Key Points
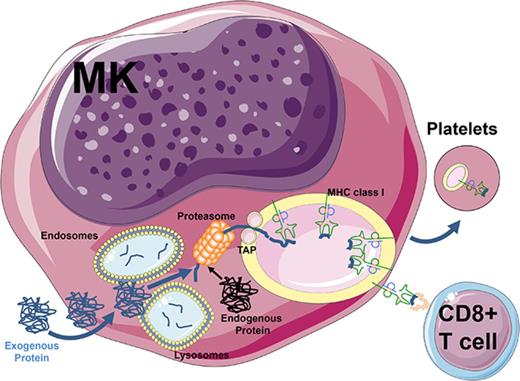
Megakaryocytes process and present endogenous/exogenous antigens on MHC class I molecules to activate CD8+ T cells.
Megakaryocytes can transfer MHC class I molecules loaded with foreign antigen to proplatelets in vitro.
Abstract
Megakaryocytes (MKs) are bone marrow–derived cells that are primarily responsible for generating platelets for the maintenance of hemostasis. Although MK can variably express major histocompatibility complex (MHC) class I and II molecules during their differentiation, little is known whether they can elicit nonhemostatic immune functions such as T-cell activation. Here, we demonstrate that mature CD34− MHC class II− CD41+ MKs can endocytose exogenous ovalbumin (OVA) and proteolytically generate its immunogenic peptide ligand, which is crosspresented on their surface in association with MHC class I molecules. This crosspresentation triggered in vitro and in vivo OVA-specific CD8+ T-cell activation and proliferation. In addition, the OVA-MHC class I complexes were transferred from MK to pro-platelets upon thrombopoiesis in vitro. MK could also present endogenous MK-associated (CD61) peptides to activate CD61-specific CD8+ T cells and mediate immune thrombocytopenia in vivo. These results suggest that, in addition to their hemostatic role, mature MKs can significantly affect antigen-specific CD8+ T-cell responses via antigen presentation and are able to spread this immunogenic information through platelets.
Introduction
Antigen presentation by molecules encoded by the major histocompatibility complex (MHC) to T lymphocytes is a key event in the detection and elimination of foreign protein antigens. Classically, exogenous proteins are engulfed by antigen-presenting cells (APCs) by pinocytosis, endocytosis, or phagocytosis1 ; once internalized, the proteins are degraded before being loaded onto MHC class II molecules for presentation to CD4+ T lymphocytes.2 Alternatively, antigen crosspresentation via MHC class I generally relies on the degradation of exogenous proteins by the cytosolic proteasome.1 Subsequently, peptides are translocated into the endoplasmic reticulum (ER) and loaded onto nascent MHC class I molecules that are transported to the cell surface, which leads to CD8+ T-cell activation via T-cell receptors.3,4 These crosspresentation pathways have also been associated with intracellular routes using vacuoles and recycled MHC class I and fusions between the ER and endosomes or the exchange of components between these compartments.5-7 The array of presented antigens at the plasma membrane can vary from tens to hundreds of thousands of copies, but the effect of the number of complexes as well as their half-life on immune response remains unclear.8 The process of crosspresentation is usually restricted to professional APCs, particularly dendritic cells9 ; however, other cell types such as activated platelets or endothelial cells in the liver and brain10-13 can also mediate crosspresentation.
The bone marrow microenvironment is the primary site where hematopoietic stem cells give rise to mature blood cells and is also a crucial niche for immunity with a reservoir of memory T cells.14,15 Megakaryocytes (MKs) are large (50 to 100 µm) and rare cells that give rise to proplatelets via a complex differentiation pathway.16-18 This pathway is driven by thrombopoietin, a hormone primarily produced in the liver,19 which leads mature MK to generate long pseudopodia and essentially transform their entire cytoplasm into proplatelets.19,20 Platelets have also been shown to mediate various immune functions such as secretion of immunomodulatory chemokines, cytokines, and adhesion molecules such as CD62P (P-selectin) and CD40L (CD154), which can trigger inflammatory responses in endothelial cells.21,22 In addition, platelets can trap infectious agents via the expression of Toll-like receptors (TLRs) 1 to 921 and have been shown to internalize exogenous protein antigens and crosspresent them in the association with MHC class I molecules to CD8+ T cells.11 The crosspresentation event, however, critically requires platelet activation because resting platelets express plasma adsorbed denatured MHC class I molecules that are inhibitory to different CD8+ T-cell responses.23-25 Major biological questions include: How do platelets acquire these immune capabilities? Are they derived from exogenous sources or do they acquire them from their parental cells, the MKs?
The immunological role of MKs has not been completely elucidated; however, they have been implicated in the pathogenesis of the autoimmune bleeding disorder immune thrombocytopenia (ITP),26,27 a disease with an incompletely understood pathogenesis involving antiplatelet antibodies and/or CD8+ T cells.28 Early MK progenitors (CD34+/CD41−/MHC class II+) have been shown to support heterologous noncognate CD4+ T-cell activation.29 In contrast, fully mature CD34− CD41+ MKs lose MHC class II expression but do express MHC class I molecules.29-31 Considering that activated platelets are able to crosspresent antigens,11,25 we examined whether this ability stemmed from their parental MK cells. We show that mature CD34− CD41+ MKs are able to engulf the exogenous protein antigen ovalbumin (OVA) and actively process and present its immunogenic peptide on MHC class I molecules, which effectively triggers antigen-specific CD8+ T-cell activation in vitro and in vivo. We also provide evidence that MK can transfer this immunogenic information to platelets during thrombopoiesis. Finally, using an in vivo murine model of ITP,32 we demonstrate endogenous CD61 antigen presentation by MKs that results in a potent in vivo CD8+ T cell–mediated immune response in the bone marrow leading to thrombocytopenia. Taken together, these results suggest a potentially important role for mature MKs as novel APCs that can present various antigens to antigen-specific CD8+ T cells. These novel findings provide insight into the ability of mature MKs to modulate T-cell responses and transfer this ability to platelets.
Methods
Detailed methods are described in the supplemental Methods.
