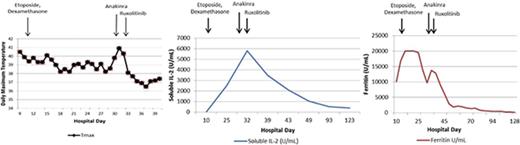
Key Points
Optimal salvage therapy for refractory HLH is unknown.
In our patient, ruxolitinib treatment led to clinical remission of refractory HLH.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a potentially fatal illness characterized by impaired natural killer and cytotoxic T-cell function. Patients present with systemic inflammation and multisystem organ dysfunction; if untreated, HLH results in death. The clinical picture can evolve, and thus, there should be a high degree of clinical suspicion for this diagnosis in critically ill patients and a low threshold for sending testing (diagnostic criteria noted in Figure 1) to ensure timely diagnosis and initiation of treatment.1
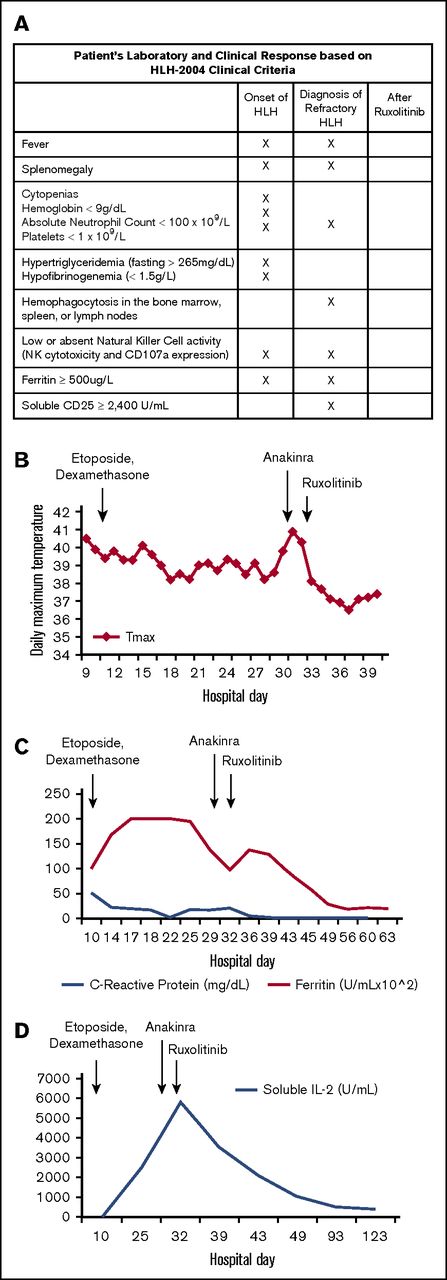
Patient’s laboratory and clinical response to treatment. (A) Our patient’s clinical and laboratory abnormalities and how he met criteria for diagnosis of HLH noted at initial diagnosis, at diagnosis of refractory disease, and after treatment with ruxolitinib. Laboratory testing was sent at each time point; X indicates that a criterion was met. (B) Temperature curve (°C). Patient remained febrile after etoposide and dexamethasone treatment and after anakinra treatment. Patient became afebrile and has remained afebrile after ruxolitinib was administered. (C) Ferritin and C-reactive protein (CRP). Ferritin remained elevated after etoposide and dexamethasone were started and, although eventually beginning to fall, rebounded at time of diagnosis of refractory HLH. Ferritin continued to decline to the normal range after the patient received ruxolitinib. Similarly, CRP, a nonspecific marker of inflammation, was elevated at the start of treatment and began to decline after etoposide and dexamethasone were started. CRP began to rise as the patient became refractory to treatment and then declined to normal range after ruxolitinib administration. (D) Soluble IL-2 receptor. Although not elevated at initial diagnosis, soluble IL-2 receptor increased with persistent fevers and inflammation and reached a maximum level just before start of ruxolitinib. After ruxolitinib initiation, soluble IL-2 receptor levels returned to normal.
Patient’s laboratory and clinical response to treatment. (A) Our patient’s clinical and laboratory abnormalities and how he met criteria for diagnosis of HLH noted at initial diagnosis, at diagnosis of refractory disease, and after treatment with ruxolitinib. Laboratory testing was sent at each time point; X indicates that a criterion was met. (B) Temperature curve (°C). Patient remained febrile after etoposide and dexamethasone treatment and after anakinra treatment. Patient became afebrile and has remained afebrile after ruxolitinib was administered. (C) Ferritin and C-reactive protein (CRP). Ferritin remained elevated after etoposide and dexamethasone were started and, although eventually beginning to fall, rebounded at time of diagnosis of refractory HLH. Ferritin continued to decline to the normal range after the patient received ruxolitinib. Similarly, CRP, a nonspecific marker of inflammation, was elevated at the start of treatment and began to decline after etoposide and dexamethasone were started. CRP began to rise as the patient became refractory to treatment and then declined to normal range after ruxolitinib administration. (D) Soluble IL-2 receptor. Although not elevated at initial diagnosis, soluble IL-2 receptor increased with persistent fevers and inflammation and reached a maximum level just before start of ruxolitinib. After ruxolitinib initiation, soluble IL-2 receptor levels returned to normal.
