Key Points
Pre–allo-HSCT age-adjusted recipient telomere length and mismatched unrelated donor predict TRM.
Abstract
Various pretransplant patient and disease characteristics are associated with treatment-related mortality (TRM) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). However, TRM cannot yet be satisfactorily predicted. We prospectively investigated the aggregate impact of pretransplant clinical variables (period, donor/recipient age, gender, cytomegalovirus status, disease risk, stem cell source, and HLA matching), comorbidity index scores (Hematopoietic Cell Transplantation Comorbidity Index), and biological markers (telomere length, ferritin, and C-reactive protein) on TRM in single-center patients receiving a first allo-HSCT. From 2006 to 2012, all variables were available for 178 patients. In multivariate analysis, only mismatched unrelated donor (hazard ratio [HR], 2.79; 95% confidence interval [CI], 1.19-6.58; P = .019) and shorter age-adjusted recipient telomere length (HR, 2.17; 95% CI, 1.03-4.57; P = .041) were independently associated with TRM. Pre–allo-HSCT age-adjusted telomere length thus appears to be a useful new predictor of TRM in the setting of HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative procedure for numerous malignant and nonmalignant hematological diseases. However, relapse and nonrelapse complications remain responsible for significant morbidity and mortality, making estimates of overall survival (OS) and treatment-related mortality (TRM) important when considering therapeutic allo-HSCT.
TRM cannot yet be satisfactorily predicted. As many patient and disease pretransplant characteristics may be associated with TRM, the Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI)1 may help physicians consider patient status, OS, and TRM. Furthermore, biological markers, including ferritin2 and C-reactive protein (CRP),3-8 have been found to correlate with OS and/or TRM. More recently, we and others have reported on the correlation of recipient or donor telomere length with OS and TRM.9,10
A number of clinical and biological factors may be used to inform transplantation-related decision making. However, clinical and biological data, including pretransplant telomere length and comorbidity index scores, are mostly assessed separately, making it difficult to gauge each factor’s contribution to TRM. In this report, we prospectively investigated the aggregate impact of all factors known to affect TRM in a single-center patient cohort.
Methods
All consecutive patients receiving a first allo-HSCT for malignant or nonmalignant disease at Saint Louis Hospital between January 2006 and June 2012 were eligible for inclusion. Umbilical cord blood recipients were excluded. HLA matching was determined by molecular biology (4-digit) for HLA-A, -B, -C, -DQ, and -DR. Donors were matched related donors (MRDs), matched unrelated donors (MUDs), or 9/10 mismatched unrelated donors (MMUDs). Supportive care remained unchanged during the study period: patients were hospitalized in single rooms ventilated with high-efficiency particulate air filtration systems. Broad spectrum antibiotics were administered for fever during neutropenia, and antifungal treatment was added for persistent fever unresponsive to antibiotic therapy. All patients received prophylactic low-dose acyclovir. All patients received anti-Candida prophylaxis during aplasia and anti-Aspergillus prophylaxis in cases where steroids were administered. Prophylaxis of Pneumocystis jiroveci was given after engraftment. Cytomegalovirus (CMV) and Epstein-Barr virus DNA polymerase chain reaction and serum galactomannan testing were performed twice weekly during the first 100 days after allo-HSCT.
Clinical variables assessed were the following: donor/recipient ages, gender and CMV status, transplant year, disease risk (American Society for Blood and Marrow Transplantation 2006 [ASBMT 2006] Request For Information classification), stem cell source, and HLA matching. Comorbidities were assessed by the HCT-CI and coded prospectively for all patients by a single observer (A.X.). HCT-CI scores were ranked into previously published categories (0, 1-2, ≥3).1 Ferritin, CRP, and biological data for HCT-CI were recorded in the fortnight before the conditioning regimen was started. Ferritin was considered < or ≥2500 ng/mL2 and CRP < or ≥9 mg/L, as previously reported.9
Recipient and donor telomere lengths were determined in peripheral blood leukocytes by quantitative polymerase chain reaction, as previously described.9 Age-adjusted patient telomere length was determined by subtracting linear predicted length from observed length. Predicted telomere length was calculated based on the predicted value (a + b × age) by fitting the linear regression model (Y = α + β × age + ε) based on donor telomere length. In a previous work on an independent patient cohort,9 we had observed a correlation between poorer clinical outcomes and lowest-quartile telomere length. In this cohort, we applied the same categorization: shorter (first quartile) vs longer telomeres (upper 3 quartiles). Patients or legal guardians signed informed consent according to protocols approved by the Saint-Louis Hospital Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki.