MK treatment
After bovine serum album (Sigma-Aldrich, St. Louis, MO) gradient enrichment, MKs were pulsed with purified EndoFit ovalbumin (InvivoGen, San Diego, CA), DQ-Ovalbumin, or Ovalbumin conjugated with an AF647 (Molecular Probes, Eugene, OR) at the various indicated concentrations and durations at 37°C and 5% CO2. Negative controls were performed in tandem by incubating cells on ice throughout the experiment. Pulsed MKs were analyzed by flow cytometry (Fortessa X-20, Becton Dickinson, Mississauga, ON, Canada) to quantitatively assess OVA intake and degradation by measuring the corresponding mean fluorescence intensity. The MK cells were labeled and assessed for CD41 and BODIPY FL MHC class I-OVA levels (mean fluorescence intensity). For the proteasome and cytoskeleton inhibition experiments, MKs were incubated with 10 µM of MG132 (MedChem Express, Monmouth Junction, NJ), Nocodazole (Sigma-Aldrich), or vehicle (dimethyl sulfoxide) for 12 hours before an OVA pulse (500 µg/mL for 24 hours). The cells were then washed and either analyzed by flow cytometry or used in coculture with OT-1 CD8+ T cells (described in the following section).
In vitro antigen-specific CD8+ T-cell activation assay
In vitro antigen-specific CD8+ T-cell activation was performed using purified OVA-specific OT-1 CD8+ T cells or wild-type (WT) BL/6 CD8+ T cells in combination with WT C57BL/6 (BL/6, H-2b) MKs or BALB/c (B/c, H-2d) MKs. Coculture experiments were adapted from a previously described method.33 Briefly, 105 BL/6 or B/c MKs were used as target cells and pulsed with OVA in 96 U-bottom well plates. After 24 hours, MKs were washed and purified OT-1 or WT naïve CD8+ T cells (effector cells) were added at the indicated effector:target ratios (1:1 and 2.5:1). Cells were cocultured for 24, 48, or 72 hours at 37°C. Interleukin-2 (IL-2) release in the culture medium was measured by ELISA (R&D systems, Minneapolis, MN) and cells were analyzed by flow cytometry. The T cells were labeled for CD8, CD69, and with a proliferation dye (violet proliferation dye 450, BD Horizon) according to the manufacturer’s instructions. The gating strategy for CD8+ T-cell activation relied on CD8+ and violet proliferation dye 45+ cells; at least 10 000 cells were analyzed. Cells were also labeled for CD41 to confirm that MKs could endocytose and process DQ-OVA. Data analysis was performed with BD FAcsdiva (BD Biosciences, Mississauga, Canada) or FlowJo software (FlowJo LLC, Ashland, OR).
Proplatelet quantification and visualization
Mouse fetal liver cells were collected from WT CD1 fetal mice (Charles River Laboratories) on day 13.5 in utero and cultured at 37°C with 5% CO2 in the presence of supernatant containing 70 ng/mL recombinant mouse c-Mpl ligand for 4 days.34 On day 4, round MKs were isolated by bovine serum album gradient sedimentation and cultured for 1 additional day to allow for proplatelet formation, as described.35,36 MKs were transferred to a 24-well plate and imaged using the IncuCyte HD system (Essen BioScience, Ann Arbor, MI). Frames were captured at 1-hour intervals from 4 separate 950 × 760 μm2 regions per well using a 10× objective lens. Cultures were maintained at 37°C in an XL-3 incubation chamber (Carl Zeiss) throughout and run in triplicate. The extent of proplatelet production over time was measured in ImageJ, version 1.45, software using investigator-coded software as previously described.37,38 Values from all 4 regions of each well were pooled and averaged across replicates. For fixed samples, MKs were purified and probed as previously described.37,38 All samples were treated with a secondary goat anti-mouse antibody conjugated to an Alexa 568 nm fluorochrome (Invitrogen Corporation, Molecular Probes). As background controls, slides were incubated with the appropriate secondary antibody alone and all images were adjusted to account for nonspecific binding of antibodies. MK imaging was done with a 60× objective and platelets and proplatelets with a 90× objective. MHC class I-OVA was imaged using a Leica DMI 6000B spinning disk confocal microscope with a 63× objective.
Results
Bone marrow–derived MKs endocytose and process exogenous OVA via the proteasome
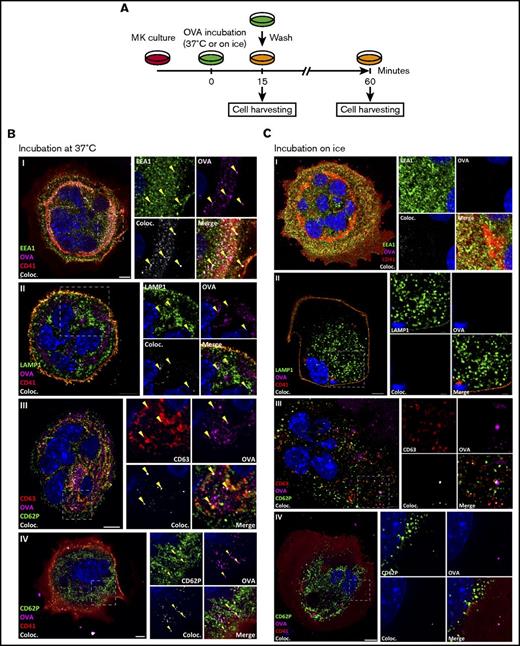
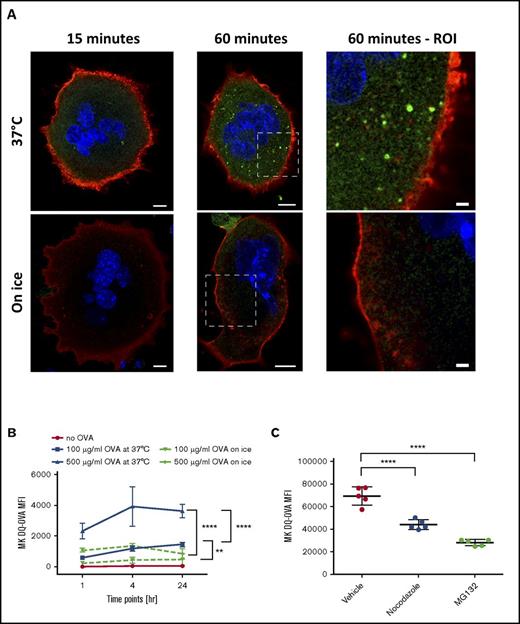
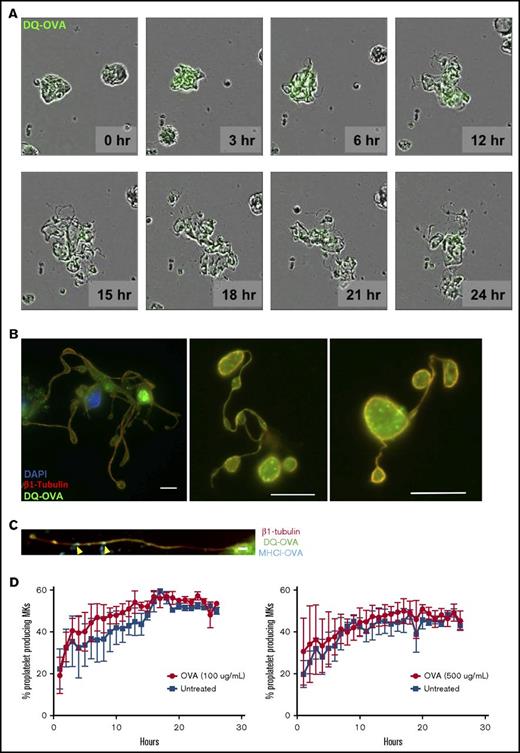