Eighty percent of patients have at least a partial response to front-line therapy with dexamethasone and etoposide.2 However, for the 20% who are refractory to therapy, there is no consensus on optimal second-line treatment. Alemtuzumab, infliximab, anakinra, and others have been suggested as possible salvage treatment options with variable success.2,3 Recently, ruxolitinib, a Janus kinase (JAK) 1/2 inhibitor, has shown promise in mouse models of primary and secondary HLH. When administered empirically to perforin-deficient mice, ruxolitinib inhibits interferon γ (IFN-γ), interleukin-6 (IL-6), and IL-12 production and prevents clinical symptoms of HLH from developing.4 Similarly, when ruxolitinib is administered after HLH symptom onset, cytokine production and tissue damage are decreased, leading to improved survival in mice.5 The dramatic effect of ruxolitinib in murine models has led to interest in its use clinically for refractory HLH.
Here we present a case of a patient with refractory HLH who was clinically deteriorating and experienced a dramatic improvement with ruxolitinib therapy.
Case description and methods
An 11-year-old previously healthy boy, recently emigrated from Burma, was admitted with complaints of extremity myalgias and difficulty walking. After admission, he developed daily high-spiking fevers of 39°C to 40°C and a pleural effusion requiring chest-tube placement. Despite initial clinical improvement, he had persistent fevers and developed liver dysfunction, respiratory failure, and acute renal insufficiency. An extensive infectious workup was negative, and his symptoms and laboratory values did not improve with broad-spectrum antimicrobials. Rheumatologic, immunodeficient, and oncologic causes were investigated and found to be negative (Table 1). Because of his persistent fevers, multisystem organ dysfunction, and continued rise in ferritin (>20 000 ng/mL), there was concern about evolving HLH (Figure 1). Although initial bone marrow evaluation did not show evidence of hemophagocytosis, he met clinical criteria for HLH, and with acute clinical decline requiring intubation and ionotropic support, HLH-directed therapy was initiated with dexamethasone (10 mg/m2 per day) and etoposide (112.5 mg/m2; dose adjusted for renal insufficiency).
Patient’s laboratory evaluation for etiology of secondary HLH
| Laboratory results . |
|---|
| Infectious |
| Blood and urine cultures: negative |
| Nasopharyngeal viral swabs: positive for enterovirus but repeat negative |
| Enterovirus blood PCR: negative |
| EBV PCR: negative |
| EBV IgG: positive; IgM: negative |
| CMV IgG: positive; IgM: negative |
| HIV antibody: negative |
| HIV RNA PCR: negative |
| Leishmaniasis antibody: negative |
| Endotracheal tube culture: normal flora |
| Bronchoalveolar lavage: negative |
| CSF encephalitis panel*: negative |
| Fungitel: negative |
| Galactomannan: negative |
| Histoplasmosis antibody: negative |
| Histoplasmosis antigen: negative |
| Blastomyces antibody: negative |
| Blastomyces antigen: negative |
| Hepatitis A IgG: positive; IgM: negative |
| Hepatitis B surface IgG: positive |
| Hepatitis B core IgG: negative |
| Hepatitis C IgG: negative |
| Malaria prep: negative |
| Strongyloides antibody: negative |
| Brucella IgG: negative; IgM: negative |
| Malaria prep: negative |
| Parvovirus: negative |
| Quantiferon gold: indeterminate |
| PPD: negative |
| Immunologic |
| IgG: 1890, IgA:400, IgE: 1124, IgM: 97 (mg/dL) |
| Normal vaccine response to tetanus and diphtheria, present but low response to Haemophilusinfluenzae type b |
| CD3+: 310/mm3 (CD4 <200/mm3) |
| CD56: 0/mm3 |
| CD19: 212/mm3 |
| Rheumatologic |
| ACE: 40 U/L (normal range, 13-100 U/L) |
| C3: 173 mg/dL (normal, 80-156 mg/dL) |
| C4: 30.5 mg/dL (normal, 12-43 mg/dL) |
| Anti-RNP: 110 AU/mL (normal < 100 AU/mL) |
