Abstract
Sickle-cell disease (SCD) is characterized by frequent and painful vaso-occlusive crises (VOCs). Various treatments have been evaluated over the years. However, a clear overview is lacking. The objective of this study was to systematically review all pharmacotherapeutical strategies in the prevention of VOCs beyond hydroxyurea. We performed a systematic literature search (MEDLINE, Embase, CENTRAL). Eligible studies were controlled clinical trials evaluating pharmacotherapeutical interventions targeting the reduction of VOCs in patients with SCD. Primary outcomes were the number or duration of SCD-related pain days, VOCs, or hospital admissions for VOCs. Secondary outcomes included time to first VOC or hospital admission for a VOC. A standardized data extraction sheet was used. The methodological quality of studies was assessed using Cochrane’s risk-of-bias tool. A total of 36 studies were included in this review, covering 26 different prophylactic interventions. The most promising interventions for reducing the frequency of either VOCs or hospitalizations were the oral antioxidants l-glutamine and ω-3 fatty acids and the IV antiadhesive agent crizanlizumab. Twenty-three studies did not show any beneficial effect of the intervention under investigation, and 6 studies were either too small or methodologically inadequate to draw conclusions. Because of the heterogeneity of interventions, no meta-analysis was performed. In conclusion, this review identified 3 promising pharmacotherapeutical strategies in the prevention of VOCs in SCD. Importantly, this study highlights the discrepancy between the significant burden of SCD worldwide and the low number of adequate trials performed. This review was registered at PROSPERO (CRD42015025250).
Introduction
Sickle-cell disease (SCD) is a chronic, hereditary hemoglobinopathy. Every year, more than 300 000 children are born with this disease, making it 1 of the most common genetic disorders in the world. This number is expected to increase even further in the coming decades.1,2 A majority of these patients are located on the African continent.
SCD is characterized by chronic anemia, progressive organ damage, and a wide spectrum of acute manifestations such as stroke, acute chest syndrome, and serious bacterial infections.3 As a result of these severe complications, SCD has been associated with a significant reduction in life expectancy.4,5 One of the most frequent and debilitating symptoms of the disease is the incidence of acute, painful vaso-occlusive crises (VOCs), which typically occur in the extremities, back, joints, abdomen, or chest.6-8 These severe and unpredictable painful attacks are responsible for >90% of acute hospital admissions in SCD.9 Moreover, patients frequently experience pain at home without seeking any health care support.10,11 Aside from the direct physical morbidity of these recurrent painful attacks, other significant outcomes such as quality of life, school attendance, and societal participation of patients are greatly affected.12-18 Moreover, high societal costs are involved. Direct costs arise from high health care utilization, and indirect costs include absence from work associated with impaired physical ability to perform daily tasks.19
Over the past few decades, our understanding of the pathophysiology of VOCs in SCD has increased greatly. In addition to the fundamental concept of sickle hemoglobin (HbS) polymerization upon deoxygenation and the subsequent sickling of red blood cells, the roles of other significant processes in the pathophysiology of VOCs have been identified. Adhesive interactions between leukocytes, red blood cells, and an activated endothelium add to vascular obstruction, triggered by factors such as inflammation, stress, and hemolysis.20 Recurrent episodes of ischemia and subsequent reperfusion stimulate the release of reactive oxidants, thereby further promoting tissue damage, inflammation, and cellular activation.20 Secondary to these processes, activation of coagulation occurs, which amplifies vascular obstruction.21 Thus, the pathophysiology of VOCs is characterized by a complex and highly dynamic interplay of various determinants.
To relieve the clinical burden of SCD, an important research focus lies in the prevention of VOCs. The currently available pharmacotherapeutical options for prevention of VOCs are still extremely limited. The only drug with proven efficacy registered for use in SCD is hydroxyurea (HU), a potent inducer of fetal hemoglobin. HU has been shown to be highly efficacious in SCD.22-24 However, patients receiving HU often do not experience complete relief from their symptoms, and it is currently the only disease-modifying therapy for SCD. Therefore, there remains an urgent need for new therapeutic approaches. Novel treatments based on recently identified pathophysiological mechanisms have emerged over the past years. Many are currently under investigation in preclinical or early-phase trials, as extensively discussed in other reviews.25-27 However, some treatments have already been evaluated in phase 2 or 3 randomized clinical trials (RCTs), but a clear overview of these studies and their findings does not exist. The objective of this study was to systematically review the currently available controlled clinical trials, investigating new pharmacotherapeutical strategies in the prevention of VOCs in SCD. Hereby, we aim to facilitate the identification of promising treatments evaluated over the years and advance their clinical implementation.
Methods
We performed a systematic review following PRISMA guidelines.28
Data sources and searches
We identified relevant trials from electronic searches in MEDLINE (Ovid MEDLINE In-Process & Other Non-Indexed Citations and Ovid MEDLINE 1946 to present), Embase (Ovid Embase Classic and Embase 1947 to present), and the Cochrane Central Register of Controlled Trials (CENTRAL). Both full-text publications as well as conference abstracts were eligible for inclusion in our study. The search in MEDLINE was performed using the terms described in supplemental Table 1. Search strategies for all other databases were modeled on the MEDLINE version. References of eligible trials and other relevant systematic reviews were screened for additional relevant studies. There were no restrictions in our search on language, publication year, or publication status to limit potential bias. Date of the last search was 30 January 2017.
Study selection
Both controlled trials as well as RCTs with a concurrent control group were eligible for this review. Studies were included if they concerned patients of all ages with either homozygous or compound heterozygous SCD and evaluated prophylactic pharmacotherapeutical interventions targeting the reduction of acute VOC pain in SCD. Studies of HU were excluded from this review because these have been discussed extensively in previous reviews.22-24,29 Both inactive control as well as active control interventions were considered.
Outcomes of this review
Primary outcomes of this review were the number and/or duration of SCD-related pain days, VOCs, or hospital admissions for VOCs. Secondary outcomes included time to first VOC or hospital admission for a VOC, intensity of pain (as expressed in pain scores), quality of life, and adverse events.
Data extraction and quality assessment
Two reviewers independently screened all titles and abstracts identified in our search using the Covidence screening tool. The full text of all potentially relevant studies was obtained. Again, 2 authors independently assessed the text for final eligibility for inclusion in this review.
A standardized and piloted electronic data extraction sheet was developed based on the checklist described in the Cochrane Handbook for Systematic Reviews of Interventions (chapter 7.3).30 In summary, data were collected on the study (eg, date and location of study), population, intervention, control, and defined outcomes. In addition, data on all results within the scope of this review were collected. A data extraction sheet template is included in supplemental Appendix II. Data extraction was performed by 1 author using predefined data fields and checked for accuracy by a second author.
Two authors independently assessed the methodological quality of the studies using the Cochrane collaboration’s risk-of-bias tool (chapter 8.5).30 In summary, the following 6 criteria were used: randomization sequence generation, concealment of treatment allocation, blinding of participants, clinicians and outcome assessors to treatment allocation, completeness of the outcome data, and selective outcome reporting. Each criterion received a score of high, unclear, or low risk of bias. In the main results, we summarize these 6 criteria as a single risk-of-bias score from 0 to 6. The higher this score, the more items were marked with a low risk of bias. Any other potential bias is reported separately, such as uncorrected baseline imbalances, industry funding, potential conflicts of interest of the authors, or potential carryover effect in crossover trials.
Any disagreements between reviewers concerning study selection, data extraction, or quality assessment were resolved by discussion or consultation with a third reviewer.
Data synthesis and analysis
All included studies were grouped according to the pathophysiological mechanism of action and are presented in table format. Relative effect measures were calculated and reported if the required data were available. For dichotomous outcomes, odds ratios with their 95% confidence intervals were calculated. For continuous variables, mean differences with corresponding confidence intervals were calculated. If these could not be calculated, results are shown as reported in the study. Because of the high clinical diversity and the small number of studies per intervention, all findings are presented solely narratively; no meta-analysis was performed.
Role of the funding source
All sponsors were public or nonprofit organizations that support science in general. They had no role in the design or execution of this review, the writing of the manuscript, or the decision to submit the paper for publication.
Results
Selection of articles
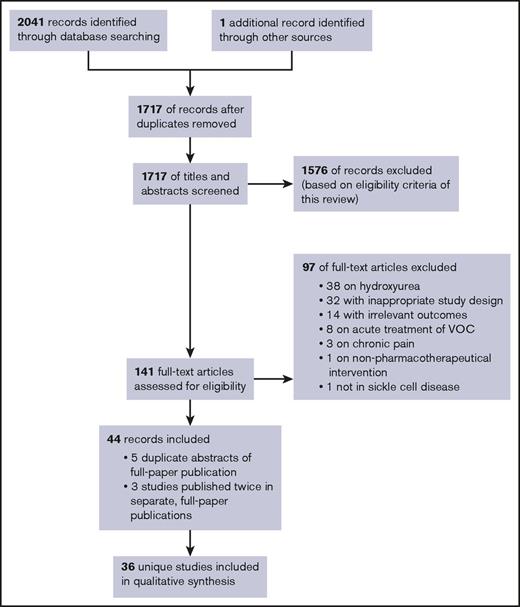
Our database searches identified a total of 1717 unique records for initial abstract screening, of which 141 were selected for full-text screening (Figure 1). Ninety-seven studies were excluded for various reasons, as noted in Figure 1 (see supplemental Appendix III for a full list of the excluded studies). Five of the included records involved conference abstracts of studies that were published separately as full papers (duplicates).31-35 Six included records involved 3 distinct studies, all published twice as separate, full-paper publications.36-41 The remaining total of 36 unique studies was included in this review, covering 26 different prophylactic interventions.
PRISMA flow diagram. Flowchart of publications included in this review. Our database searches identified a total of 1,717 unique records for initial abstract screening, of which 141 were selected for full-text screening. Subsequently, 97 studies were excluded for various reasons. Five of the included records involved conference abstracts of studies that were published separately as full papers (duplicates). Six included records involved 3 distinct studies that were all published twice as separate, full-paper publications. A remaining total of 36 unique studies were included in this review, covering 26 different prophylactic interventions.
PRISMA flow diagram. Flowchart of publications included in this review. Our database searches identified a total of 1,717 unique records for initial abstract screening, of which 141 were selected for full-text screening. Subsequently, 97 studies were excluded for various reasons. Five of the included records involved conference abstracts of studies that were published separately as full papers (duplicates). Six included records involved 3 distinct studies that were all published twice as separate, full-paper publications. A remaining total of 36 unique studies were included in this review, covering 26 different prophylactic interventions.