To assess the ability of MKs to crosspresent, we tested their capacity to endocytose OVA as a surrogate exogenous antigen. MKs were pulsed with fluorescent OVA and analyzed immediately after pulsing or after an additional 1-hour incubation at 37°C (Figure 1A). Immediately after pulsing, OVA colocalized with the endosome marker early endosome antigen 1 and the multivesicular body marker CD63 and partially with the lysosome marker lysosome-associated membrane glycoprotein 1 (Manders split colocalization coefficient = 0.40 ± 0.18, n = 4) (Figure 1BI-III). One hour later, OVA was colocalized with CD62P, confirming its presence in α-granules (Figure 1CIV; Manders split colocalization coefficient = 0.51 ± 0.08, n = 5). Endocytosis of fluorescent protein was decreased when MK metabolism was reduced by incubation on ice (Figure 1B). Proteolytic degradation of the endocytosed OVA was queried using DQ-OVA conjugated protein, which only becomes fluorescent upon proteolytic processing. In this assay, DQ-OVA intracellular granule fluorescence amplified over time (Figure 2A) with escalating doses of DQ-OVA (100 vs 500 μg/mL P < .0001, Figure 2B; 31 μg/mL vs 0 μg/mL P < .05 and 62 to 500 μg/mL vs 0 μg/mL P < .0001; supplemental Figure 1A) as well as the duration of exposure at 37°C (2-way analysis of variance [ANOVA] P < .01), yet was significantly reduced when MKs were incubated on ice (Figure 2A; 100 μg/mL P < .01 and 500 μg/mL P < .0001, Figure 2B).
MKs endocytose OVA. As described in the timeline (A), MKs were pulsed with fluorescent OVA for 15 minutes at 37°C (B) or on ice (C) and the cells were either fixed immediately or incubated for an additional 60 minutes. After pulse, OVA was tested for colocalization with the endosomal marker early endosome antigen 1 (EEA1) (I), lysosomal marker lysosome-associated membrane glycoprotein 1 (LAMP1) (II), and multivesicular body marker CD63 (III), and, after 60 minutes, with α-granule marker CD62P (IV). The colocalization (Coloc.) channel was reconstructed in silico based on the colocalizing voxels (3-dimensional pixels) of OVA and each marker and summarizes the colocalization in each condition. Yellow arrows highlight some of the colocalizations in each panel. (Zeiss LSM 700 confocal microscope, oil-immersion objective 63×; whole cell scale bar = 10 µm, region of interest scale bar = 2 µm.)
MKs endocytose OVA. As described in the timeline (A), MKs were pulsed with fluorescent OVA for 15 minutes at 37°C (B) or on ice (C) and the cells were either fixed immediately or incubated for an additional 60 minutes. After pulse, OVA was tested for colocalization with the endosomal marker early endosome antigen 1 (EEA1) (I), lysosomal marker lysosome-associated membrane glycoprotein 1 (LAMP1) (II), and multivesicular body marker CD63 (III), and, after 60 minutes, with α-granule marker CD62P (IV). The colocalization (Coloc.) channel was reconstructed in silico based on the colocalizing voxels (3-dimensional pixels) of OVA and each marker and summarizes the colocalization in each condition. Yellow arrows highlight some of the colocalizations in each panel. (Zeiss LSM 700 confocal microscope, oil-immersion objective 63×; whole cell scale bar = 10 µm, region of interest scale bar = 2 µm.)
MKs can process OVA into peptides. MKs were pulsed with DQ-OVA and fluorescence was monitored over 60 minutes at 37°C (A). Scale bars represent 10 µm, except in 2 right panels scale bars represent 2 μm. MKs were incubated with 0, 100, or 500 µg/mL of DQ-OVA for 1, 4, or 24 hours at 37°C or on ice (B). Two-way ANOVA P < .01 and 1-way ANOVA with a Tukey correction for multiple testing at 24 hours; n = 4; mean with standard deviation (SD). Incubation of MKs with the proteasome inhibitor, MG132, or with the tubulin dynamic inhibitor, Nocodazole, was used to assess the proteasome and cytoskeleton contribution, respectively, to OVA processing (C). One-way ANOVA with a Tukey correction for multiple testing; n = 5; mean with SD; ****P < .0001; **P < .01; each n corresponds to an independent MK donor mouse.
MKs can process OVA into peptides. MKs were pulsed with DQ-OVA and fluorescence was monitored over 60 minutes at 37°C (A). Scale bars represent 10 µm, except in 2 right panels scale bars represent 2 μm. MKs were incubated with 0, 100, or 500 µg/mL of DQ-OVA for 1, 4, or 24 hours at 37°C or on ice (B). Two-way ANOVA P < .01 and 1-way ANOVA with a Tukey correction for multiple testing at 24 hours; n = 4; mean with standard deviation (SD). Incubation of MKs with the proteasome inhibitor, MG132, or with the tubulin dynamic inhibitor, Nocodazole, was used to assess the proteasome and cytoskeleton contribution, respectively, to OVA processing (C). One-way ANOVA with a Tukey correction for multiple testing; n = 5; mean with SD; ****P < .0001; **P < .01; each n corresponds to an independent MK donor mouse.
