CaCl2) or 2 mM calcium, both in the presence of 1.6M urea. VWF-A2 FRET (C) and mVWF FRET (D) were incubated with different calcium concentrations. 1.6M urea was then added to trigger time-dependent protein unfolding. Changes in FRET ratio were measured. (E-F) 0.025 U/mL ADAMTS13 was added to samples at the end of the runs in panels C and D, respectively. The proteolysis reaction proceeded for 45 minutes (E) and 16 hours (F). Reaction products were analyzed using anti-His mAb to detect the cleavage band (arrow). Proteolysis is reduced at high calcium. Thus, VWF-A2 is predominantly unfolded/open at low calcium and folded/closed at high calcium. Unfolding and proteolysis of single domain VWF-A2 proceeds more efficiently compared with the full multimeric mVWF protein.
VWF-A2 FRET calcium dependence. (A-B) Schematic of VWF-A2 FRET and mVWF FRET proteins. Emission spectra of these FRET proteins were collected in the milieu of either 10 mM EDTA (0 mM CaCl2) or 2 mM calcium, both in the presence of 1.6M urea. VWF-A2 FRET (C) and mVWF FRET (D) were incubated with different calcium concentrations. 1.6M urea was then added to trigger time-dependent protein unfolding. Changes in FRET ratio were measured. (E-F) 0.025 U/mL ADAMTS13 was added to samples at the end of the runs in panels C and D, respectively. The proteolysis reaction proceeded for 45 minutes (E) and 16 hours (F). Reaction products were analyzed using anti-His mAb to detect the cleavage band (arrow). Proteolysis is reduced at high calcium. Thus, VWF-A2 is predominantly unfolded/open at low calcium and folded/closed at high calcium. Unfolding and proteolysis of single domain VWF-A2 proceeds more efficiently compared with the full multimeric mVWF protein.
Prominent calcium dependent VWF-A2 unfolding was apparent for both VWF-A2 FRET and mVWF FRET in the range from 0 to 500 μM Ca2+, when 1.6M urea was added to abruptly unfold proteins (Figure 5C-D). Similar calcium-dependent refolding was also observed when the 2 proteins were first unfolded using 4M urea initially before refolding was initiated by abruptly reduced urea from 4M to 1.6M (supplemental Figure 8). Whereas the final A2-domain equilibrium conformation was similar for VWF-A2 FRET and mVWF FRET, the unfolding and refolding kinetics of VWF-A2 FRET proceeded more rapidly compared with mVWF FRET (Figure 5; supplemental Figure 8). In this regard, at 25 μM calcium, 50% unfolding of VWF-A2 FRET took 5 minutes (Figure 5B) compared with 45 minutes for mVWF FRET (Figure 5C). Comparable levels of A2-domain cleavage also required only 45 minutes for VWF-A2 FRET, whereas this extended to 16 hours for mVWF FRET (Figure 5E-F; supplemental Figure 8C-D).
A 2-step model was used to fit the time-course data to quantify VWF-A2 folding-unfolding dynamics (supplemental Methods; supplemental Figure 9). This analysis estimates VWF-A2 FRET and mVWF FRET unfolding rate constants of 0.027/s and 0.0068/s. Thus, the single domain refolds fourfold faster compared with the multimeric protein. Further, the ratio of the A2-domain in closed vs open conformations (ie, KD2) increased from 0.033 for VWF-A2 FRET to 0.095 for mVWF FRET, suggesting that approximately threefold higher amounts of mVWF FRET exist in a closed conformation compared with VWF-A2 FRET at equilibrium. Overall, calcium in the range observed in the ER/Golgi can trigger A2-conformation change in the presence of additional agonists. VWF structural features outside the A2-domain may also contribute to multimeric VWF conformation stability and proteolysis.
Calcium does not regulate VWF-string cleavage
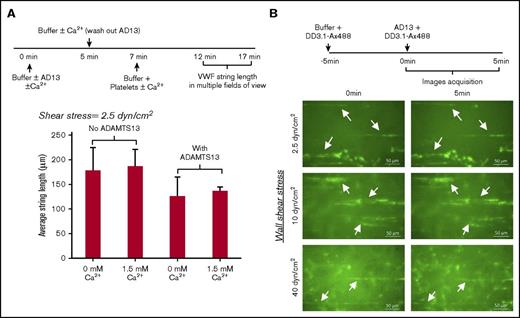
The proteolytic cleavage of VWF strings assembled on the endothelium, in part, regulates VWF multimer distribution in circulation.32,33 To determine the effect of calcium on this process, 1U/mL ADAMTS13 was perfused over histamine stimulated ECs for 5 minutes without platelets, in buffer containing either 1.5 mM CaCl2 or no calcium (Figure 6A). Subsequently, after a brief wash to remove unbound ADAMTS13, platelets were perfused to measure VWF string length. After 5 minutes, the average string length was similar both in runs with and without exogenous calcium. Thus, the added calcium did not affect VWF string formation, and ADAMTS13 in the perfusion buffer only reduced string length by ∼25%.