In the further presentation of our results, we have classified treatments per main mechanism of action: anticoagulation, antioxidants, antiadhesion, antisickling, and other interventions. Treatments with multiple pathophysiological targets were classified by their primary mechanism of action. The included trials were published between 1968 and 2016, of which 15 trials were published before the year 200037,42-55 and 8 in the last 5 years.56-63 Three studies did not use a placebo intervention for their control group.43,54,56 A majority of studies were performed in North America (n = 10),33,46,47,51,61,62,64-68 Africa (n = 9),38,42,52,55,56,59,69-71 and Latin America (n = 8).40,44,48,49,53,54,57,72 Four studies were performed on multiple continents.58,60,63,73 The number of patients per study ranged from 9 to 341. A majority of studies were limited to patients with the HbSS or HbSβ0 genotype (n = 26).37,38,42,44,45,48-57,59-61,63,64,66,67,69-71,74
A summary overview of the characteristics, risk of bias, and primary outcomes of the included studies is provided in Table 1. The complete results of our risk-of-bias assessment and a detailed overview of the primary and secondary outcomes of this review per study are provided in supplemental Tables 2 and 3, respectively.
Overview of included trials
| Study, reference (location) . | Design (phase) . | Participants . | Duration of intervention . | Intervention vs comparator (n of randomly assigned patients) . | Risk of bias score . | Primary review outcomes . | Measure of effect . | |
|---|---|---|---|---|---|---|---|---|
| Main criteria . | Age, y; male sex, %; HU, % . | |||||||
| Anticoagulation | ||||||||
| Aspirin | ||||||||
| 51 (United States) | CT, crossover (phase 2) | HbSS, HbSβ0, children | Mean, 7.7; 37%; NA | 21 months (crossover at 6 and 18 months, no washout) | Aspirin 3-6 mg/kg in tablets of 81 mg bd (n = 49*) orally | 3/6 | Days per painful event, mean (SD): 3.9 (±1.4) during intervention phase vs 3.2 (±1.6) during placebo phase | NA† |
| Placebo (n = 49*), oral | ||||||||
| Cumulative n of crises: 43 during intervention phase vs 49 during placebo phase | NA† | |||||||
| Cumulative n of days in pain: 140 during intervention phase vs 153 during placebo phase | NA† | |||||||
| 49 (Brazil) | RCT, crossover (phase 2) | HbSS, HbSβ0 | Range, 4-31; NA; NA | 10 months (5 months per treatment, no washout) | Aspirin 17-45 mg/kg od (n = 29*) orally | 0/6 | Frequency of painful events: 40 events in 17 patients during aspirin vs 40 events in 16 patients during placebo treatment | NA† |
| Placebo (n = 29*) orally | ||||||||
| Ticlopidine | ||||||||
| 55 (Senegal, Cote d'Ivoire, Benin) | RCT (phase 2) | HbSS | Range, 15-45; NA; NA | 6 months | Ticlopidine 250 mg <45 kg bd, >45 kg td (n = 70) orally | 5/6 | Total n of crises during 6-month study observation: 42 crises in 70 patients in ticlopidine (1.2 crises per patient-year) vs 125 crises in 70 patients in placebo group (3.5 crises per patient-year) | P < .001‡ |
| Placebo (n = 70) orally | ||||||||
| Pentoxifylline | ||||||||
| 44 (Brazil) | RCT (phase 2) | HbSS | Mean, 17.7§; 67§; NA | 6 weeks | Pentoxifylline ≥18 years 400 mg td, <18 years 400-600 mg per day depending on body weight (n = 28*) orally | 1/6 | N of patients with pain events: | OR (CI): 0.18 (0.05-0.67); P < .01|| |
| Observation days 2-7: 15 (54%) of 28 patients in pentoxifylline group vs 25 (86%) of 29 patients in control group | ||||||||
| Placebo (n = 29*) orally | Observation days 8-42: 14 (50%) of 28 patients in intervention group vs 25 (86%) of 29 patients in placebo group | OR (CI): 0.09 (0.03-0.31); P < .01|| | ||||||
| N of pain events per group: | P < .05‡ | |||||||
| Observation days 2-7: 32 in pentoxifylline group vs 60 in placebo group | ||||||||
| Observation days 8-42: 57 in intervention group vs 219 in placebo group | P < .05‡ | |||||||
| Acenocoumarol | ||||||||
| 72 (Netherlands, Antilles) | RCT, crossover (phase 2) | HbSS, HbSC, HbSβ; age ≥16 years | Mean, 37 (HbSS group); NA; NA | 33 weeks (14 weeks per treatment, 5-week washout in between) | Acenocoumarol 1 mg, dosage based on targeted INR range of 1.6-2.0 (n = 22*) orally | 6/6 | Frequency of acute vaso-occlusive events: 3 events in acenocoumarol vs 5 events in placebo group | NS‡ |
| Placebo (n = 22*) orally | ||||||||
| Prasugrel | ||||||||
| 32, 33, 62 (United States, Canada) | RCT (phase 2) | HbSS, HbSC, HbSβ0/+; age 18-55 years | Mean, 32.9§; 49§; 44§ | 30 days (+ 30-day follow-up) | Prasugrel 5 mg od (n = 41) orally | 4/6 | Proportion of patients reporting no pain or infrequent pain: 50% in prasugrel vs 22% in placebo group | NS‡ |
| Placebo (n = 21) orally | N of patients having painful episodes: 9 (23%) of 40 patients in prasugrel vs 7 (37%) of 19 patients in placebo group | OR (CI): 0.50 (0.15-1.64); NS|| | ||||||
| Proportion of days with pain, least-squares mean: 42% in prasugrel vs 54% in placebo group | NS‡ | |||||||
| 60 (United States, United Kingdom, Ghana, Saudi Arabia, Lebanon, Kenya, Italy, Egypt, Canada, Belgium, Brazil, Turkey, Oman) | RCT (phase 3) | HbSS, HbSβ0; age 2-17 years; ≥2 crises in past yr | Mean, 10.6§; 49§; 45§ | 9-24 months | Prasugrel 0.04-0.12 mg/kg od (n = 171) orally | 6/6 | N of patients with painful crises: 113 (66%) of 171 patients in prasugrel vs 122 (72%) of 170 patients in placebo group | OR (CI): 0.77 (0.48-1.21); NS|| |
| Placebo (n = 170) orally | N of patients with VOCs: 115 (67%) of 171 patients in prasugrel vs 123 (72%) of 170 patients in placebo group | OR (CI): 0.78 (0.49-1.25); NS|| | ||||||
| N of patients with hospital admission: 69 (40%) of 171 patients in prasugrel vs 76 (45%) of 170 patients in placebo group | OR (CI): 0.84 (0.54-1.29); NS|| | |||||||
| Monthly rate of daily pain, mean (SD): 17.5 (NA) in prasugrel group vs 17.7 (NA) in placebo group | Mean difference (CI): 0.24 (4.6–4.1); NS‡ | |||||||
| Antioxidants | ||||||||
| ω-3 fatty acids | ||||||||
| 31, 65 (United States) | RCT (phase 2) | Any sickle-cell syndrome; adults; ≥3 pain episodes per year | NA; NA; 0 | 6-12 months | Fish oil 0.25 g/kg per day in 3 daily doses containing 0.1 g/kg per day ω-3 fatty acids (EPA and DHA; n = 5*) orally | 3/6 | Frequency of pain episodes per year, mean: 3.8 episodes in ω-3 fatty acids vs 7.1 episodes in placebo group | P < .01‡ |
| Placebo (n = 5*) orally | ||||||||
| 59 (Sudan) | RCT (phase 2) | HbSS; age 2-24 years | Mean, 8.1§; 59§; 0 | 1 year | ω-3 fatty acids (EPA and DHA) 25 mg/kg od (n = 70) orally | 6/6 | Annual VOC rate, median (IQR): | P < .01‡ |
| 2.7 (0.9-4.8) in ω-3 vs 4.6 (3.0-6.4) in placebo group | ||||||||
| Placebo (n = 70) orally | Annual clinical VOC rate, median (IQR): 0 (0-0.9) in ω-3 vs 1 (0-2.4) in placebo group | P < .001‡ | ||||||
| N of hospitalization days, median (IQR): 0 (6) in ω-3 vs 0 (6) in placebo group | P < .05‡ | |||||||
| N-acetylcysteine | ||||||||
| 64 (United States) | RCT (phase 2) | HbSS, HbSβ0; age ≥15 years; ≥2 VOCs per year in past 2 years | Mean, 18.1§; 60§; NA | 7 months | N-acetylcysteine in 3 daily doses orally: 600 mg (n = 5*), 1200 mg (n = 5*), 2400 mg (n = 6*) | 3/6 | N of acute VOC episodes per person-days, mean: 0.006 episodes in 2400 mg N-acetylcysteine vs 0.011 in 1200 mg vs 1.24 in 600 mg vs 0.017 in placebo group | NA† |
| Placebo (n = 5*) orally | ||||||||
| Vitamin C-E | ||||||||
| 57 (Brazil) | RCT (phase 2) | HbSS, HbSβ0; adults | Median, 28.3§; 34§; 57§ | 6 months | Vitamin C 1400 mg and vitamin E 800 mg od (n = 44*) orally | 3/6 | Incidence of acute SCD complications: 16 (36%) of 44 patients in vitamin C-E vs 12 (31%) of 39 patients in placebo group | OR (CI): 1.29 (0.51-3.21); NS|| |
| Placebo (n = 39*) orally | ||||||||
| l-glutamine | ||||||||
| 34, 61 (United States) | RCT (phase 3) | HbSS, HbSβ0; age ≥5 years; ≥2 crises in past 12 months | Range, 5-58; 46; HU+ (% NA, stratified) | 48 weeks (+ 3-week tapering + 2-week follow-up) | l-glutamine: 0.6g/kg per day in 2 doses (n = 152) orally | 1/6 | N of sickle cell crises, median: 3 in l-glutamine vs 4 in placebo group | P = .008‡ |
| Placebo (n = 78) orally | N of hospitalizations, median: 2 in l-glutamine group vs 3 in placebo group | P = .005‡ | ||||||
| Cumulative n of hospital days, median: 6.5 in l-glutamine group vs 11 in placebo group | P = .022‡ | |||||||
| Antiadhesion | ||||||||
| Crizanlizumab | ||||||||