To confirm contributions of the tubulin cytoskeleton and the proteasome in DQ-OVA processing, cells were incubated with either Nocodazole or MG132, respectively, before being pulsed. Intracellular DQ-OVA fluorescence was significantly decreased with inhibition of MK tubulin dynamics (Nocodazole) and even more so with inhibited proteasome function (MG132) (P < .0001, Figure 2C), validating that the machinery needed for antigen endocytosis and crosspresentation is functional in MKs.
MKs load OVA antigenic peptides onto their MHC class I molecules
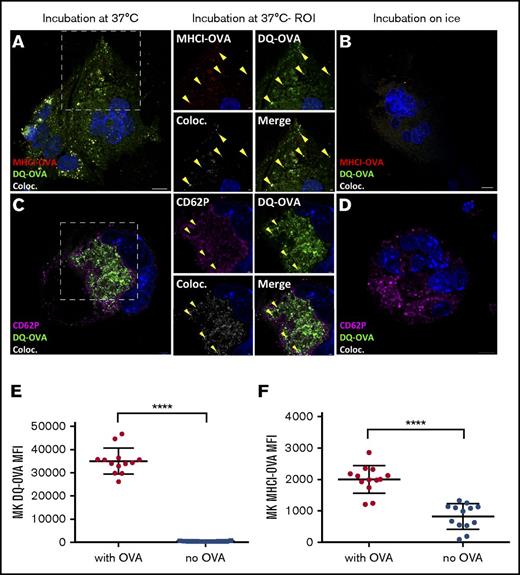
Since gradient-enriched CD41+ MK cells express MHC class I molecules and the co-stimulatory molecules CD80 and CD86 but not MHC class II molecules (supplemental Figure 2), we addressed whether MKs could present antigenic peptides on MHC class I molecules. One hour after being pulsed, the DQ-OVA granular pattern colocalized with an anti-MHC class I-OVA antibody (clone 25 D1.16), which specifically recognizes the immunogenic OVA peptide SIINFEKL in the MHC class I antigen-binding groove39 (Figure 3A-B), confirming the presence of OVA peptide binding to MHC class I. DQ-OVA also colocalized with CD62P (Figure 3C-D), similar to the α-granule colocalization results observed with fluorescent OVA (Figure 1BIV). The dose-dependent increase of DQ-OVA intracellular fluorescence observed by flow cytometry mirrored a significant increase in plasma membrane MHC class I-OVA of nonpermeabilized MKs (P < .0001, Figure 3E; P < .05 to < .0001; supplemental Figure 1A-B), confirming the efficiency of MKs at loading antigens onto MHC class I molecules.
MKs present OVA peptides in MHC class I (MHCI) molecules. After a 60-minute chase, DQ-OVA and anti–MHCI-OVA (binds to the OVA SIINFEKL antigen in the groove of MHC class I molecules) colocalization was tested at 37°C (A) and on ice (B). In the same conditions, DQ-OVA colocalization with the α-granule marker CD62P was measured by confocal microscopy (C-D). DQ-OVA fluorescence and consistently expressed MHCI-OVA level at plasma membrane upon a pulse of 24 hours was determined by flow cytometry (500 µg/mL) (E-F). Unpaired t test; n = 13; mean with SD; ****P < .0001; each n corresponds to an independent MK donor mouse. Original magnification ×63 for panels A-D. Scale bars represent 10 μm, except in ROI images scale bars represent 2 μm.
MKs present OVA peptides in MHC class I (MHCI) molecules. After a 60-minute chase, DQ-OVA and anti–MHCI-OVA (binds to the OVA SIINFEKL antigen in the groove of MHC class I molecules) colocalization was tested at 37°C (A) and on ice (B). In the same conditions, DQ-OVA colocalization with the α-granule marker CD62P was measured by confocal microscopy (C-D). DQ-OVA fluorescence and consistently expressed MHCI-OVA level at plasma membrane upon a pulse of 24 hours was determined by flow cytometry (500 µg/mL) (E-F). Unpaired t test; n = 13; mean with SD; ****P < .0001; each n corresponds to an independent MK donor mouse. Original magnification ×63 for panels A-D. Scale bars represent 10 μm, except in ROI images scale bars represent 2 μm.
OVA-pulsed MK presentation induces antigen-specific CD8+ T-cell responses
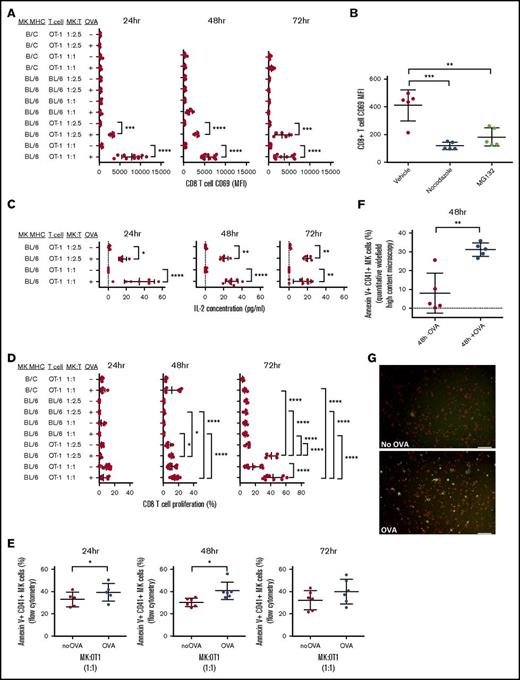
To determine if MK MHC class I presentation was functionally able to trigger CD8+ T-cell activation and proliferation, OVA SIINFEKL peptide-specific OT-1 CD8+ T cells were cocultured with OVA-pulsed or unpulsed MKs. Compared with OT-1 cells cultured with unpulsed MKs, OVA-pulsed MK/OT-1 cocultures showed a significant dose-dependent increase in expression of the early activation marker CD69 (P < .001 to < .0001, Figure 4A; P < .05 to < .0001, supplemental Figure 1C; supplemental Figure 3A); this was significantly decreased by incubation with either nocodazole or MG132 compared with dimethyl sulfoxide (vehicle) (P < .001 and P < .01, respectively, Figure 4B). The OT-1 activation was MHC restricted because there was no increase in CD69 expression when incubated with pulsed MKs from a different MHC class I haplotype (Figure 4A). In addition, naive WT BL/6 CD8+ T cells were not sensitive to MK OVA MHC class I presentation (Figure 4A) despite similar levels of MK DQ-OVA (supplemental Figure 3B). Functionally, the T-cell activation pattern in the OVA-pulsed cultures correlated with significantly higher levels of IL-2 production (P < .05 to P < .0001, Figure 4C) and significant CD8+ T-cell proliferation (P < .05 to P < .0001, Figure 4D, and P < .05 to P < .0001; supplemental Figure 3C). Using Annexin V to assess phosphatidylserine on MKs as a measure of early sign of apoptosis, we found that at 24 and 48 hours, the proliferating CD8+ OT-1 T cells mediated a significant cytotoxic response against the OVA-presenting MKs (P < .05, Figure 4E, P < .01, Figure 4F-G).