TRM and OS probabilities were assessed using age-adjusted telomere length quartiles. TRM was defined as death without previous relapse/progression (Facilitating International Prospective Clinical Trials in Stem Cell Transplantation, European Society for Blood and Marrow Transplantation). Patient age, sex, and other baseline characteristics were described using summary statistics (means, proportions, and standard deviations) stratified by age-adjusted telomere quartiles. P values based on multisample tests for proportions and the analysis of variance tests or Kruskal-Wallis nonparametric tests were used to compare baseline characteristics. Survival rates were calculated using Kaplan-Meier analysis. Fine and Gray univariate and multivariate methods for competing risks studies were used to assess prognostic TRM factors, with relapse as a competing event. All tests were 2-sided, with a type I error rate of 0.05. Statistical analyses were performed with SPSS 19, Stata 11, and R package “cmprsk.”
Results
Characteristics of study patients
A total of 488 patients were eligible. Clinical and biological data and good-quality DNA to assess telomere length were available in 178 cases. Patient demographic, disease, and transplant characteristics are summarized in Table 1. Demographic characteristics, median follow-up, OS, and TRM were comparable between patients with (n = 178) or without (n = 310) telomere data. We therefore limited our analysis to the cohort of 178 patients with all data available. Median age was 41 years (range, 6-68), and 12% of patients were younger than 15. Median donor age was 33 years (range, 6-67). Most patients (87.1%) were transplanted for a malignant disease. Donors were MRD, MUD, and MMUD in 54.5%, 33.7%, and 11.8% of cases, respectively. Conditioning regimen was reduced-intensity or sequential in 54.5% of cases. Stem cell source was peripheral blood (77%) or bone marrow (23%). Median follow-up was 35.1 months (range, 1.1-105.3); 5-year OS, 63% (95% CI, 55-71); and 5-year TRM, 20% (95% CI, 14-26), in line with previous reports.11
Patient characteristics
| Variable . | Total . | Telomeres . | P . | |
|---|---|---|---|---|
| (n = 488), no. (%) . | No (n = 310), no. (%) . | Yes (n = 178), no. (%) . | ||
| Recipient sex, M/F | 291/197 (59.6/40.4) | 184/126 (59.4/40.6) | 107/71 (60.1/39.9) | .87 |
| Donor sex, M/F | 298/190 (61.1/38.9) | 191/119 (61.6/38.4) | 107/71 (60.1/39.9) | .74 |
| Donor/recipient sex mismatch | ||||
| F donor/M recipient | 105 (21.5) | 65 (21) | 40 (22.5) | .7 |
| Recipient median age, y (range) | 43 (5-68) | 44 (5-26) | 41 (6-68) | .11 |
| Patients <15 y | 31 (6.4) | 19 (6.1) | 12 (6.7) | .79 |
| Donor median age, y (range) | 34 (2-67) | 35 (2-66) | 33 (6-67) | .44 |
| CMV recipient, positive/negative/unknown | 268/210/10 (55/43/2) | 182/125/3 (26.4/40.3/1) | 86/85/7 (48.3/47.7/3.9) | .058 |
| CMV donor, positive/negative/unknown | 227/249/12 (46.5/51/2.5) | 154/149/7 (49.7/48.1/2.3) | 73/100/5 (41/56.2/2.8) | .07 |