| Anti-SSA/SSB: <100 AU/mL (normal <100 AU/mL) |
| ANA titer: 80, nucleolar (normal <40) |
| Anti-dsDNA: 2 iU/mL (normal ≤4 iU/mL) |
| Anticardiolipin IgG and IgM: negative |
| Lupus anticoagulant: negative |
| Antiproteinase 3 antibody: <1.0 AI (normal <1.0 AI) |
| Antimyeloperoxidase antibody: <1.0 AI (normal <1.0 AI) |
| Periodic fever syndrome panel†: negative |
| ADAMTS13 activity: 55% (assay can be inhibited by hyperbilirubinemia, normal >67%) |
| Creatine kinase: 49 iU/L (normal, 30-150 iU/L) |
| Cryoglobulins: positive |
| Haptoglobin: 151 mg/dL (normal, 43-212 mg/dL) |
| Oncologic |
| CT head, chest, abdomen negative for malignancy |
| Bone marrow flow cytometry negative for leukemia; cytogenetics normal |
| Laboratory results . |
|---|
| Infectious |
| Blood and urine cultures: negative |
| Nasopharyngeal viral swabs: positive for enterovirus but repeat negative |
| Enterovirus blood PCR: negative |
| EBV PCR: negative |
| EBV IgG: positive; IgM: negative |
| CMV IgG: positive; IgM: negative |
| HIV antibody: negative |
| HIV RNA PCR: negative |
| Leishmaniasis antibody: negative |
| Endotracheal tube culture: normal flora |
| Bronchoalveolar lavage: negative |
| CSF encephalitis panel*: negative |
| Fungitel: negative |
| Galactomannan: negative |
| Histoplasmosis antibody: negative |
| Histoplasmosis antigen: negative |
| Blastomyces antibody: negative |
| Blastomyces antigen: negative |
| Hepatitis A IgG: positive; IgM: negative |
| Hepatitis B surface IgG: positive |
| Hepatitis B core IgG: negative |
| Hepatitis C IgG: negative |
| Malaria prep: negative |
| Strongyloides antibody: negative |
| Brucella IgG: negative; IgM: negative |
| Malaria prep: negative |
| Parvovirus: negative |
| Quantiferon gold: indeterminate |
| PPD: negative |
| Immunologic |
| IgG: 1890, IgA:400, IgE: 1124, IgM: 97 (mg/dL) |
| Normal vaccine response to tetanus and diphtheria, present but low response to Haemophilusinfluenzae type b |
| CD3+: 310/mm3 (CD4 <200/mm3) |
| CD56: 0/mm3 |
| CD19: 212/mm3 |
| Rheumatologic |
| ACE: 40 U/L (normal range, 13-100 U/L) |
| C3: 173 mg/dL (normal, 80-156 mg/dL) |
| C4: 30.5 mg/dL (normal, 12-43 mg/dL) |
| Anti-RNP: 110 AU/mL (normal < 100 AU/mL) |
| Anti-SSA/SSB: <100 AU/mL (normal <100 AU/mL) |
| ANA titer: 80, nucleolar (normal <40) |
| Anti-dsDNA: 2 iU/mL (normal ≤4 iU/mL) |
| Anticardiolipin IgG and IgM: negative |
| Lupus anticoagulant: negative |
| Antiproteinase 3 antibody: <1.0 AI (normal <1.0 AI) |
| Antimyeloperoxidase antibody: <1.0 AI (normal <1.0 AI) |
| Periodic fever syndrome panel†: negative |
| ADAMTS13 activity: 55% (assay can be inhibited by hyperbilirubinemia, normal >67%) |
| Creatine kinase: 49 iU/L (normal, 30-150 iU/L) |
| Cryoglobulins: positive |
| Haptoglobin: 151 mg/dL (normal, 43-212 mg/dL) |
| Oncologic |
| CT head, chest, abdomen negative for malignancy |
| Bone marrow flow cytometry negative for leukemia; cytogenetics normal |
ACE, angiotensin converting enzyme; AI, antibody index; ANA, antinuclear antibodies; CMV, cytomegalovirus; CSF, cerebrospinal fluid; CT, computed tomography; EBV, Epstein-Barr virus; Ig, immunoglobulin; PCR, polymerase chain reaction; PPD, tuberculosis skin test.
Encephalitis panel tested: NMDA receptor antibody, VGKC-complex antibody, CAD65 antibody, GABA-B receptor antibody, AMPA receptor antibody, ANNA-1, ANNA-2, ANNA-3, AGNA-1, PCA-1, PCA-2, PCA-Tr, amphiphysin antibody, CRMP-5-IgG.
Periodic fever panel genes tested: ELA2, LPIN2, MEFV, MVK, NLRP3, PSTP1P1, TNFRSF1A.
Initially, he had evidence of clinical response, and 3 days after the start of etoposide, he was extubated and weaned off of ionotropic support and had modest improvement in his coagulopathy and renal function. However, he continued to have daily fevers, splenomegaly, and laboratory criteria for HLH.