MKs activate CD8+ T cells via OVA crosspresentation. MKs were pulsed with or without OVA (+OVA or –OVA, respectively) and cocultured with OT-1 CD8+ T cells at 2 different cell ratio MK:CD8+ T cells (effector:target ratios = 1:1 and 1:2.5) for 24, 48, or 72 hours. In addition, B/c MKs were cocultured with OT-1 T cells to verify the MHC class I haplotype specificity of the CD8+ T-cell activation. MKs were also cocultured with WT BL/6 CD8+ T cells as a negative control with naïve CD8+ T cells. CD69 surface expression was assessed to monitor CD8+ T-cell activation (A). CD8+ T-cell activation was also measured with and without tubulin cytoskeleton or proteasome inhibitors (nocodazole and MG132, respectively) during OVA pulse (B). IL-2 was measured in coculture medium to confirm CD8+ T-cell activation (C). CD8+ T-cell proliferation was also measured (D). One-way ANOVA with a Tukey correction for multiple testing; n ≥ 5; mean with SD (A-D). Cytotoxic response was quantified in MKs using Annexin V as a marker of apoptosis by flow cytometry (E). Paired t or Wilcoxon test; n = 5, mean with SD. These results were confirmed at 48 hours by quantitative widefield high-content microscopy. Unpaired t test; n = 5; mean with SD (F). A representative image of the coculture used for the quantification shows the staining for Annexin V (green) in CD41+ MKs (red) in coculture with CD8+ T cells (unlabeled) in the presence or absence of OVA (G). Scale bar represents 50 μm. ****P < .0001; ***P < .001; **P < .01; *P < .05; each n corresponds to an independent MK donor and CD8+ T-cell donor coculture.
MKs activate CD8+ T cells via OVA crosspresentation. MKs were pulsed with or without OVA (+OVA or –OVA, respectively) and cocultured with OT-1 CD8+ T cells at 2 different cell ratio MK:CD8+ T cells (effector:target ratios = 1:1 and 1:2.5) for 24, 48, or 72 hours. In addition, B/c MKs were cocultured with OT-1 T cells to verify the MHC class I haplotype specificity of the CD8+ T-cell activation. MKs were also cocultured with WT BL/6 CD8+ T cells as a negative control with naïve CD8+ T cells. CD69 surface expression was assessed to monitor CD8+ T-cell activation (A). CD8+ T-cell activation was also measured with and without tubulin cytoskeleton or proteasome inhibitors (nocodazole and MG132, respectively) during OVA pulse (B). IL-2 was measured in coculture medium to confirm CD8+ T-cell activation (C). CD8+ T-cell proliferation was also measured (D). One-way ANOVA with a Tukey correction for multiple testing; n ≥ 5; mean with SD (A-D). Cytotoxic response was quantified in MKs using Annexin V as a marker of apoptosis by flow cytometry (E). Paired t or Wilcoxon test; n = 5, mean with SD. These results were confirmed at 48 hours by quantitative widefield high-content microscopy. Unpaired t test; n = 5; mean with SD (F). A representative image of the coculture used for the quantification shows the staining for Annexin V (green) in CD41+ MKs (red) in coculture with CD8+ T cells (unlabeled) in the presence or absence of OVA (G). Scale bar represents 50 μm. ****P < .0001; ***P < .001; **P < .01; *P < .05; each n corresponds to an independent MK donor and CD8+ T-cell donor coculture.
MKs trigger CD8+ T-cell activation in vivo
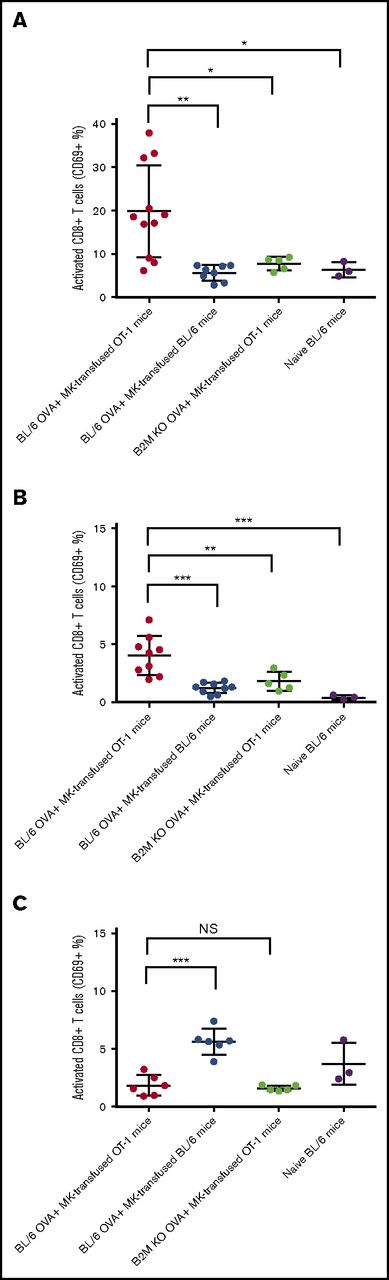
To evaluate MK APC capability in vivo, we transfused DQ-OVA–pulsed MKs into OT-1 or WT C57BL/6 mice and assessed the percentage of activated CD8+ T cells in their spleens after 48 hours. Compared with transfused WT mice, the spleens of transfused OT-1 mice had a significantly higher percentage of activated (CD69+) CD8+ T cells (4.78 ± 0.8108 and 19.47 ± 4.72, respectively, P < .05, Figure 5A; supplemental Figure 4A). The level of circulating CD69+ CD8+ T cells was also higher in this group (P < .001, Figure 5B) but not in lymph nodes at this time point (Figure 5C). When MK from β-2-microglobulin (B2M) KO mice were pulsed with OVA and injected into OT-1 mice, the CD8+ T-cell activation was abolished (P < .05, Figure 5A, and P < .01, Figure 5B) confirming that the in vivo activation was due to the transfused MK and not potential host crosspresentation mechanisms.