| 58 (United States, Brazil, Jamaica) | RCT (phase 2) | Any SCD genotype (including HbSS, HbSC, HbSβ0/+); age 16-65 years; 2-10 VOCs in 12 months before enrollment | Median, 29§; 48§; 63§ | 52 weeks | Crizanlizumab, 2 loading doses 2 weeks apart, then every 4 weeks IV: high dose, 5 mg/kg (n = 67); low dose, 2.5 mg/kg (n = 66) | 6/6 | Annual rate of sickle-cell–related pain crises, median (IQR): 1.63 (0.00-3.97) in high-dose vs 2.01 (1.25-5.87) in low-dose vs 2.98 (1.25-5.87) in placebo group | High dose vs placebo: −45.3%; P = .01‡; low dose vs placebo: −32.6%; NS‡ |
| Annual rate of uncomplicated sickle-cell–related pain crises: 1.08 (0.00-3.96) in high-dose vs 2.00 (0.00-3.02) in low-dose vs 2.91 (1.00-5.00) in placebo group | High dose vs placebo: −62.9%; P = .02‡; low dose vs placebo: 31.3%; NS‡ | |||||||
| Annual rate of days hospitalized, median (IQR): 4.00 (0.00-25.72) in high-dose vs 6.87 (0.00-28.30) in low-dose vs 6.87 (0.00-28.30) in placebo group | High dose vs placebo: −41.8%; NS‡; low dose vs placebo: 0%; NS‡ | |||||||
| Placebo (n = 65) IV | ||||||||
| Antisickling | ||||||||
| Promazine hydrochloride | ||||||||
| 46 (United States) | RCT, crossover (phase 2) | HbSS, HbSC; ≥2 painful episodes in past 2 years | NA; 64; NA | 2 years (crossover every 3 months, no washout) | Promazine hydrochloride 25 mg, dosage based on body weight; 40-80 lb bd, 80-120 lb td, ≥120 lb daily (n = 14) orally | 3/6 | N of hospital admissions for painful episodes per patient-month: 0.09 admissions (based on 16 admissions over 180 patient-months) in promazine hydrochloride group vs 0.05 admissions (based on 8 admissions over 152 patient-months in placebo group) | NA† |
| Placebo (n = 14) orally | N of diary recorded painful episodes per patient-month: 0.22 episodes (based on 40 episodes over 180 patient-months) in promazine hydrochloride group vs 0.28 episodes (based on 42 episodes over 152 patient-months) in placebo group | NA† | ||||||
| Steroids (including oral contraceptives) | ||||||||
| 42 (Nigeria) | RCT, crossover (phase 2; preliminary report before crossover) | HbSS; ≥1 episode of moderately severe pain in 3 months | Range, 2-35; 36; NA | 4-6 months | Men, testosterone 10 mg once per week im; women, progesterone 10 mg once per week im (n = 35*) | 1/6 | Primary review outcome: NA | |
| Secondary review outcomes: | ||||||||
| Pain score, mean (range): 57 (0-330) in testosterone/progesterone vs 153 (130-200) in placebo group | NA† | |||||||
| Placebo (n = 9*) im | ||||||||
| 53 (Jamaica) | RCT, crossover (phase 2) | HbSS; female sex | Range, 20-40; 0; NA | 2 years (9 months per treatment, 6-month washout in between) | Medroxyprogesterone 150 mg every 3 months (n = 10*) im | 1/6 | Primary review outcome: NA | |
| Placebo (saline; n = 13*) im | Secondary review outcomes: | |||||||
| Bone pain episodes: 14 (61%) of 23 in medroxyprogesterone vs 20 (87%) of 23 in placebo group | OR (CI): 0.23 (0.05-1.01); NS|| | |||||||
| Severity score of bone pain episodes, mean: 2.0 in medroxyprogesterone vs 1.8 in placebo group | NA† | |||||||
| 54 (Panama) | CT (phase 2) | HbSS; 1 crisis per month; female sex; desire for reversible method of contraception | Range, 17-39; 0; NA | 12 months | Levonorgestrel 0.15 mg and ethinyl estradiol 0.03 mg od (n = 14) orally | 0/6 | Proportion of patients with painful crisis at end of study: 50% in control group vs 30% of progestogen-only group vs 45.5% of combined oral contraceptive group | NA† |
| Medroxyprogesterone acetate 150 mg, first 3 months monthly, last 9 months every 3 months (n = 13) im | ||||||||
| Surgically sterilized (n = 16) | ||||||||
| Sodium bicarbonate | ||||||||
| 43 (United Kingdom) | RCT, crossover (phase 2) | HbSS, HbSC, HbSβ0/+; age 5-17 years | Mean, 8.4; 33; NA | 2 years (crossover after 1 year, no washout) | Sodium bicarbonate 0.1-0.4 mg/kg od, dosage based on targeted urine alkalinization (n = 18) orally | 1/6 | N of crises per 50 patient-weeks, mean (SD): 2.2 (±1.8) in sodium bicarbonate vs 3.0 (±2.3) in routine group | Mean difference (CI): −0.8 (−2.2-0.6); NS|| |
| Routine care (n = 18) | ||||||||
| Urea | ||||||||
| 52 (Ghana) | RCT (phase 2) | HbSS | <5 years, 23%§; 5-14 years, 38%§; >14 years, 40%§; 47§; NA | Average, 13.7 months | Urea 0.266 g/kg (n = 40) orally: low dose twice per week; high dose daily | 5/6 | N of patients with >1 crisis: 18 (45%) of 40 of urea vs 15 (38%) of 40 of placebo group | OR (CI): 1.4 (0.6-3.3); NS|| |
| N of patients with >2 crises: 8 (20%) of 40 of urea vs 5 (13%) of 39 of placebo group | OR (CI): 1.7 (0.5-5.7); NS|| | |||||||
| Crisis rate per person-year in patients with >1 crisis, mean: 0.596 in urea vs 0.506 in placebo group | Crisis rate ratio, 0.849 (placebo vs urea); NS‡ | |||||||
| Placebo (n = 39) orally | Crisis rate per person-year in patients with >2 crises, mean: 0.189 in urea vs 0.136 in placebo group | Crisis rate ratio, 0.720 (placebo vs urea); NS‡ | ||||||
| Piracetam | ||||||||
| 45 (Lebanon) | RCT, crossover (phase 2) | Sickle-cell anemia; children | Range, 4-15; 44; NA | 10 months (5 months per treatment, no washout) | Piracetam 80 mg/kg per day in 4 daily doses (n = 9*) orally | 2/6 | N of pain crises per patient per month, average: 0.89 in piracetam group vs 1.85 in placebo | P < .05‡ |
| Placebo (n = 9*) orally | ||||||||
| 36, 37 (Saudi Arabia) | RCT (phase 2) | HbSS, HbSβ0, children | Range, 3-12; 57; NA | 1 year | Piracetam 160 mg/kg per day (n = 48*) orally; during admission 300 mg/kg per day IV | 2/6 | N of painful crisis per year, mean (SD): 2.4 (±1.9) in piracetam vs 4.3 (±3.1) in placebo group | Mean difference (CI): −1.9 (−3.0 to −0.8); P < .001|| |
| Placebo (n = 39*) orally; during admission IV | N of hospitalizations per year in total group, mean (SD): 2.07 (±2.4) in piracetam group vs 3.20 (±2.9) in placebo group | Mean difference (CI): −1.1 (−2.3-0.0); P < .05|| | ||||||
| 40, 41 (Brazil) | RCT, crossover (phase 2) | HbSS, HbSC, HbSβ0/+; age 5-20 years | Median, 12.1; 45; 0 | 13 months (6 months per treatment, 1-month washout in between) | Piracetam 4.8g/m2 per day in 4 daily doses (n = 73*) orally | 1/6 | Absolute n of painful VOCs, median: 3 in both groups | NS‡ |
| Placebo (n = 73*) orally | N of pain days per month, median: 1.47 in both groups | NS‡ | ||||||
| Hospitalization days: data NA | NS‡ | |||||||
| Niprisan | ||||||||
| 38, 39 (Nigeria) | RCT, crossover (phase 2) | HbSS; ≥3 crises in past year; age 2-45 years | Mean, 15.5; 45; NA | 12 months (6 months per treatment, no washout) | Niprisan 12 mg/kg od (n = 70*) | 3/6 | N of episodes of mild to moderate pain over first 6 months, mean (SD): 9.1 (±9.6) in niprisan vs 20.6 in placebo group (±32.9) | NS‡ |
| Placebo (n = 70*) | N of episodes of severe pain first 6 months, mean (SD): 7.9 (±8.4) in niprisan vs 22.1 (±32.9) in placebo group | P < .05‡ | ||||||
| N of painful crises first 6 months, mean (SD): 0.27 (±0.6) in niprisan vs 1.17 (±1.8) in placebo group | NS‡ | |||||||
| N of hospital admissions per month in first 6 months, mean (SD): 0.15 (±0.6) in niprisan vs 0.22 (±0.6) in placebo group | NS‡ | |||||||
| NB. No summary statistics possible because of skewed data | ||||||||
| Ciklavit | ||||||||
| 71 (Nigeria) | RCT (phase 2) | HbSS; age 1-15 years | Mean, 8.09§; 57§; NA | 6 months | Ciklavit ≤5 years 10 mL twice daily, >5 years 20 mL bd (n = 47*) | 3/6 | N of painful crises in 6 months, mean (SD): 4.2 (4.2) in Ciklavit vs 3.9 (4.3) in placebo group | Mean difference (CI): 0.30 (−1.49-2.09); NS|| |
| Placebo (n = 42*) | ||||||||
| Cromolyn sodium | ||||||||
| 74 (Iran) | CT, crossover (phase 2) | HbSS | Range, 5-34; 41; HU was part of intervention | 12 months (crossover every 3 months, unclear washout) | Subsequent treatment regimens 1-4 (n = 17*): (1) cromolyn sodium nasal spray 2%, 4 puffs each nostril, frequency NA; (2) placebo nasal spray; (3) as in 1, plus HU 10-15 mg/kg per day orally; and (4) as in 2, plus HU 10-15 mg/kg per day orally | 0/6 | Primary review outcome: NA | |
| Secondary review outcomes: | ||||||||
| Pain score: data NA, pain score in cromolyn sodium and HU group lower than cromolyn sodium–only group | P < .05‡ | |||||||
| Other comparisons between groups | NS‡ | |||||||
| Senicapoc | | | |||||||
| 66 (United States) | RCT (phase 2) | HbSS; age 18-60 years | Mean, 37.2§; 59§; 27 | 12 weeks | Senicapoc loading dose on day 1, subsequently: low dose, 6 mg od (n = 29) orally; high dose, 10 mg od (n = 31) orally | 6/6 | N of patients with painful crisis: 5 (17%) of 29 in low-dose senicapoc vs 5 (16%) of 31 in high-dose senicapoc vs 5 (17%) of 30 in placebo group | OR (CI): low dose vs placebo, 1.04 (0.27-4.06); NS||; high dose vs placebo, 0.96 (0.24-3.73); NS|| |