| CMV R+/D− | 109 (23.2) | 68 (22.6) | 41 (24.3) | .086 |
| Disease | .17 | |||
| Acute leukemia | 211 (43.2) | 133 (42.9) | 80 (44.9) | |
| MPN | 42 (8.6) | 22 (7.1) | 20 (11.2) | |
| MDS | 52 (10.7) | 38 (12.3) | 14 (7.9) | |
| Lymphoma | 66 (13.5) | 47 (15.2) | 19 (10.7) | |
| Chronic lymphoproliferative disease | 59 (12.1) | 37 (11.9) | 22 (12.3) | |
| Other | 58 (11.9) | 33 (10.6) | 23 (12.9) | |
| Disease risk (ASBMT 2006) | .62 | |||
| Standard | 259 (53.1) | 163 (52.6) | 96 (53.9) | |
| High | 173 (35.5) | 114 (36.8) | 59 (33.1) | |
| Nonmalignant | 56 (11.5) | 33 (10.6) | 23 (12.9) | |
| HLA matching | .46 | |||
| Matched related | 251 (51.4) | 154 (49.7) | 97 (54.5) | |
| Matched unrelated | 182 (37.3) | 122 (39.4) | 60 (33.7) | |
| Mismatched unrelated | 55 (11.3) | 34 (11) | 21 (11.8) | |
| Stem cell source, BM/PBSC | 108/380 (22.1/77.9) | 67/243 (21.6/78.4) | 41/137 (23/77) | .72 |
| Conditioning regimen | .124 | |||
| MAC | 200 (41) | 119 (38.4) | 81 (45.5) | |
| RIC or sequential | 288 (59) | 191 (61.6) | 97 (54.5) | |
| ATG in conditioning | 175 (35.9) | 118 (38.1) | 57 (32) | .18 |
| Conditioning | .38 | |||
| MAC no TBI | 123 (25.2) | 71 (22.9) | 52 (29.2) | |
| MAC TBI 12 Gy | 77 (15.8) | 45 (15.5) | 26 (16.3) | |
| RIC no TBI | 184 (37.7) | 124 (40) | 60 (33.7) | |
| RIC TBI 2 Gy | 104 (21.3) | 67 (21.6) | 37 (20.8) | |
| GVHD prophylaxis | .121 | |||
| CsA/MTX | 211 (43.3) | 125 (40.3) | 86 (48.6) | |
| CsA/MMF | 263 (54) | 177 (57.1) | 86 (48.6) | |
| Other | 13 (2.7) | 8 (2.6) | 5 (2.8) | |
| Transplantation year | <10e-10 | |||
| 2006 | 62 (12.7) | 48 (15.5) | 14 (7.9) | |
| 2007 | 56 (11.5) | 22 (7.1) | 34 (19.1) | |
| 2008 | 61 (12.5) | 27 (8.7) | 34 (19.1) | |
| 2009 | 71 (14.5) | 67 (21.6) | 4 (2.2) | |
| 2010 | 89 (18.2) | 81 (26.1) | 8 (4.5) | |
| 2011 | 94 (19.3) | 59 (19) | 35 (19.7) | |
| 2012 | 55 (11.3) | 6 (1.9) | 49 (27.5) | |
| Ferritin | .54 | |||
| <2500 ng/mL | 236 (86.1) | 161 (87) | 75 (84.3) | |
| ≥2500 ng/mL | 38 (13.9) | 24 (13) | 14 (15.7) | |
| Missing data | 214 | 125 | 89 | |
| CRP | .77 | |||
| ≤9 mg/L | 339 (81.5) | 213 (81.9) | 126 (80.8) | |
| >9 mg/L | 77 (18.5) | 47 (18.1) | 30 (19.2) | |
| Missing data | 72 | 50 | 22 | |
| HCT-CI | .83 | |||
| 0 | 39 (8) | 26 (8.4) | 13 (7.3) | |
| 1 or 2 | 101 (20.7) | 62 (20) | 39 (21.9) | |
| ≥3 | 348 (71.3) | 222 (71.6) | 126 (70.8) | |
| Variable . | Total . | Telomeres . | P . | |
|---|---|---|---|---|
| (n = 488), no. (%) . | No (n = 310), no. (%) . | Yes (n = 178), no. (%) . | ||
| Recipient sex, M/F | 291/197 (59.6/40.4) | 184/126 (59.4/40.6) | 107/71 (60.1/39.9) | .87 |
| Donor sex, M/F | 298/190 (61.1/38.9) | 191/119 (61.6/38.4) | 107/71 (60.1/39.9) | .74 |
| Donor/recipient sex mismatch | ||||
| F donor/M recipient | 105 (21.5) | 65 (21) | 40 (22.5) | .7 |
| Recipient median age, y (range) | 43 (5-68) | 44 (5-26) | 41 (6-68) | .11 |
| Patients <15 y | 31 (6.4) | 19 (6.1) | 12 (6.7) | .79 |
| Donor median age, y (range) | 34 (2-67) | 35 (2-66) | 33 (6-67) | .44 |