MKs presentation activates CD8+ T cells in vivo. MKs from WT or B2M KO mouse donors were pulsed with OVA and transfused in OT-1 or WT BL/6 mice. After 48 hours, mice were sacrificed and the percentage of activated CD8+ T cells was measured considering the CD69 expression in spleen (A), blood (B), and lymph nodes (C). NS, not significant. One-way ANOVA with a Tukey correction for multiple testing; n ≥ 3; mean with SD; ***P < .001; **P < .01; *P < .05; each n corresponds to an independent MK donor and recipient mouse.
MKs presentation activates CD8+ T cells in vivo. MKs from WT or B2M KO mouse donors were pulsed with OVA and transfused in OT-1 or WT BL/6 mice. After 48 hours, mice were sacrificed and the percentage of activated CD8+ T cells was measured considering the CD69 expression in spleen (A), blood (B), and lymph nodes (C). NS, not significant. One-way ANOVA with a Tukey correction for multiple testing; n ≥ 3; mean with SD; ***P < .001; **P < .01; *P < .05; each n corresponds to an independent MK donor and recipient mouse.
MKs deliver OVA-MHC class I complexes to proplatelets in vitro
MKs may directly interact with CD8 T cells, but they may also deliver antigen-MHC class I complexes to proplatelets. OVA peptides appeared to be packaged into α-granules in addition to their presentation at the plasma membrane (Figures 1BIV and 3C). To elucidate the fate of these antigenic complexes during the terminal stages of megakaryopoiesis, we used cultured murine fetal liver–derived MKs to assess the DQ-OVA pattern during proplatelet formation. We observed OVA peptide being transferred to newly formed proplatelets over time (Figure 6A-B), as well as MHC class I-OVA complexes (Figure 6C). Moreover, OVA processing did not affect the ability of MKs to generate and release proplatelets at any tested concentration (Figure 6D), pointing to a dissemination of exogenous antigen-MHC class I complexes from MKs through to platelets.
MKs deliver MHC class I complexes containing OVA antigen to proplatelets in vitro. MKs were pulsed with OVA (green) and proplatelet formation (A) was monitored for 24 hours as OVA antigens were gradually packaged into proplatelets during their formation. This observation was confirmed at 24 hours postpulse (B). OVA signal was found in mature proplatelets, presenting the typical β1 tubulin peripheral coil (B). Scale bar represents 10 μM. MHCI-OVA complexes were also transferred to proplatelet during thrombopoiesis (C). Scale bar represents 2 μM. The processing of OVA antigens did not affect MK proplatelet formation (D).
MKs deliver MHC class I complexes containing OVA antigen to proplatelets in vitro. MKs were pulsed with OVA (green) and proplatelet formation (A) was monitored for 24 hours as OVA antigens were gradually packaged into proplatelets during their formation. This observation was confirmed at 24 hours postpulse (B). OVA signal was found in mature proplatelets, presenting the typical β1 tubulin peripheral coil (B). Scale bar represents 10 μM. MHCI-OVA complexes were also transferred to proplatelet during thrombopoiesis (C). Scale bar represents 2 μM. The processing of OVA antigens did not affect MK proplatelet formation (D).
MKs have the ability to present endogenous CD61 antigens
Given the implication of both CD8+ T cells and MKs in ITP, we verified whether MKs could suffice in mounting thrombocytopenia in a well-established in vivo murine ITP model.32 In this model, the immune response and resulting thrombocytopenia rely on the transfer of splenocytes from GPIIIa (CD61) KO mice immunized with WT (CD61+) platelets into severe combined immunodeficient (SCID) mice (supplemental Figure 5A). Here, rather than using platelets, we used WT (CD61+) MKs as source of CD61 antigen and used platelet transfusions for comparison. When CD61 KO mice were transfused weekly with intact CD61+ platelets or MKs for 5 weeks, both induced the production of immunoglobulin G antibodies specific for CD61+ platelets with the platelet transfusions generating a higher antibody titer than MK transfusions (supplemental Figure 5B). The immunized mice were euthanized and their spleen cells were either depleted of CD19+ B cells or not (CD19-depleted or nondepleted groups, respectively), triggering T cell–mediated or both antibody- and T cell–mediated ITP, respectively, as previously demonstrated.32 Compared with the SCID mice transferred with naïve spleen cells, mice transferred with platelet-immune spleen cells induced both antibody- and T cell–mediated ITP (P < .0001, Figure 7A). In contrast, when SCID mice were transferred with spleen cells form MK-immune mice, a predominant T cell–mediated ITP was observed in the recipients (P < .001, Figure 7A). Furthermore, only these mice demonstrated significantly increased CD8+ T cells in the bone marrow (P < .05, Figure 7B). In addition, the percentages of bone marrow CD8+ T cells correlated with platelet counts in the MK-immune groups with increased CD8 T-cell counts causing a more severe thrombocytopenia (P < .01 and not significant, Figure 7C-D, respectively). These data confirmed that MKs, in addition to their ability to crosspresent exogenous antigen, can also crosspresent endogenous antigens in vivo and induce a significant thrombocytopenia.
MK presentation of endogenous CD61 antigens induces CD8+ T-cell activation in an ITP murine model. Platelet counts were performed in the different SCID recipients in the ITP model and, on day 14, showed a significantly lower count in the platelet-immune groups (CD19-dep [dep] and nondepleted [ND]) and in MK-immune CD19-dep group (A). At that time point, CD8+ CD3+ T cells were quantified in the bone marrow of the recipient group (B). One-way ANOVA with a Tukey correction for multiple testing; n = 5; mean with SD (A-B). The correlation between CD8+ CD3+ T-cell percentage in the bone marrow and platelet count was assessed in both MK-immune (C) and platelet-immune groups (D). Pearson correlation; n = 9 (C). Spearman correlation; n = 10 (D). ****P < .0001; ***P < .001; **P < .01; *P < .05; each n corresponds to a different recipient SCID mouse.