Low cleavage of VWF-string by ADAMTS13 in the absence of platelets at all calcium concentrations. (A) HEPES buffer sometimes containing 1 U/mL ADAMTS13, with or without 1.5 mM calcium, was perfused over stimulated HUVECs at 2.5 dyn/cm2 for 5 minutes. Following a 2-minute wash, 108 platelets/mL were introduced under identical buffer and shear conditions. VWF-string length was measured in multiple fields of view 5 minutes after platelet perfusion. VWF-string length decreased by 30% upon inclusion of ADAMTS13, although the difference was not statistically significant. (B) Alexa-488–labeled mAb DD3.1 (DD3.1-Ax488) was perfused over stimulated HUVECs for 5 minutes at indicated wall shear stress (2.5, 10, 40 dyn/cm2) before introduction of 1 U/mL ADAMTS13 for additional 5 minutes. VWF strings (stretched green lines, sometimes denoted by white arrows) were typically no different before and after ADAMTS13 perfusion. Data are from the analysis of 17 to 71 strings in each independent run in different fields of view.
Low cleavage of VWF-string by ADAMTS13 in the absence of platelets at all calcium concentrations. (A) HEPES buffer sometimes containing 1 U/mL ADAMTS13, with or without 1.5 mM calcium, was perfused over stimulated HUVECs at 2.5 dyn/cm2 for 5 minutes. Following a 2-minute wash, 108 platelets/mL were introduced under identical buffer and shear conditions. VWF-string length was measured in multiple fields of view 5 minutes after platelet perfusion. VWF-string length decreased by 30% upon inclusion of ADAMTS13, although the difference was not statistically significant. (B) Alexa-488–labeled mAb DD3.1 (DD3.1-Ax488) was perfused over stimulated HUVECs for 5 minutes at indicated wall shear stress (2.5, 10, 40 dyn/cm2) before introduction of 1 U/mL ADAMTS13 for additional 5 minutes. VWF strings (stretched green lines, sometimes denoted by white arrows) were typically no different before and after ADAMTS13 perfusion. Data are from the analysis of 17 to 71 strings in each independent run in different fields of view.
Consistent with the notion that string cleavage is negligible in the absence of platelets, the length of platelet-free VWF strings labeled with a fluorescence anti-D′D3 domain mAb did not change substantially upon perfusion of 1 U/mL ADAMTS13 alone for 5 minutes over shear stresses from 2.5 to 40 dyn/cm2 (Figure 6B). Indeed, more cleavage was possible upon application of supraphysiological ADAMTS13 concentrations (10 U/mL), although endothelial adherent VWF still remain, even after 10 minutes (supplemental Figure 10). In contrast, when platelets were included, strings bearing platelets were 90% cleaved well within 5 minutes of ADAMTS13 perfusion (supplemental Figure 11). Finally, the observations in Figure 6B are not due to mAb DD3.1, because this reagent does not affect VWF proteolysis by ADAMTS13 either under static (supplemental Figure 12) or shear conditions (supplemental Figure 13).
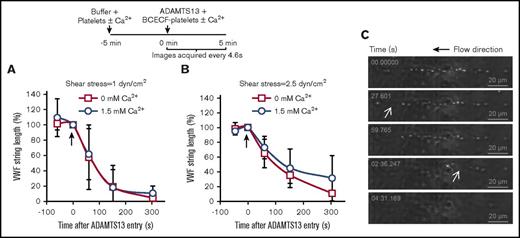
Next, VWF strings bearing platelets were analyzed for cleavage in the presence or absence of calcium (Figure 7). Here, 108 platelets/mL were perfused over stimulated HUVECs to form VWF-platelet strings of stable length over 5 minutes, in buffer with either 1.5 or 0 mM CaCl2. A total of 1 U/mL ADAMTS13 was then introduced in the same buffer with fluorescent platelets being used to mark the time of ADAMTS13 entry into the flow device. Such studies were performed at 1 (Figure 7A), 2.5 (Figure 7B), and 10 dyn/cm2 (supplemental Figure 13). No significant effect of calcium was observed. The micrographs in Figure 7C and supplemental video A present a typical sequence where the interplatelet distance within a VWF string is extended before ADAMTS13-mediated proteolysis. Overall, VWF-platelet string cleavage was independent of calcium under the conditions tested.