He then experienced acute clinical deterioration after 10 days of HLH treatment, despite receiving a dose of anakinra (1 mg/kg IV); he developed severe pulmonary edema leading to respiratory failure, recurrent hemodynamic instability, and worsening liver and renal dysfunction. A bone marrow biopsy at that time demonstrated significant hemophagocytosis, which led to the diagnosis of refractory HLH.
Because alemtuzumab was not available for 72 hours and the patient continued to deteriorate, the decision was made to administer oral ruxolitinib in addition to dexamethasone. Ruxolitinib 2.5 mg twice per day was started based on dosing used for graft-versus-host disease treatment.6,7 Within 24 hours of starting ruxolitinib, our patient became afebrile, with rapid improvement in respiratory, liver, and hemodynamic function, improvement in inflammatory markers, and decrease in transfusion requirements (Figure 1). He no longer required ionotropic support after 24 hours and was extubated within 3 days. Because he was refractory to etoposide, he did not receive any additional doses after the initiation of ruxolitinib. Genetic testing was performed and was negative for any known mutations in genes causing HLH (AP3B1, BLOC1S6, CD27, ITK, LYST, MAGT1, PRF1, RAB27A, SH2D1A, SLC7A7, STX11, STXBP2, UNC13D, and XIAP). Additionally, targeted exome sequencing was performed at our institution on a research protocol and was negative for any known mutations associated with immune deficiencies. The trigger for this patient’s HLH remains unknown. The patient is currently well and successfully underwent treosulfan-based unrelated-donor bone marrow transplantation for treatment of his refractory HLH.
Results and discussion
There are few data surrounding salvage therapy for patients with refractory HLH. Patients for whom initial therapy fails have a poor prognosis, with a mortality rate of >50%.2,8 There are case reports and small case series of patients with refractory HLH treated with infliximab, anakinra, alemtuzamab, or daclizumab and a currently ongoing clinical trial using anti–IFN-γ.2,9-13 This is the first report of a patient with HLH who has been successfully treated with ruxolitinib.
Ruxolitinib inhibits activation of JAK1/2 and its downstream signaling pathways.14 The JAK1/2 pathway is activated by cytokines, specifically IFN-γ, IL-2, and IL-6, which are key contributors to inflammation in HLH.4 These cytokines bind to JAK receptors, leading to activation of the STAT family of transcription factors and regulation of downstream target genes.14 Blockade of this pathway decreases cytokine signaling and inflammation. JAK1/2 inhibitors, such as ruxolitinib, are approved by the US Food and Drug Administration for the treatment of polycythemia vera and myelofibrosis in adults. Recent data support the efficacy of ruxolitinib in reducing the symptoms of proinflammatory diseases including rheumatoid arthritis, ulcerative colitis, myeloproliferative disorders, psoriasis, and graft-versus-host disease.6,7,15-22 In murine models, ruxolitinib has been shown to both prevent and treat HLH by decreasing cytokine production and inflammation via inhibition of STAT1 signaling.4,5 There is an active trial using ruxolitinib in adults with secondary HLH, but there have been no reports of pediatric patients who have received ruxolitinib for the treatment of HLH.
Our patient had a rapid clinical decline while receiving first-line therapy with etoposide and dexamethasone, necessitating urgent escalation in HLH treatment. Alemtuzumab was unavailable, and anakinra was ineffective, prompting further escalation to ruxolitinib. Within 24 hours of the first dose of ruxolitinib, the patient became afebrile, with dramatic improvement in inflammatory markers and organ function.
Whether our patient’s improvement was due to ruxolitinib alone or in combination with anakinra is uncertain. However, the patient did not show clinical improvement until after the addition of ruxolitinib. We hypothesize that the cytokine blockade from ruxolitinib either alone or in combination with anakinra effectively halted the ongoing inflammatory dysregulation from HLH. Our patient tolerated ruxolitinib with only mild nausea that was well controlled with antiemetics. This symptom has subsequently resolved.
Our patient continued to receive ruxolitinib with weaning doses of dexamethasone biweekly as therapy for refractory HLH with normalization of HLH markers. He then underwent successful treosulfan-based unrelated-donor transplantation and was recently discharged from the hospital. Given this dramatic response, ruxolitinib should be evaluated further in pediatric clinical trials as targeted treatment for HLH.
Acknowledgments
This work was supported by the National Institutes of Health, National Center for Advancing Translational Sciences through grants UL1TR001436 and 1TL1TR001437.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: L.B. and L.P. drafted the initial manuscript; M.T., D.M., R.P., S.R., and J.T. revised the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julie Talano, Department of Pediatrics, Division of Hematology, Oncology, Blood and Marrow Transplant, Medical College of Wisconsin, 8701 Watertown Plank Rd, MFRC 3018, Milwaukee, WI 53226; e-mail: jtalano@mcw.edu.
References
Author notes
L.B. and L.P. contributed equally to this work.