| Placebo (n = 30) orally | ||||||||
| 73 (United States, United Kingdom, Jamaica, Brazil, France, Trinidad) | RCT (phase 3) | HbSS, HbSC, HbSβ0/+; age 16-65 years; ≥2 crises in past 12 months | Mean, 28.5§; 43§; 58§ | 52 weeks | Senicapoc 1 mg od, initial loading dose 20 mg bd for 4 days (n = 145*) orally | 6/6 | N of patients with acute sickle-cell–related painful crises: 106 (73%) of 145 in senicapoc vs 89 (62%) of 144 in placebo group | OR (CI): 1.68 (1.02-2.76); P = .045|| |
| Placebo (n = 144*) orally | ||||||||
| Painful crisis rate, mean (SD): 0.38 (±0.36) in senicapoc group vs 0.31 (±0.48) in placebo group | Mean difference (CI): 0.07 (−0.03-0.17); NS|| | |||||||
| N of days in hospital per patient, mean: 18.6 in senicapoc group vs 15.1 in placebo group | P = .03‡ | |||||||
| Magnesium pidolate | ||||||||
| 68 (United States) | RCT (phase 2) | HbSC; age ≥2 years; ≥1 vaso-occlusive event in past 12 months | Mean, 13.6; 57; HU was part of intervention | 44 weeks | HU 20 mg/kg per day plus magnesium pidolate 0.3 mmol/kg per day bd orally | 2/6 | N of vaso-occlusive pain crises: no significant differences observed between treatment groups (data NA) | NA† |
| HU 20 mg/kg per day plus placebo orally | ||||||||
| Placebo plus magnesium pidolate 0.3 mmol/kg per day bd orally | ||||||||
| Placebo plus placebo orally | ||||||||
| N per group, NA (total N = 44) | ||||||||
| Lime juice | ||||||||
| 56 (Nigeria) | RCT (phase 2) | Sickle-cell anemia; children | Mean, 4.6§; 53§; 0 | 6 months | Lime juice td ≤10 kg 5 mL, 11-20 kg 10 mL, >20 kg 15 mL (n = 58*) | 1/6 | N of children with significant painful episodes: 29 (50%) of 58 in lime juice vs 51 (93%) of 55 in routine care group | OR (CI): 0.078 (0.02-0.24); P < .001|| |
| Routine care (n = 55*) | ||||||||
| N of children with hospitalizations: 2 (3%) of 58 in lime juice vs 19 (35%) of 55 in routine care group | OR (CI): 0.068 (0.01-0.3); P < .001|| | |||||||
| Frequency of significant painful episodes, mean (SD): 0.64 (±0.11) in lime juice vs 1.51 (±0.34) in routine care group | Mean difference (CI): −0.87 (−0.96 to −0.78); P < .001|| | |||||||
| Frequency of hospitalizations, mean (SD): 0.03 (±0.01) in lime juice vs 0.35 (±0.07) in routine care group | Mean difference (CI): −0.32 (−0.33 to −0.30); P < .001|| | |||||||
| HQK-1001 | ||||||||
| 63 (United States, Egypt, Jamaica, Canada, Lebanon) | RCT (phase 2) | HbSS, HbSβ0; age 12-60 years; ≥1 acute SCD-related event in past 12 months | Mean, 27.8§; 34§; 0 | 48 weeks | HQK-1001 15 mg/kg bd (n = 38*) orally | 4/6 | Patients with at least 1 pain crisis: 22 (58%) of 38 in HQK-1001 vs 19 (50%) of 38 in placebo group | OR (CI): 1.38 (0.56-3.40); NS|| |
| Placebo (n = 38*) orally | Patients with acute chest syndrome: 6 (16%) of 38 in HQK-1001 vs 2 (5%) of 38 in placebo group | OR (CI): 3.4 (0.6-17.9); NS|| | ||||||
| Annualized rate of pain crises, mean (SD): 3.5 (±5.2) in HQK-1001 group vs 1.7 (±2.2) in placebo group | Mean difference (CI): 1.80 (−0.03-3.63); NS|| | |||||||
| Other interventions | ||||||||
| Folate | ||||||||
| 48 (Jamaica) | CT (phase 2) | HbSS; age 0.5-4 years | Range, 0.5-4; 56; NA | 1 year | Folate 5 mg od (n = 59*) | 3/6 | Patients with painful episodes: 22 (37%) of 59 in folate vs 18 (32%) of 56 in placebo group | OR (CI): 1.26 (0.58-2.71); NS|| |
| Placebo (n = 56*) | ||||||||
| Zinc | ||||||||
| 50 (India) | RCT (phase 2) | HbSS; age >5 years | Mean, 16.4§; 71§; NA | 1.5 years | Zinc sulfate 220 mg td (n = 65*) | 4/6 | N of crisis episodes, mean (SD): 2.46 (±1.04) in zinc vs 5.29 (±2.58) in placebo group | Mean difference (CI): −2.83 (−3.51 to −2.15); P < .0001|| |
| Placebo (n = 65*) | ||||||||
| 47 (United States) | RCT, partial crossover (phase 2) | HbSS, HbSC, HbSβ; adults | Range, 19-49; 50; NA | 3 years (excluding 1-year follow-up prior) | Zinc acetate 50-75 mg daily (n = 11; zinc deficient) orally | 2/6 | Incidence of hospital admission, mean (SD): 6.90 (±3.60) in zinc vs 5.36 (±5.30) in routine care vs 8.00 (±5.2) in placebo group | Mean difference (CI): zinc vs routine care, 1.54 (−2.49-5.57); NS||; zinc vs placebo, −1.1 (−5.15-2.95); NS|| |
| Placebo with crossover to zinc acetate 50-75 mg daily after 1 year (n = 10; zinc deficient) orally | ||||||||
| Routine care (n = 11; zinc sufficient) | Incidence of VOCs, mean (SD): 6.72 (±3.60) in zinc vs 5.18 (±4.80) in routine care vs 7.6 (±5.4) in placebo group | Mean difference (CI): zinc vs routine care, 1.54 (−2.23-5.31); NS||; zinc vs placebo, −0.88 (−5.03-3.27); NS|| | ||||||
| 67 (United States) | RCT (phase 2) | HbSS; adults | Mean, 33.6§; 61§; 0 | 3 months | Zinc acetate 25 mg td (n = 18) orally | 4/6 | N of sickle pain episodes: 1 episode in 18 patients in zinc vs 3 episodes in 18 patients in placebo group | NA† |
| Placebo (n = 18) orally | ||||||||
| Antimalarials | ||||||||
| 70 (Nigeria) | RCT, open label (phase 2) | HbSS; age 1-16 years | Mean, 8.1§; 52§; NA | 9 months | Antimalarial prophylaxis | 4/6 | N of patients with bone pain crisis during study: 2 (3%) of 36 in pyrimethamine vs 0 (0%) of 32 in proguanil vs 5 (17%) of 29 in placebo group | OR (CI): pyrimethamine vs placebo, 0.28 (0.05-1.58); NS||; proguanil vs placebo, NA; Fisher’s exact P = .02||; pyrimethamine vs placebo, 0.09 (0.02-0.48); P = .001||; proguanil vs placebo, 0.30 (0.09-1.02); NS|| |
| Pyrimethamine 0.5 mg/kg once per week (n = 36*) orally | ||||||||
| Proguanil 1.5 mg/kg od (n = 32*) orally | N of patients with hospitalizations during study: 2 (6%) of 36 in pyrimethamine vs 5 (16%) of 32 in proguanil vs 11 (38%) of 29 in placebo group | |||||||
| Placebo (vitamin C 7 mg/kg od; n = 29*) orally | ||||||||
| 35, 69 (Senegal) | RCT (phase 2) | Sickle-cell anemia | Mean, 24.5§; 47§; NA | 2 months | Seasonal intermittent antimalarial treatment with sulfadoxine 25 mg/kg and pyrimethamine 1.25 mg/kg once per month (n = 30) orally | 6/6 | N of VOC per year, total: 5 in sulfadoxine-pyrimethamine vs 5 in placebo group | Relative risk (CI): 1.0 (0.3-3.1); NS‡ |
| Placebo (n = 30) orally | ||||||||
| N of hospitalizations, total: 5 in sulfadoxine-pyrimethamine vs 5 in placebo group | Relative risk (CI): 1.0 (0.3-3.1); NS‡ | |||||||
| Study, reference (location) . | Design (phase) . | Participants . | Duration of intervention . | Intervention vs comparator (n of randomly assigned patients) . | Risk of bias score . | Primary review outcomes . | Measure of effect . | |
|---|---|---|---|---|---|---|---|---|
| Main criteria . | Age, y; male sex, %; HU, % . | |||||||
| Anticoagulation | ||||||||
| Aspirin | ||||||||
| 51 (United States) | CT, crossover (phase 2) | HbSS, HbSβ0, children | Mean, 7.7; 37%; NA | 21 months (crossover at 6 and 18 months, no washout) | Aspirin 3-6 mg/kg in tablets of 81 mg bd (n = 49*) orally | 3/6 | Days per painful event, mean (SD): 3.9 (±1.4) during intervention phase vs 3.2 (±1.6) during placebo phase | NA† |
| Placebo (n = 49*), oral | ||||||||
| Cumulative n of crises: 43 during intervention phase vs 49 during placebo phase | NA† | |||||||
| Cumulative n of days in pain: 140 during intervention phase vs 153 during placebo phase | NA† | |||||||
| 49 (Brazil) | RCT, crossover (phase 2) | HbSS, HbSβ0 | Range, 4-31; NA; NA | 10 months (5 months per treatment, no washout) | Aspirin 17-45 mg/kg od (n = 29*) orally | 0/6 | Frequency of painful events: 40 events in 17 patients during aspirin vs 40 events in 16 patients during placebo treatment | NA† |
| Placebo (n = 29*) orally | ||||||||
| Ticlopidine | ||||||||
| 55 (Senegal, Cote d'Ivoire, Benin) | RCT (phase 2) | HbSS | Range, 15-45; NA; NA | 6 months | Ticlopidine 250 mg <45 kg bd, >45 kg td (n = 70) orally | 5/6 | Total n of crises during 6-month study observation: 42 crises in 70 patients in ticlopidine (1.2 crises per patient-year) vs 125 crises in 70 patients in placebo group (3.5 crises per patient-year) | P < .001‡ |
| Placebo (n = 70) orally | ||||||||
| Pentoxifylline | ||||||||
| 44 (Brazil) | RCT (phase 2) | HbSS | Mean, 17.7§; 67§; NA | 6 weeks | Pentoxifylline ≥18 years 400 mg td, <18 years 400-600 mg per day depending on body weight (n = 28*) orally | 1/6 | N of patients with pain events: | OR (CI): 0.18 (0.05-0.67); P < .01|| |
| Observation days 2-7: 15 (54%) of 28 patients in pentoxifylline group vs 25 (86%) of 29 patients in control group | ||||||||