VWF-string cleavage in the presence of platelets is calcium-independent. (A-B) HEPES buffer containing platelets with and without 1.5 mM calcium was perfused over stimulated HUVECs for 5 minutes. 1 U/mL ADAMTS13 along with 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein–labeled platelets was then introduced (shown by arrow at t = 0 s) in the same buffer at either 1 dyn/cm2 (A) or 2.5 dyn/cm2 (B). Percent change/decrease in VWF string length after ADAMTS13 addition was measured. No significant difference was noted between calcium and calcium-free runs. (C) Representative time course of VWF-string cleavage, marked by successive loss of platelets. White arrows indicate stretching of interplatelet distance before cleavage. Data are from 4 to 7 experiments under each condition.
VWF-string cleavage in the presence of platelets is calcium-independent. (A-B) HEPES buffer containing platelets with and without 1.5 mM calcium was perfused over stimulated HUVECs for 5 minutes. 1 U/mL ADAMTS13 along with 2′,7′-Bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein–labeled platelets was then introduced (shown by arrow at t = 0 s) in the same buffer at either 1 dyn/cm2 (A) or 2.5 dyn/cm2 (B). Percent change/decrease in VWF string length after ADAMTS13 addition was measured. No significant difference was noted between calcium and calcium-free runs. (C) Representative time course of VWF-string cleavage, marked by successive loss of platelets. White arrows indicate stretching of interplatelet distance before cleavage. Data are from 4 to 7 experiments under each condition.
Discussion
This study determines physiological conditions in which calcium may control VWF proteolysis. It complements previous biophysical investigations that apply optical tweezers,14,15 isothermal calorimetry,15 thermal denaturation14,34 and molecular-dynamics simulations13,35,36 to either characterize force-dependent VWF-A2 unfolding in the presence and absence of calcium or the structural stability of VWF-A2 calcium-binding mutants. It concludes that calcium-dependent VWF proteolysis by ADAMTS13 may occur intracellularly, particularly following VWD type 2A mutations. Calcium is not the primary driver of wild-type VWF string-cleavage when bound on ECs.
Intracellular VWF cleavage
Consistent with prior literature, this study reports the expression of ADAMTS13 in ECs/HUVECs,7,9 with colocalization with VWF being observed in the perinuclear region.9 The estimated ADAMTS13 activity in HUVECs was ∼0.039 ± 0.018 U/mL when considered on a whole-cell basis and ∼0.81 ± 0.37 U/mL if the enzyme is assumed to be localized in the ER. These calculations are based on the measured kinetics of XS-VWF substrate proteolysis by HUVEC lysates vs similar assays performed using recombinant ADAMTS13 calibrants,20 and assuming that HUVECs have a mean radius of 12.5 µm with the ER constituting 4.8% of the cell volume.37 Such ADAMTS13 levels may be physiologically relevant because proteolytically digested VWF was observed in HUVEC lysates and secretions. Further, both wild-type VWF and VWF-Lock were cleaved to an equal extent when expressed in HUVECs and in HEK293Ts coexpressing ADAMTS13. Thus, ADAMTS13-mediated proteolysis likely occurs before disulfide bond formation.29 Others have also shown that pro-ADAMTS13 (ADAMTS13 with propeptide) is active when expressed in HeLa cells and it can cleave pro-VWF (VWF with propeptide).38 A total of 5 to 60 μM Ca2+ is sufficient for robust ADAMTS13 activity.39
Although some intracellular cleavage was observed for wild-type VWF in HUVECs, the proteolysis was more prominent for VWF-A2 calcium-binding mutants, especially VWD type 2A R1597W. In intracellular compartments where calcium concentrations are low (80-500 μM18 ), the calcium-binding mutants consistently appeared in an open conformation using both confocal microscopy and flow cytometry, both in HUVECs and HEK293Ts. Ex vivo studies with recombinant VWF-A2 FRET and mVWF FRET show that A2-conformation is dynamically regulated in the range from 0 to 500 μM calcium. Although urea was necessary to facilitate protein unfolding in this study, other intracellular molecular interactions may control the VWF-A2 open-closed transitions in situ.