MK presentation of endogenous CD61 antigens induces CD8+ T-cell activation in an ITP murine model. Platelet counts were performed in the different SCID recipients in the ITP model and, on day 14, showed a significantly lower count in the platelet-immune groups (CD19-dep [dep] and nondepleted [ND]) and in MK-immune CD19-dep group (A). At that time point, CD8+ CD3+ T cells were quantified in the bone marrow of the recipient group (B). One-way ANOVA with a Tukey correction for multiple testing; n = 5; mean with SD (A-B). The correlation between CD8+ CD3+ T-cell percentage in the bone marrow and platelet count was assessed in both MK-immune (C) and platelet-immune groups (D). Pearson correlation; n = 9 (C). Spearman correlation; n = 10 (D). ****P < .0001; ***P < .001; **P < .01; *P < .05; each n corresponds to a different recipient SCID mouse.
Discussion
MK play a crucial role in creating platelets for hemostasis, but little is known of their immunomodulatory function. There is evidence that multipotent progenitor cells and early MK progenitor cells (CD34+, CD117+, CD41+, MHC class II+) are able to activate CD4+ Th17 cells 29-31 ; however, because MHC class II expression is lost during MK differentiation, it was unclear if mature MKs possess any capability of activating T cells (supplemental Figure 2A). In addition platelets have been shown to crosspresent antigens to antigen-specific CD8+ T cells11 and modulate immune responses.21,22 This CD8+ T-cell activation only occurred when the platelets were activated in contrast to resting platelets which are generally unable to activate CD8+ T cells and may actually suppress CD8+ T cell–mediated functions such as skin graft rejection.40 This apparent discrepancy appears to be due to different pools of MHC class I molecules associated with platelets; resting platelets have denatured MHC class I molecules on their surface adsorbed from plasma, whereas activated platelets express intact MHC class I from their α granules.23-25,41 The origins of this crosspresenting ability of activated platelets remain unknown; therefore, we addressed whether MKs may be responsible and if they can act as crosspresenting APC in an antigen-specific manner. We thus tested the ability of mature MHC class II−/MHC class I+ MKs to crosspresent protein antigens to CD8+ T cells in an antigen-specific manner. We show that mature MKs are not only able to crosspresent the exogenous protein antigen OVA in vitro and in vivo, but that they also have the ability to present their own endogenous antigens to CD8+ T cells via MHC class I molecules in vivo. Of clinical significance, MK presentation of MK-derived peptides triggered CD8+ T cell–mediated thrombocytopenia in vivo. Perhaps more important, we also demonstrate that exogenous peptide antigens loaded onto the MK MHC class I molecules are transferred to proplatelets. Taken together, these results suggest MKs can act as potent antigen-specific APCs to modulate CD8+ T-cell responses in vivo and may have the ability to spread these antigenic complexes to the periphery via platelet production.
Multiple lines of evidence support that MKs are responsible for conveying immunomodulatory functions to platelets. For example, MKs express TLR42,43 and other immunomodulatory molecules such as CD40L, which they transmit to platelets during thrombogenesis.44 Furthermore, MK maturation has been shown to be affected by infections via mechanisms involving TLR2 and TLR442,43 and can also be directly targeted by certain infections (eg, Dengue virus), thereby causing reduced platelet production and thrombocytopenia.45 Our work provides additional evidence that mature MKs can act as potent APCs that significantly affect CD8+ T-cell responses. This also provides a potential explanation for how activated platelets cross-present antigens.
Our results suggest that all the necessary molecular machinery to properly process and present antigens is present in mature MKs. For our study, we used OVA, a well-characterized protein antigen,46-49 in a manner that would favor crosspresentation to OVA antigen-specific CD8+ T cells via MHC class I molecules. Even when used at nonphysiological high doses (eg, 500 μg/mL), MKs did not show any sign of saturation in terms of antigen processing or MHC class I antigen loading at the plasma membrane (supplemental Figure 1). Although these observations point to a remarkable capacity of crosspresentation by MKs, possibly from their large dimensions, their presentation potency and how it compares to other traditional APC remains to be established.
The CD8+ T-cell stimulation was extremely sensitive in vitro; very low doses of OVA were sufficient to trigger a lymphocyte response (supplemental Figure 1). This may reflect the efficiency of MHC class I presentation to specifically activate CD8+ T cells, which only requires a few MHC class I–antigen complexes.50 The specific activation of CD8+ T cells by MK presentation was also confirmed in vivo. It has been shown that when MKs are administered intravenously to mice, they localize principally in the lungs, where they release functional platelets.51 Although beyond the scope of this paper, whether CD8+ T-cell activation may be occurring in the lungs is undoubtedly a worthwhile topic for future studies. In addition, the potential indirect immune effects that would be mediated by phagocytosis of the transfused MKs by host professional APCs have been ruled out because there was no CD69 expression on CD8+ T cells from OT-1 mice transfused with MKs from B2M KO OVA+ mice (Figure 5A-B). Furthermore, no OVA+ platelets could be detected in the blood of recipient OT-1 mice (data not shown), pointing toward a direct immunomodulation by the transfused MKs. On the other hand, 48 hours after injection, no CD8+ T-lymphocyte activation was detectable in the lymph nodes of OT-1 mice, unlike in the WT BL/6 recipient mice (Figure 5C), which may reflect a primary immune response pattern against OVA by the naïve T-cell repertoire in the recipient mice.
OVA antigens were located in α-granules (Figures 1BIV and 3C) and at the plasma membrane (Figure 3F). We therefore hypothesized that MKs could “pass down” this immunogenic information to their progeny platelets. The massive transfer of secretory granules during thrombopoiesis,19 as well as a similar observation about self-antigens,41 supports the hypothesis. Indeed, our results suggest that MKs are able to package exogenous antigens loaded onto MHC class I molecules into platelets, thus effectively spreading them throughout the organism, which may constitute an efficient way to modulate distant immune cells. In addition, OVA processing does not require any specific stimulus and has no impact on proplatelet formation (Figure 6C), which suggests that these different pathways occur concomitantly.