| Placebo (n = 29*) orally | Observation days 8-42: 14 (50%) of 28 patients in intervention group vs 25 (86%) of 29 patients in placebo group | OR (CI): 0.09 (0.03-0.31); P < .01|| | ||||||
| N of pain events per group: | P < .05‡ | |||||||
| Observation days 2-7: 32 in pentoxifylline group vs 60 in placebo group | ||||||||
| Observation days 8-42: 57 in intervention group vs 219 in placebo group | P < .05‡ | |||||||
| Acenocoumarol | ||||||||
| 72 (Netherlands, Antilles) | RCT, crossover (phase 2) | HbSS, HbSC, HbSβ; age ≥16 years | Mean, 37 (HbSS group); NA; NA | 33 weeks (14 weeks per treatment, 5-week washout in between) | Acenocoumarol 1 mg, dosage based on targeted INR range of 1.6-2.0 (n = 22*) orally | 6/6 | Frequency of acute vaso-occlusive events: 3 events in acenocoumarol vs 5 events in placebo group | NS‡ |
| Placebo (n = 22*) orally | ||||||||
| Prasugrel | ||||||||
| 32, 33, 62 (United States, Canada) | RCT (phase 2) | HbSS, HbSC, HbSβ0/+; age 18-55 years | Mean, 32.9§; 49§; 44§ | 30 days (+ 30-day follow-up) | Prasugrel 5 mg od (n = 41) orally | 4/6 | Proportion of patients reporting no pain or infrequent pain: 50% in prasugrel vs 22% in placebo group | NS‡ |
| Placebo (n = 21) orally | N of patients having painful episodes: 9 (23%) of 40 patients in prasugrel vs 7 (37%) of 19 patients in placebo group | OR (CI): 0.50 (0.15-1.64); NS|| | ||||||
| Proportion of days with pain, least-squares mean: 42% in prasugrel vs 54% in placebo group | NS‡ | |||||||
| 60 (United States, United Kingdom, Ghana, Saudi Arabia, Lebanon, Kenya, Italy, Egypt, Canada, Belgium, Brazil, Turkey, Oman) | RCT (phase 3) | HbSS, HbSβ0; age 2-17 years; ≥2 crises in past yr | Mean, 10.6§; 49§; 45§ | 9-24 months | Prasugrel 0.04-0.12 mg/kg od (n = 171) orally | 6/6 | N of patients with painful crises: 113 (66%) of 171 patients in prasugrel vs 122 (72%) of 170 patients in placebo group | OR (CI): 0.77 (0.48-1.21); NS|| |
| Placebo (n = 170) orally | N of patients with VOCs: 115 (67%) of 171 patients in prasugrel vs 123 (72%) of 170 patients in placebo group | OR (CI): 0.78 (0.49-1.25); NS|| | ||||||
| N of patients with hospital admission: 69 (40%) of 171 patients in prasugrel vs 76 (45%) of 170 patients in placebo group | OR (CI): 0.84 (0.54-1.29); NS|| | |||||||
| Monthly rate of daily pain, mean (SD): 17.5 (NA) in prasugrel group vs 17.7 (NA) in placebo group | Mean difference (CI): 0.24 (4.6–4.1); NS‡ | |||||||
| Antioxidants | ||||||||
| ω-3 fatty acids | ||||||||
| 31, 65 (United States) | RCT (phase 2) | Any sickle-cell syndrome; adults; ≥3 pain episodes per year | NA; NA; 0 | 6-12 months | Fish oil 0.25 g/kg per day in 3 daily doses containing 0.1 g/kg per day ω-3 fatty acids (EPA and DHA; n = 5*) orally | 3/6 | Frequency of pain episodes per year, mean: 3.8 episodes in ω-3 fatty acids vs 7.1 episodes in placebo group | P < .01‡ |
| Placebo (n = 5*) orally | ||||||||
| 59 (Sudan) | RCT (phase 2) | HbSS; age 2-24 years | Mean, 8.1§; 59§; 0 | 1 year | ω-3 fatty acids (EPA and DHA) 25 mg/kg od (n = 70) orally | 6/6 | Annual VOC rate, median (IQR): | P < .01‡ |
| 2.7 (0.9-4.8) in ω-3 vs 4.6 (3.0-6.4) in placebo group | ||||||||
| Placebo (n = 70) orally | Annual clinical VOC rate, median (IQR): 0 (0-0.9) in ω-3 vs 1 (0-2.4) in placebo group | P < .001‡ | ||||||
| N of hospitalization days, median (IQR): 0 (6) in ω-3 vs 0 (6) in placebo group | P < .05‡ | |||||||
| N-acetylcysteine | ||||||||
| 64 (United States) | RCT (phase 2) | HbSS, HbSβ0; age ≥15 years; ≥2 VOCs per year in past 2 years | Mean, 18.1§; 60§; NA | 7 months | N-acetylcysteine in 3 daily doses orally: 600 mg (n = 5*), 1200 mg (n = 5*), 2400 mg (n = 6*) | 3/6 | N of acute VOC episodes per person-days, mean: 0.006 episodes in 2400 mg N-acetylcysteine vs 0.011 in 1200 mg vs 1.24 in 600 mg vs 0.017 in placebo group | NA† |
| Placebo (n = 5*) orally | ||||||||
| Vitamin C-E | ||||||||
| 57 (Brazil) | RCT (phase 2) | HbSS, HbSβ0; adults | Median, 28.3§; 34§; 57§ | 6 months | Vitamin C 1400 mg and vitamin E 800 mg od (n = 44*) orally | 3/6 | Incidence of acute SCD complications: 16 (36%) of 44 patients in vitamin C-E vs 12 (31%) of 39 patients in placebo group | OR (CI): 1.29 (0.51-3.21); NS|| |
| Placebo (n = 39*) orally | ||||||||
| l-glutamine | ||||||||
| 34, 61 (United States) | RCT (phase 3) | HbSS, HbSβ0; age ≥5 years; ≥2 crises in past 12 months | Range, 5-58; 46; HU+ (% NA, stratified) | 48 weeks (+ 3-week tapering + 2-week follow-up) | l-glutamine: 0.6g/kg per day in 2 doses (n = 152) orally | 1/6 | N of sickle cell crises, median: 3 in l-glutamine vs 4 in placebo group | P = .008‡ |
| Placebo (n = 78) orally | N of hospitalizations, median: 2 in l-glutamine group vs 3 in placebo group | P = .005‡ | ||||||
| Cumulative n of hospital days, median: 6.5 in l-glutamine group vs 11 in placebo group | P = .022‡ | |||||||
| Antiadhesion | ||||||||
| Crizanlizumab | ||||||||
| 58 (United States, Brazil, Jamaica) | RCT (phase 2) | Any SCD genotype (including HbSS, HbSC, HbSβ0/+); age 16-65 years; 2-10 VOCs in 12 months before enrollment | Median, 29§; 48§; 63§ | 52 weeks | Crizanlizumab, 2 loading doses 2 weeks apart, then every 4 weeks IV: high dose, 5 mg/kg (n = 67); low dose, 2.5 mg/kg (n = 66) | 6/6 | Annual rate of sickle-cell–related pain crises, median (IQR): 1.63 (0.00-3.97) in high-dose vs 2.01 (1.25-5.87) in low-dose vs 2.98 (1.25-5.87) in placebo group | High dose vs placebo: −45.3%; P = .01‡; low dose vs placebo: −32.6%; NS‡ |
| Annual rate of uncomplicated sickle-cell–related pain crises: 1.08 (0.00-3.96) in high-dose vs 2.00 (0.00-3.02) in low-dose vs 2.91 (1.00-5.00) in placebo group | High dose vs placebo: −62.9%; P = .02‡; low dose vs placebo: 31.3%; NS‡ | |||||||
| Annual rate of days hospitalized, median (IQR): 4.00 (0.00-25.72) in high-dose vs 6.87 (0.00-28.30) in low-dose vs 6.87 (0.00-28.30) in placebo group | High dose vs placebo: −41.8%; NS‡; low dose vs placebo: 0%; NS‡ | |||||||
| Placebo (n = 65) IV | ||||||||
| Antisickling | ||||||||
| Promazine hydrochloride | ||||||||
| 46 (United States) | RCT, crossover (phase 2) | HbSS, HbSC; ≥2 painful episodes in past 2 years | NA; 64; NA | 2 years (crossover every 3 months, no washout) | Promazine hydrochloride 25 mg, dosage based on body weight; 40-80 lb bd, 80-120 lb td, ≥120 lb daily (n = 14) orally | 3/6 | N of hospital admissions for painful episodes per patient-month: 0.09 admissions (based on 16 admissions over 180 patient-months) in promazine hydrochloride group vs 0.05 admissions (based on 8 admissions over 152 patient-months in placebo group) | NA† |
| Placebo (n = 14) orally | N of diary recorded painful episodes per patient-month: 0.22 episodes (based on 40 episodes over 180 patient-months) in promazine hydrochloride group vs 0.28 episodes (based on 42 episodes over 152 patient-months) in placebo group | NA† | ||||||
| Steroids (including oral contraceptives) | ||||||||
| 42 (Nigeria) | RCT, crossover (phase 2; preliminary report before crossover) | HbSS; ≥1 episode of moderately severe pain in 3 months | Range, 2-35; 36; NA | 4-6 months | Men, testosterone 10 mg once per week im; women, progesterone 10 mg once per week im (n = 35*) | 1/6 | Primary review outcome: NA | |
| Secondary review outcomes: | ||||||||
| Pain score, mean (range): 57 (0-330) in testosterone/progesterone vs 153 (130-200) in placebo group | NA† | |||||||
| Placebo (n = 9*) im | ||||||||
| 53 (Jamaica) | RCT, crossover (phase 2) | HbSS; female sex | Range, 20-40; 0; NA | 2 years (9 months per treatment, 6-month washout in between) | Medroxyprogesterone 150 mg every 3 months (n = 10*) im | 1/6 | Primary review outcome: NA | |
| Placebo (saline; n = 13*) im | Secondary review outcomes: | |||||||
| Bone pain episodes: 14 (61%) of 23 in medroxyprogesterone vs 20 (87%) of 23 in placebo group | OR (CI): 0.23 (0.05-1.01); NS|| | |||||||
| Severity score of bone pain episodes, mean: 2.0 in medroxyprogesterone vs 1.8 in placebo group | NA† | |||||||
| 54 (Panama) | CT (phase 2) | HbSS; 1 crisis per month; female sex; desire for reversible method of contraception | Range, 17-39; 0; NA | 12 months | Levonorgestrel 0.15 mg and ethinyl estradiol 0.03 mg od (n = 14) orally | 0/6 | Proportion of patients with painful crisis at end of study: 50% in control group vs 30% of progestogen-only group vs 45.5% of combined oral contraceptive group | NA† |