Calcium binding and VWF-A2 structure changes are separable based on a 2-step model (supplemental Material) because Ca2+ binding to VWF-A2 occurs at low KD ∼3.8 µM,15 whereas higher Ca2+ (∼0-500 µM) is necessary for domain unfolding. According to this model, KD1 = 3.8 µM controls the transition of the closed/folded form of VWF-A2 from a calcium-bound to calcium-free state. Thus, whereas ∼1% of VWF-A2 is in its closed, calcium-free form at 400 μM Ca2+, this increases to ∼3% at 50 μM Ca2+. Following calcium release, VWF-A2 transitions from its closed to open form with KD2 ∼0.033 to 0.095. Here, VWF-A2 unfolding rates are 4 times greater for the single domain compared with multimeric VWF, suggesting that additional features beyond the A2-domain may stabilize VWF-A2 in multimeric VWF. Consistent with this, western blot results show that the conditions for monomer VWF-A2 proteolysis by ADAMTS13 is less stringent compared with multimeric VWF.20 Others also highlight the complex nature of the VWF multimer structure including intermonomer interaction involving the D4 domain.40 Finally, in addition to calcium, notable VWF-A2 structural features such as cis-proline and vicinal cysteines,41 molecular chaperones and local pH may also regulate ADAMTS13-mediated proteolysis rates. These features likely control multimeric wild-type and mutant VWF proteolysis rates in vivo.
Extracellular VWF cleavage
Previous single-molecule biophysics studies showed that the force required for VWF A2-domain unfolding increases two- to threefold in the presence of calcium,14 with calcium also accelerating A2-domain refolding upon stress relaxation.15 Calcium was titrated in flow chamber studies to determine if the calcium-dependent force spectroscopy measurements also contribute to VWF-string cleavage under physiological flow. Although buffers without calcium were applied, EDTA was not used because it dislodges ECs and inhibits ADAMTS13 activity.39
These studies noted that platelets are necessary for VWF-string cleavage. In the absence of platelets, VWF bound to stimulated HUVECs remained uncleaved by ADAMTS13 even after prolonged shear exposure up to 40 dyn/cm2. Simultaneous infusion of platelets with ADAMTS13, however, resulted in rapid VWF cleavage and loss of VWF-platelet strings within 60 seconds at wall shear stress >1 dyn/cm2. Our conclusions are different from that of others who report VWF-string cleavage in the absence of platelets,33 likely because of the nature of their experiment design. Nevertheless, these results are consistent with hydrodynamic calculations that predict that forces on the order of 0.02 pN are applied on platelet-free VWF strings at 1 dyn/cm2 42 (supplemental Methods; supplemental Figure 14). Such forces are small compared with the minimal force necessary to unfold VWF-A2 both in the absence (5-10 pN43 ) and presence of calcium (∼14 pN14 ). Platelet capture augments the applied force by 100-fold to 3.8 to 8 pN at 1 dyn/cm2. The attachment of more than 1 platelet to a VWF-string further enhances the magnitude of applied force. Further, the applied force increases substantially if platelets liftoff or are physically separated from the endothelium because of sparse/weak VWF interactions with the vessel wall (supplemental Figure 14; supplemental Video A). These observations are also consistent with in vivo findings that suggest that VWF strings are short-lived in vivo.44
VWF-platelet string cleavage proceeded with similar kinetics in the absence and presence of Ca2+, above 1 dyn/cm2. This is consistent with the concept that platelet binding, platelet separation distance from the endothelium and unidirectional force application that prevent calcium-dependent refolding at zero stress, is the primary parameter controlling VWF-string cleavage. Thus, although it is possible that calcium may reduce proteolysis when VWF is subjected to oscillating forces when the protein is either freely tumbling in solution43,45,46 or when it is bound to platelets,26,47 this divalent ion may not protect EC-bound VWF-platelet strings from cleavage because the applied force is unidirectional in the microvessels.
Overall, the data suggest that calcium may play a role in regulating intracellular VWF cleavage but it may not control VWF-platelet string proteolysis. The physiological and in vivo significance of this observation may be expanded using a wider range of VWD type 2A mutants and by implementing targeted deletion of ADAMTS13 in ECs both in normal and VWD animal models.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Alan Siegel (Buffalo, NY) for assistance with confocal microscopy data acquisition.
This work was funded by a grant from the National Institutes of Health, National Heart, Lung, and Blood Institute (grant HL77258).
Authorship
Contribution: S.G., A.K., and C.Z. designed experiments, performed experiments, analyzed data, and wrote the manuscript. K.M.D. designed and performed experiments. S.N. designed experiments, analyzed results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sriram Neelamegham, 906 Furnas Hall, Buffalo, NY 14260; e-mail: neel@buffalo.edu.