MKs can be affected in immune-mediated diseases that target platelets such as the autoimmune disorder ITP or fetal/neonatal alloimmune thrombocytopenia.26,27 Thus, to determine whether MKs could also present endogenously derived antigens to CD8+ T cells, we assessed their ability to generate immunity against CD61 and determined whether they could induce thrombocytopenia in vivo.32 The splenocytes from the MK immune mice had the ability to induce significant thrombocytopenia when transferred to SCID mice (Figure 5A). This appeared to be predominantly the result of CD8+ T cell generation, because CD19-depleted splenocytes from the MK immune mice generated significant thrombocytopenia (Figure 7B). Interestingly, platelet counts in SCID mice transferred with nondepleted splenocytes from the MK-immune mice were not significantly lower when compared with the negative control group (Figure 7B). This suggests that the presence of B cells may somehow reduce the effect of the immune cells activated by MK antigen presentation. Taken together, our data suggest that MKs may promote a stronger T- vs B-cell immune response compared with platelets. This may be because resting platelets have plasma adsorbed denatured MHC class I molecules on their surface,11,22,25 which may allow for increased antibody responsiveness but decreased T-cell activation. Despite this, the contribution of transfused MK-derived platelets on T- and B-cell immunity cannot be excluded. The higher percentage of CD8+ T cells in the bone marrow of SCID mice transferred with MK-immune (CD19-depleted) splenocytes further supports an MK-dominated T-cell response (Figure 5C). Moreover, a large proportion of the platelet count variance (R2 = 0.6816, **P < .01) was associated with the presence of CD8+ T cells in the bone marrow upon MK immunization (Figure 7C), whereas no significant correlation was observed with platelet immunization (Spearman r = 0.07951, not significant; Figure 7D). Furthermore, platelets can directly modulate the immune response in various disease states such as ITP.52 Our data support an immune-modulatory role of MKs, which may contribute to the pathophysiology of ITP such as in tolerance-like effects in patients with ITP after thrombopoietin receptor agonist therapies.53,54
In summary, our results demonstrate a novel antigen-specific crosspresentation function of mature MKs (CD34− CD41+) with both exogenous and endogenous antigens. We confirm that in conditions where the ability to modulate CD4+ T cells is lost, MKs still have an important role to play in adaptive immunity via CD8+ T-cell activation. These events may have a profound impact on thrombopoiesis and the pathogenesis of platelet autoimmune disorders, such as ITP26,27 and infectious settings leading to thrombocytopenia such as Dengue or sepsis.45,55
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Walter H. A. Kahr and Fred G. Pluthero from the Department of Paediatrics, University of Toronto, Division of Haematology/Oncology, and Program in Cell Biology, Research Institute, The Hospital for Sick Children, Toronto, Canada, for their fruitful suggestions during the course of the project. The authors also thank Caterina Di Ciano-Oliveira and Christopher M. Spring from the Keenan Research Centre for Biomedical Science, St. Michael's Hospital, Toronto, Canada, for their technical support in confocal microscopy and flow cytometry, respectively.
This work was supported by grants from Health Canada and Canadian Blood Services (CBS) (J.W.S.); a CBS Graduate Research Fellowship (M.J.M.); and the National Institutes of Health, National Heart, Lung, and Blood Institute (5F32HL118865) (K.R.M.) and National Institute of Diabetes and Digestive and Kidney Diseases (1K01DK111515) (K.R.M.). A.Z. is the recipient of a postdoctoral fellowship from Swiss National Science Foundation (P300PB_164760). R.K. received a Post-Doctoral Fellowship from the CBS. L.G. received an Ontario Trillium Scholarship and a China Scholarship Council PhD Scholarship. K.R.M. is an American Society of Hematology Fellow Scholar.
Authorship
Contribution: A.Z. designed and performed all experiments, collected data, analyzed and interpreted data, performed statistical analyses, made the figures, and wrote the paper; E.R.S. designed and performed experiments, collected and interpreted data, and edited the manuscript; K.R.M. and J.E.I. performed the proplatelet experiments and edited the manuscript; R.A., L.G., M.J.M., M.K., E.B., and R.K. contributed to certain experiments, collected the subsequent data, and edited the manuscript; and J.W.S. provided financial resources, analyzed and interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.Z. is Centre de Recherche du CHU de Québec, CHUL-UL, Québec City, QC, Canada.
The current affiliation for R.K. is Division of Hematology and Transfusion Medicine, Lund University, Lund, Sweden.
Correspondence: John W. Semple, Transfusion Medicine, Lund University, BMC C14, Klinikgatan 26, 221 84 Lund, Sweden; e-mail: john_w.semple@med.lu.se.

![Figure 7. MK presentation of endogenous CD61 antigens induces CD8+ T-cell activation in an ITP murine model. Platelet counts were performed in the different SCID recipients in the ITP model and, on day 14, showed a significantly lower count in the platelet-immune groups (CD19-dep [dep] and nondepleted [ND]) and in MK-immune CD19-dep group (A). At that time point, CD8+ CD3+ T cells were quantified in the bone marrow of the recipient group (B). One-way ANOVA with a Tukey correction for multiple testing; n = 5; mean with SD (A-B). The correlation between CD8+ CD3+ T-cell percentage in the bone marrow and platelet count was assessed in both MK-immune (C) and platelet-immune groups (D). Pearson correlation; n = 9 (C). Spearman correlation; n = 10 (D). ****P < .0001; ***P < .001; **P < .01; *P < .05; each n corresponds to a different recipient SCID mouse.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/20/10.1182_bloodadvances.2017007021/3/m_advances007021f7.jpeg?Expires=1770537049&Signature=OuEysYA~W8ArK3~VXLG7XdsDigumIo7cE64xUOSdDRwRG34wmQ~k9RYger176Q5ry8gKaGsaoznLFMqXE7cV1Xx05fG7rjaMzUHrOna2zpRsrYPQ48-qk1XK~tASK7XUxCj3IXQkppLjsRn3DHJ7EUhNWW8OgFKprtdyXzb0KjvbxDaJrkDaHvCsULRXgr3ic~5OpZFECB2wZEIT~fWHtM6GHYJjg8Iw3KzcP9mJrN5r1MEcF5JCf-LyUxyfTm~KXSkegMWx5vhbV4EXZcEKHihZ27Zmyk-rlhYOxrQbKf-mhHtI9T-cEBRFGWytBACeVvg4-ftsNR2Fs6EVU3kgMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)