| Medroxyprogesterone acetate 150 mg, first 3 months monthly, last 9 months every 3 months (n = 13) im | ||||||||
| Surgically sterilized (n = 16) | ||||||||
| Sodium bicarbonate | ||||||||
| 43 (United Kingdom) | RCT, crossover (phase 2) | HbSS, HbSC, HbSβ0/+; age 5-17 years | Mean, 8.4; 33; NA | 2 years (crossover after 1 year, no washout) | Sodium bicarbonate 0.1-0.4 mg/kg od, dosage based on targeted urine alkalinization (n = 18) orally | 1/6 | N of crises per 50 patient-weeks, mean (SD): 2.2 (±1.8) in sodium bicarbonate vs 3.0 (±2.3) in routine group | Mean difference (CI): −0.8 (−2.2-0.6); NS|| |
| Routine care (n = 18) | ||||||||
| Urea | ||||||||
| 52 (Ghana) | RCT (phase 2) | HbSS | <5 years, 23%§; 5-14 years, 38%§; >14 years, 40%§; 47§; NA | Average, 13.7 months | Urea 0.266 g/kg (n = 40) orally: low dose twice per week; high dose daily | 5/6 | N of patients with >1 crisis: 18 (45%) of 40 of urea vs 15 (38%) of 40 of placebo group | OR (CI): 1.4 (0.6-3.3); NS|| |
| N of patients with >2 crises: 8 (20%) of 40 of urea vs 5 (13%) of 39 of placebo group | OR (CI): 1.7 (0.5-5.7); NS|| | |||||||
| Crisis rate per person-year in patients with >1 crisis, mean: 0.596 in urea vs 0.506 in placebo group | Crisis rate ratio, 0.849 (placebo vs urea); NS‡ | |||||||
| Placebo (n = 39) orally | Crisis rate per person-year in patients with >2 crises, mean: 0.189 in urea vs 0.136 in placebo group | Crisis rate ratio, 0.720 (placebo vs urea); NS‡ | ||||||
| Piracetam | ||||||||
| 45 (Lebanon) | RCT, crossover (phase 2) | Sickle-cell anemia; children | Range, 4-15; 44; NA | 10 months (5 months per treatment, no washout) | Piracetam 80 mg/kg per day in 4 daily doses (n = 9*) orally | 2/6 | N of pain crises per patient per month, average: 0.89 in piracetam group vs 1.85 in placebo | P < .05‡ |
| Placebo (n = 9*) orally | ||||||||
| 36, 37 (Saudi Arabia) | RCT (phase 2) | HbSS, HbSβ0, children | Range, 3-12; 57; NA | 1 year | Piracetam 160 mg/kg per day (n = 48*) orally; during admission 300 mg/kg per day IV | 2/6 | N of painful crisis per year, mean (SD): 2.4 (±1.9) in piracetam vs 4.3 (±3.1) in placebo group | Mean difference (CI): −1.9 (−3.0 to −0.8); P < .001|| |
| Placebo (n = 39*) orally; during admission IV | N of hospitalizations per year in total group, mean (SD): 2.07 (±2.4) in piracetam group vs 3.20 (±2.9) in placebo group | Mean difference (CI): −1.1 (−2.3-0.0); P < .05|| | ||||||
| 40, 41 (Brazil) | RCT, crossover (phase 2) | HbSS, HbSC, HbSβ0/+; age 5-20 years | Median, 12.1; 45; 0 | 13 months (6 months per treatment, 1-month washout in between) | Piracetam 4.8g/m2 per day in 4 daily doses (n = 73*) orally | 1/6 | Absolute n of painful VOCs, median: 3 in both groups | NS‡ |
| Placebo (n = 73*) orally | N of pain days per month, median: 1.47 in both groups | NS‡ | ||||||
| Hospitalization days: data NA | NS‡ | |||||||
| Niprisan | ||||||||
| 38, 39 (Nigeria) | RCT, crossover (phase 2) | HbSS; ≥3 crises in past year; age 2-45 years | Mean, 15.5; 45; NA | 12 months (6 months per treatment, no washout) | Niprisan 12 mg/kg od (n = 70*) | 3/6 | N of episodes of mild to moderate pain over first 6 months, mean (SD): 9.1 (±9.6) in niprisan vs 20.6 in placebo group (±32.9) | NS‡ |
| Placebo (n = 70*) | N of episodes of severe pain first 6 months, mean (SD): 7.9 (±8.4) in niprisan vs 22.1 (±32.9) in placebo group | P < .05‡ | ||||||
| N of painful crises first 6 months, mean (SD): 0.27 (±0.6) in niprisan vs 1.17 (±1.8) in placebo group | NS‡ | |||||||
| N of hospital admissions per month in first 6 months, mean (SD): 0.15 (±0.6) in niprisan vs 0.22 (±0.6) in placebo group | NS‡ | |||||||
| NB. No summary statistics possible because of skewed data | ||||||||
| Ciklavit | ||||||||
| 71 (Nigeria) | RCT (phase 2) | HbSS; age 1-15 years | Mean, 8.09§; 57§; NA | 6 months | Ciklavit ≤5 years 10 mL twice daily, >5 years 20 mL bd (n = 47*) | 3/6 | N of painful crises in 6 months, mean (SD): 4.2 (4.2) in Ciklavit vs 3.9 (4.3) in placebo group | Mean difference (CI): 0.30 (−1.49-2.09); NS|| |
| Placebo (n = 42*) | ||||||||
| Cromolyn sodium | ||||||||
| 74 (Iran) | CT, crossover (phase 2) | HbSS | Range, 5-34; 41; HU was part of intervention | 12 months (crossover every 3 months, unclear washout) | Subsequent treatment regimens 1-4 (n = 17*): (1) cromolyn sodium nasal spray 2%, 4 puffs each nostril, frequency NA; (2) placebo nasal spray; (3) as in 1, plus HU 10-15 mg/kg per day orally; and (4) as in 2, plus HU 10-15 mg/kg per day orally | 0/6 | Primary review outcome: NA | |
| Secondary review outcomes: | ||||||||
| Pain score: data NA, pain score in cromolyn sodium and HU group lower than cromolyn sodium–only group | P < .05‡ | |||||||
| Other comparisons between groups | NS‡ | |||||||
| Senicapoc | | | |||||||
| 66 (United States) | RCT (phase 2) | HbSS; age 18-60 years | Mean, 37.2§; 59§; 27 | 12 weeks | Senicapoc loading dose on day 1, subsequently: low dose, 6 mg od (n = 29) orally; high dose, 10 mg od (n = 31) orally | 6/6 | N of patients with painful crisis: 5 (17%) of 29 in low-dose senicapoc vs 5 (16%) of 31 in high-dose senicapoc vs 5 (17%) of 30 in placebo group | OR (CI): low dose vs placebo, 1.04 (0.27-4.06); NS||; high dose vs placebo, 0.96 (0.24-3.73); NS|| |
| Placebo (n = 30) orally | ||||||||
| 73 (United States, United Kingdom, Jamaica, Brazil, France, Trinidad) | RCT (phase 3) | HbSS, HbSC, HbSβ0/+; age 16-65 years; ≥2 crises in past 12 months | Mean, 28.5§; 43§; 58§ | 52 weeks | Senicapoc 1 mg od, initial loading dose 20 mg bd for 4 days (n = 145*) orally | 6/6 | N of patients with acute sickle-cell–related painful crises: 106 (73%) of 145 in senicapoc vs 89 (62%) of 144 in placebo group | OR (CI): 1.68 (1.02-2.76); P = .045|| |
| Placebo (n = 144*) orally | ||||||||
| Painful crisis rate, mean (SD): 0.38 (±0.36) in senicapoc group vs 0.31 (±0.48) in placebo group | Mean difference (CI): 0.07 (−0.03-0.17); NS|| | |||||||
| N of days in hospital per patient, mean: 18.6 in senicapoc group vs 15.1 in placebo group | P = .03‡ | |||||||
| Magnesium pidolate | ||||||||
| 68 (United States) | RCT (phase 2) | HbSC; age ≥2 years; ≥1 vaso-occlusive event in past 12 months | Mean, 13.6; 57; HU was part of intervention | 44 weeks | HU 20 mg/kg per day plus magnesium pidolate 0.3 mmol/kg per day bd orally | 2/6 | N of vaso-occlusive pain crises: no significant differences observed between treatment groups (data NA) | NA† |
| HU 20 mg/kg per day plus placebo orally | ||||||||
| Placebo plus magnesium pidolate 0.3 mmol/kg per day bd orally | ||||||||
| Placebo plus placebo orally | ||||||||
| N per group, NA (total N = 44) | ||||||||
| Lime juice | ||||||||
| 56 (Nigeria) | RCT (phase 2) | Sickle-cell anemia; children | Mean, 4.6§; 53§; 0 | 6 months | Lime juice td ≤10 kg 5 mL, 11-20 kg 10 mL, >20 kg 15 mL (n = 58*) | 1/6 | N of children with significant painful episodes: 29 (50%) of 58 in lime juice vs 51 (93%) of 55 in routine care group | OR (CI): 0.078 (0.02-0.24); P < .001|| |
| Routine care (n = 55*) | ||||||||
| N of children with hospitalizations: 2 (3%) of 58 in lime juice vs 19 (35%) of 55 in routine care group | OR (CI): 0.068 (0.01-0.3); P < .001|| | |||||||
| Frequency of significant painful episodes, mean (SD): 0.64 (±0.11) in lime juice vs 1.51 (±0.34) in routine care group | Mean difference (CI): −0.87 (−0.96 to −0.78); P < .001|| | |||||||
| Frequency of hospitalizations, mean (SD): 0.03 (±0.01) in lime juice vs 0.35 (±0.07) in routine care group | Mean difference (CI): −0.32 (−0.33 to −0.30); P < .001|| | |||||||
| HQK-1001 | ||||||||
| 63 (United States, Egypt, Jamaica, Canada, Lebanon) | RCT (phase 2) | HbSS, HbSβ0; age 12-60 years; ≥1 acute SCD-related event in past 12 months | Mean, 27.8§; 34§; 0 | 48 weeks | HQK-1001 15 mg/kg bd (n = 38*) orally | 4/6 | Patients with at least 1 pain crisis: 22 (58%) of 38 in HQK-1001 vs 19 (50%) of 38 in placebo group | OR (CI): 1.38 (0.56-3.40); NS|| |
| Placebo (n = 38*) orally | Patients with acute chest syndrome: 6 (16%) of 38 in HQK-1001 vs 2 (5%) of 38 in placebo group | OR (CI): 3.4 (0.6-17.9); NS|| | ||||||
| Annualized rate of pain crises, mean (SD): 3.5 (±5.2) in HQK-1001 group vs 1.7 (±2.2) in placebo group | Mean difference (CI): 1.80 (−0.03-3.63); NS|| | |||||||
| Other interventions | ||||||||
| Folate | ||||||||
| 48 (Jamaica) | CT (phase 2) | HbSS; age 0.5-4 years | Range, 0.5-4; 56; NA | 1 year | Folate 5 mg od (n = 59*) | 3/6 | Patients with painful episodes: 22 (37%) of 59 in folate vs 18 (32%) of 56 in placebo group | OR (CI): 1.26 (0.58-2.71); NS|| |
| Placebo (n = 56*) | ||||||||
| Zinc | ||||||||
| 50 (India) | RCT (phase 2) | HbSS; age >5 years | Mean, 16.4§; 71§; NA | 1.5 years | Zinc sulfate 220 mg td (n = 65*) | 4/6 | N of crisis episodes, mean (SD): 2.46 (±1.04) in zinc vs 5.29 (±2.58) in placebo group | Mean difference (CI): −2.83 (−3.51 to −2.15); P < .0001|| |
| Placebo (n = 65*) | ||||||||
| 47 (United States) | RCT, partial crossover (phase 2) | HbSS, HbSC, HbSβ; adults | Range, 19-49; 50; NA | 3 years (excluding 1-year follow-up prior) | Zinc acetate 50-75 mg daily (n = 11; zinc deficient) orally | 2/6 | Incidence of hospital admission, mean (SD): 6.90 (±3.60) in zinc vs 5.36 (±5.30) in routine care vs 8.00 (±5.2) in placebo group | Mean difference (CI): zinc vs routine care, 1.54 (−2.49-5.57); NS||; zinc vs placebo, −1.1 (−5.15-2.95); NS|| |
| Placebo with crossover to zinc acetate 50-75 mg daily after 1 year (n = 10; zinc deficient) orally | ||||||||
| Routine care (n = 11; zinc sufficient) | Incidence of VOCs, mean (SD): 6.72 (±3.60) in zinc vs 5.18 (±4.80) in routine care vs 7.6 (±5.4) in placebo group | Mean difference (CI): zinc vs routine care, 1.54 (−2.23-5.31); NS||; zinc vs placebo, −0.88 (−5.03-3.27); NS|| | ||||||
| 67 (United States) | RCT (phase 2) | HbSS; adults | Mean, 33.6§; 61§; 0 | 3 months | Zinc acetate 25 mg td (n = 18) orally | 4/6 | N of sickle pain episodes: 1 episode in 18 patients in zinc vs 3 episodes in 18 patients in placebo group | NA† |
| Placebo (n = 18) orally | ||||||||
| Antimalarials | ||||||||
| 70 (Nigeria) | RCT, open label (phase 2) | HbSS; age 1-16 years | Mean, 8.1§; 52§; NA | 9 months | Antimalarial prophylaxis | 4/6 | N of patients with bone pain crisis during study: 2 (3%) of 36 in pyrimethamine vs 0 (0%) of 32 in proguanil vs 5 (17%) of 29 in placebo group | OR (CI): pyrimethamine vs placebo, 0.28 (0.05-1.58); NS||; proguanil vs placebo, NA; Fisher’s exact P = .02||; pyrimethamine vs placebo, 0.09 (0.02-0.48); P = .001||; proguanil vs placebo, 0.30 (0.09-1.02); NS|| |
| Pyrimethamine 0.5 mg/kg once per week (n = 36*) orally | ||||||||
| Proguanil 1.5 mg/kg od (n = 32*) orally | N of patients with hospitalizations during study: 2 (6%) of 36 in pyrimethamine vs 5 (16%) of 32 in proguanil vs 11 (38%) of 29 in placebo group | |||||||
| Placebo (vitamin C 7 mg/kg od; n = 29*) orally | ||||||||
| 35, 69 (Senegal) | RCT (phase 2) | Sickle-cell anemia | Mean, 24.5§; 47§; NA | 2 months | Seasonal intermittent antimalarial treatment with sulfadoxine 25 mg/kg and pyrimethamine 1.25 mg/kg once per month (n = 30) orally | 6/6 | N of VOC per year, total: 5 in sulfadoxine-pyrimethamine vs 5 in placebo group | Relative risk (CI): 1.0 (0.3-3.1); NS‡ |
| Placebo (n = 30) orally | ||||||||
| N of hospitalizations, total: 5 in sulfadoxine-pyrimethamine vs 5 in placebo group | Relative risk (CI): 1.0 (0.3-3.1); NS‡ | |||||||
This table provides a summary of the characteristics, risks of bias, and primary review outcomes of included studies. The 6 risk-of-bias criteria were summarized into a single risk-of-bias score from 0 to 6; the higher this score, the more items were marked with a low risk of bias. The full results of the risk-of-bias assessment per study can be found in supplemental Table 2. A complete overview of the primary and secondary review outcomes and the outcome definitions per study is provided in supplemental Table 3.
bd, twice daily; CI, confidence interval; CT, controlled trial; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; im, intramuscularly; IQR, interquartile range; NA, not available; NS, not significant; od, once daily; OR, odds ratio; SD, standard deviation; td, three times per day.
Patients included in final analysis.
Data did not allow post hoc summary statistics or were not available in paper.
(Summary) statistic copied from paper.
In intervention group (the first stated in case of multiple intervention groups).
Summary statistic calculated post hoc with data from the original paper.
Quality of the included studies
Overall, the reporting of the included trials on methodological practices was poor. Only 7 of the 35 studies scored an overall low risk of bias on all items in the quality assessment.58-60,66,69,72,73 Fourteen studies scored a high risk of bias on at least 1 of the items of the quality assessment, in particular on incomplete outcome data (n = 8) and blinding of participants (n = 6) and personnel for intervention (n = 6).37,43-45,48,49,51,54,56,63,64,70,71,74
Anticoagulation.
A total of 7 studies assessed interventions targeting coagulation activation. In 2 crossover studies in children and adults (N = 49 and N = 29) performed in the 1980s, oral treatment with the antiplatelet agent aspirin (acetylsalicylic acid) did not provide benefit over placebo in the outcomes of this review.49,51
Cabannes et al55 evaluated the clinical efficacy of the antiplatelet drug ticlopidine (adenosine diphosphate receptor inhibitor) in adolescent and adult patients with HbSS disease (N = 140). The authors reported a significant decrease in the total number and duration of VOCs and a nonvalidated VOC severity index (Table 1; supplemental Table 3).
In an early study in patients with HbSS disease in Brazil (N = 57), the platelet inhibitor pentoxifylline significantly reduced the number and duration of pain events and the number of patients with pain events as compared with placebo.44 The study scored an unclear or high risk of bias for multiple items in the quality assessment. Schnog et al72 evaluated the effects of the oral vitamin K antagonist acenocoumarol in a pilot crossover study (N = 22). No differences in the frequency of vaso-occlusive events were observed between the acenocoumarol and placebo arms.
The effects of the platelet inhibitor prasugrel were evaluated in both a small phase 2 study in adult patients (N = 62)62 and a large multicenter phase 3 study in pediatric patients (N = 341).60 In both studies, oral treatment with prasugrel did not provide benefit over placebo in the outcomes of this review.
Antioxidants.
Five studies assessed treatments with antioxidants. Two studies evaluated the effects of daily ω-3 fatty acid supplementation. In a pilot study by Tomer et al65 (N = 10) in the United States, a significantly lower frequency of pain episodes was found. From 2009 to 2010, Daak et al59 performed a large single-center RCT in Sudan among patients with HbSS disease (N = 140). The annual VOC rate, number of hospitalization days for VOC rate, and rate of hospitalizations were significantly lower in the ω-3 group as compared with placebo (Table 1). Both studies scored an overall low risk of bias.
A small pilot study by Pace et al64 (N = 21) assessed the effect of the oral antioxidant N-acetylcysteine in SCD. The mean number of VOC episodes per person-days seemed lower in the 2 N-acetylcysteine arms with the highest doses (1200 and 2400 mg per day) as compared with the 600-mg intervention arm and the placebo arm. However, this study did not provide sufficient data for post hoc statistical comparison between treatments arms.
Daily supplementation with vitamins C and E did not have any beneficial effect on the incidence of acute SCD complications or quality of life as compared with placebo in a population of Brazilian patients with HbSS and HbSβ0 disease (N = 83).57
Niihara et al34,61 (N = 230) reported that treatment with the oral amino acid l-glutamine over 48 weeks in patients with HbSS and HbSβ0 disease reduced the number of sickle-cell crises, hospitalizations, and hospital days when compared with placebo (Table 1). Also, the number of days to first sickle-cell crisis was significantly higher and crisis severity seemed to be lower in the l-glutamine group. However, only conference abstracts of this study are currently available.
Antiadhesion.
Ataga et al58 performed an international trial assessing the effects of the monthly IV administration of high-dose and low-dose crizanlizumab (N = 198), a monoclonal antibody binding the adhesion molecule P-selectin.58 In this study, high-dose crizanlizumab compared with placebo resulted in a decrease in the annual rate of VOCs and uncomplicated VOCs and a longer time until first VOC (Table 1; supplemental Table 3). There was no significant reduction in the annual rate of hospital admission days. The study scored a low risk of bias.
Antisickling.
A majority of included studies assessed interventions with antisickling properties (n = 16). Three crossover studies, evaluating the effects of oral sodium bicarbonate (N = 18), promazine hydrochloride (N = 14), and cromolyn sodium nasal spray (N = 17), respectively, did not provide any beneficial effect over placebo.43,46,74 Oral administration of the organic compound urea in an RCT in Ghana (N = 79)52 and of magnesium pidolate in an RCT in the United States (N = 44; with or without HU)68 did not show any differences between treatments arms in the outcomes of this review either.
Three studies assessed the effects of sex steroid hormones on pain in SCD. Two of these studies did not provide adequate statistical comparisons between the intervention groups (N = 4442 and N = 4354 ). De Ceulaer et al53 (N = 23) did demonstrate a trend of reduction in the number of patients with bone pain episodes. However, all 3 studies scored an unclear or high risk of bias for multiple items in the quality assessment.
The clinical effects of the oral antisickling agent piracetam were assessed in 3 studies in children. The first was an early crossover study in Lebanon (N = 9) suggesting a reduction in the number of pain crises per patient per month in the piracetam group.45 The second study (N = 87), performed in Saudi Arabia, demonstrated a significant decrease in the mean number of painful crises and hospitalizations per year and in a composite nonvalidated severity index (Table 1; supplemental Table 3).36,37 The third study was a crossover trial in Brazil (N = 73) and did not show any benefit.40,41 All 3 studies scored an unclear or high risk of bias for multiple items in the quality assessment.
The efficacy of 3 different phytomedicines was assessed in a randomized controlled setting, all in Nigeria. In a crossover study, Wambebe et al38,39 compared the effects of the oral herbal preparation Niprisan with those of placebo (N = 70). The authors reported a significant decrease in the number of episodes of severe pain. However, differences in other outcomes relevant to this review did not reach significance. Akinsulie et al71 assessed the effect of the oral antisickling agent Ciklavit in children with HbSS disease (N = 100). Despite the conclusions of the authors, post hoc comparison did not show a significant difference in the number of VOCs during the study between the 2 intervention arms. Adegoke et al56 compared the effects of oral lime juice, rich in the presumed antisickling agents ascorbic acid and phenylalanine, with those of routine care in children with HbSS disease (N = 113). A significant decrease was observed in the frequency and the number of patients with VOCs and hospitalizations. All 3 studies scored an unclear or high risk of bias for various items in the quality assessment.
Lastly, Ataga et al66,73 performed both a phase 2 (N = 90) and phase 3 (N = 289) multicenter trial, each assessing the effects of oral treatment with the gardos channel blocker senicapoc. Both studies showed no improvement in the rate of VOCs as compared with placebo. In fact, senicapoc even seemed to increase the number of VOCs in our post hoc analysis of the latter trial. Similarly, an international multicenter trial of the oral fetal globin inducer HQK-1001 was stopped early (N = 76) because of lack of effect.63
Other interventions.
Four studies assessed the therapeutic value of dietary supplements. Rabb et al48 assessed the effect of folic acid supplementation on VOCs and dactylitis in children with HbSS disease in Jamaica (N = 115).48 In this non-RCT, there was no effect of folate on the proportion of patients with VOCs.
Three trials involved oral zinc supplementation, based on the observation that zinc may have an immunoregulatory and anti-inflammatory effect in SCD.75-77 A large, placebo-controlled study in India (N = 130) demonstrated a significant decrease in the number of sickle-cell–related crises (including nonpain events) in the zinc sulfate group, but no effect on length of hospital stay (Table 1; supplemental Table 3).50 Prasad et al47 compared zinc acetate supplementation with routine care and placebo and did not observe any differences between treatments arms (N = 32). Data from an additional study by this group did not allow post hoc comparison of the number of VOCs between the treatment arms (N = 36).67
Lastly, we identified 2 studies evaluating the effect of antimalarial therapies on the occurrence of VOCs in patients with HbSS disease in Nigeria and Senegal (N = 97 and N = 60), respectively. Malaria infections are an important trigger for VOCs in SCD. In the open-label, placebo-controlled study by Eke et al,70 a significantly lower proportion of patients with VOCs was observed in the proguanil arm, along with a lower proportion of patients with hospitalizations in the pyrimethamine arm as compared with placebo (Table 1). In the study by Diop et al,69 monthly sulfadoxine-pyrimethamine treatment as compared with placebo during high-transmission malaria season did not affect the frequency of VOCs or hospitalizations.
Discussion
In this systematic review, we have formally examined the range of pharmacotherapeutical strategies beyond HU that have been evaluated over the years for the prevention of VOCs in SCD in a controlled setting. This is the first study providing a complete overview by a systematic approach, including a quality assessment.
Despite our broad search, only 36 unique studies were identified, covering 26 different interventions. A significant proportion of the included studies were relatively older and of poor quality. Eight trials were published in the past 5 years. The most promising interventions with efficacy on the frequency of VOCs or hospitalizations in SCD were the antioxidants l-glutamine and ω-3 fatty acids and the antiadhesive P-selectin inhibitor crizanlizumab.58,59,61,65 Also, studies on the antiplatelet agent ticlopidine, the dietary supplement zinc, and antimalarial prophylaxis for patients in malaria-endemic regions provided encouraging results.50,55,70 However, 23 of the 36 studies included in this review did not show any beneficial effect of the intervention under investigation.38,40,43,46-49,51-54,57,60,62,63,66-69,71-74 The other studies were either too small or methodologically inadequate to draw strong conclusions.37,42,44,45,56,64
The amino acid l-glutamine seemed to be of great efficacy in patients with HbSS or HbSβ0 disease on the relevant outcomes of this review.61 The pathophysiological rationale for its effect in SCD has not yet been extensively studied. It is based on the observation that supplementation with l-glutamine enhances the nicotinamide adenine dinucleotide redox potential and may thereby exert an antioxidative effect in SCD.78 Currently, the results of this phase 3 trial have only been presented in conference abstracts, limiting reliable assessment of the quality and results of the study and further clinical implementation.
A beneficial effect of ω-3 fatty acid supplementation in SCD was observed in 2 trials. A pilot study by Tomer et al65 showed a significant decrease in the frequency of painful episodes compared with placebo. These findings were confirmed by a large, methodologically sound phase 3 trial by Daak et al59 demonstrating a significant decrease in the annual VOC rate, hospitalization rate for VOCs, and number of days admitted in patients with HbSS in Sudan. These results are highly promising and are supported by multiple preclinical studies. Interestingly, ω-3 fatty acids are considered to be a multitarget intervention; in addition to having an antioxidative effect, they have been shown to inhibit platelet activation and blood-cell aggregation and inflammation and improve cell-membrane deformability.65,79-82 However, further confirmation of its efficacy on clinical outcomes is needed. The fact that 2 new phase 3 multicenter studies are expected to start enrollment soon therefore offers hope (registered at www.clinicaltrials.gov as #NCT02604368 and #NCT02525107).
A high-dose regimen of the antiadhesive agent crizanlizumab was demonstrated to be an effective therapy in reducing the annual rate of VOCs and prolonging the time to first VOC.58 Unfortunately, no significant reduction in the rate of admission days was reached. Possibly, this intervention is most effective in milder episodes of VOC. Also, the rate of admission days is a multifactorial outcome that is not solely determined by the severity of a VOC. Crizanlizumab is a monoclonal antibody binding the adhesion molecule P-selectin that mediates adhesion of sickle erythrocytes to the vascular endothelium.83,84 This phase 2 study provides proof of concept that antiadhesive therapy can reduce the incidence of VOCs. Despite these encouraging results, the monthly IV administration of the drug and the likely high costs involved may prove to be barriers to widespread clinical implementation.
Oral treatment with the antiplatelet drug ticlopidine resulted in a significant reduction in the number and duration of crises and a nonvalidated severity index of crises.55 However, outcomes were not clearly defined, baseline imbalances were not properly assessed, and it was unclear how missing data and dropouts were handled in this trial from 1984. The study by Gupta et al50 on daily zinc sulfate supplementation in patients with HbSS disease showed a significant reduction in the number of sickle-related events.50 However, nonpain events were also included in this definition, there were no data on zinc status at baseline, and there was an unclear risk of bias for 2 items in the quality assessment. Further research is required to assess the effect of these interventions on SCD. Eke et al70 provided proof of concept for the efficacy of malarial prophylaxis in the reduction of VOCs and hospitalizations in patients with HbSS disease. However, these findings need confirmation in a double-blind trial design. Moreover, this intervention is only relevant to patients with SCD living in malaria-endemic regions of the world.
Our review has some limitations. Firstly, the range of pharmacotherapeutical interventions among the included studies varied significantly, hampering data synthesis by meta-analysis. Therefore, the focus of this review was to provide a broad overview, describing the quality and applicability of the included studies. Secondly, a significant portion of the included studies was of suboptimal methodological quality, limiting the reliable interpretation of these results. Lastly, by including only controlled trials, we may have missed other promising interventions that are still in earlier phases of development. However, preclinical developments and early trials have already been extensively discussed in other reviews.25-27 In this review, we aimed to focus on interventions in advanced stages of evaluation that are closer to clinical implementation.
This comprehensive review has identified various promising pharmacotherapeutical strategies in the prevention of VOCs in SCD. Sadly, this study highlights the discrepancy between the significant burden of this disease worldwide and the low number of well-designed and adequately powered trials published so far. In future trials, we should draw lessons from prior studies and focus phase 2 to 3 trial efforts on interventions with the highest potential and a solid preclinical evidence base.85 In addition, international consensus on a standardized set of outcomes is imperative. In conclusion, there remains a significant unmet need for new, accessible therapies for SCD. Encouragingly, the number of clinical trials in SCD has increased recently, and many novel therapies are currently under investigation.25-27
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Arnold Leenders and René Spijker, medical information specialists at the medical library of the Academic Medical Center, for their assistance in the systematic search for this review and Lena Václavů for her critical editing of the manuscript for grammar and style.
J.W.R.S. was supported by grants from The Netherlands Organisation for Health Research and Development, the Academic Medical Center, JANIVO Stichting, Egbers Stichting, and Fonds NutsOhra, that have been awarded to K.F. and B.J.B.
Authorship
Contribution: J.W.R.S. performed the search and wrote the manuscript; J.W.R.S., D.J.M., and S.C.A.T.D. performed abstract and full-text screening; J.W.R.S. and D.J.M. performed data extraction; J.W.R.S., B.J.B. and K.F. designed the study, performed quality assessments, and interpreted the data; B.J.B. and K.F. supervised the study and edited the manuscript; and all authors revised and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joep W. R. Sins, Academic Medical Center, Department of Hematology and Pediatric Hematology, Room H7-227, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: j.w.sins@amc.nl.