| CMV recipient, positive/negative/unknown | 268/210/10 (55/43/2) | 182/125/3 (26.4/40.3/1) | 86/85/7 (48.3/47.7/3.9) | .058 |
| CMV donor, positive/negative/unknown | 227/249/12 (46.5/51/2.5) | 154/149/7 (49.7/48.1/2.3) | 73/100/5 (41/56.2/2.8) | .07 |
| CMV R+/D− | 109 (23.2) | 68 (22.6) | 41 (24.3) | .086 |
| Disease | .17 | |||
| Acute leukemia | 211 (43.2) | 133 (42.9) | 80 (44.9) | |
| MPN | 42 (8.6) | 22 (7.1) | 20 (11.2) | |
| MDS | 52 (10.7) | 38 (12.3) | 14 (7.9) | |
| Lymphoma | 66 (13.5) | 47 (15.2) | 19 (10.7) | |
| Chronic lymphoproliferative disease | 59 (12.1) | 37 (11.9) | 22 (12.3) | |
| Other | 58 (11.9) | 33 (10.6) | 23 (12.9) | |
| Disease risk (ASBMT 2006) | .62 | |||
| Standard | 259 (53.1) | 163 (52.6) | 96 (53.9) | |
| High | 173 (35.5) | 114 (36.8) | 59 (33.1) | |
| Nonmalignant | 56 (11.5) | 33 (10.6) | 23 (12.9) | |
| HLA matching | .46 | |||
| Matched related | 251 (51.4) | 154 (49.7) | 97 (54.5) | |
| Matched unrelated | 182 (37.3) | 122 (39.4) | 60 (33.7) | |
| Mismatched unrelated | 55 (11.3) | 34 (11) | 21 (11.8) | |
| Stem cell source, BM/PBSC | 108/380 (22.1/77.9) | 67/243 (21.6/78.4) | 41/137 (23/77) | .72 |
| Conditioning regimen | .124 | |||
| MAC | 200 (41) | 119 (38.4) | 81 (45.5) | |
| RIC or sequential | 288 (59) | 191 (61.6) | 97 (54.5) | |
| ATG in conditioning | 175 (35.9) | 118 (38.1) | 57 (32) | .18 |
| Conditioning | .38 | |||
| MAC no TBI | 123 (25.2) | 71 (22.9) | 52 (29.2) | |
| MAC TBI 12 Gy | 77 (15.8) | 45 (15.5) | 26 (16.3) | |
| RIC no TBI | 184 (37.7) | 124 (40) | 60 (33.7) | |
| RIC TBI 2 Gy | 104 (21.3) | 67 (21.6) | 37 (20.8) | |
| GVHD prophylaxis | .121 | |||
| CsA/MTX | 211 (43.3) | 125 (40.3) | 86 (48.6) | |
| CsA/MMF | 263 (54) | 177 (57.1) | 86 (48.6) | |
| Other | 13 (2.7) | 8 (2.6) | 5 (2.8) | |
| Transplantation year | <10e-10 | |||
| 2006 | 62 (12.7) | 48 (15.5) | 14 (7.9) | |
| 2007 | 56 (11.5) | 22 (7.1) | 34 (19.1) | |
| 2008 | 61 (12.5) | 27 (8.7) | 34 (19.1) | |
| 2009 | 71 (14.5) | 67 (21.6) | 4 (2.2) | |
| 2010 | 89 (18.2) | 81 (26.1) | 8 (4.5) | |
| 2011 | 94 (19.3) | 59 (19) | 35 (19.7) | |
| 2012 | 55 (11.3) | 6 (1.9) | 49 (27.5) | |
| Ferritin | .54 | |||
| <2500 ng/mL | 236 (86.1) | 161 (87) | 75 (84.3) | |
| ≥2500 ng/mL | 38 (13.9) | 24 (13) | 14 (15.7) | |
| Missing data | 214 | 125 | 89 | |
| CRP | .77 | |||
| ≤9 mg/L | 339 (81.5) | 213 (81.9) | 126 (80.8) | |
| >9 mg/L | 77 (18.5) | 47 (18.1) | 30 (19.2) | |
| Missing data | 72 | 50 | 22 | |
| HCT-CI | .83 | |||
| 0 | 39 (8) | 26 (8.4) | 13 (7.3) | |
| 1 or 2 | 101 (20.7) | 62 (20) | 39 (21.9) | |
| ≥3 | 348 (71.3) | 222 (71.6) | 126 (70.8) | |
ATG, anti-thymocyte globulin; BM, bone marrow; CsA, cyclosporine A; F, female; GVHD, graft-versus-host disease; M, male; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MPN, myeloproliferative neoplasm; MTX, methotrexate; PBSC, peripheral blood stem cell; RIC, reduced-intensity conditioning; TBI, total-body irradiation.
Telomere length in patients before allo-HSCT
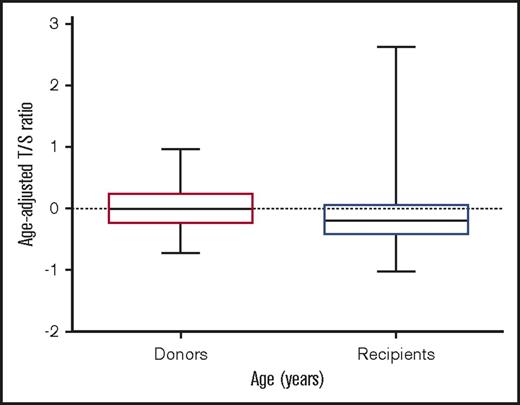
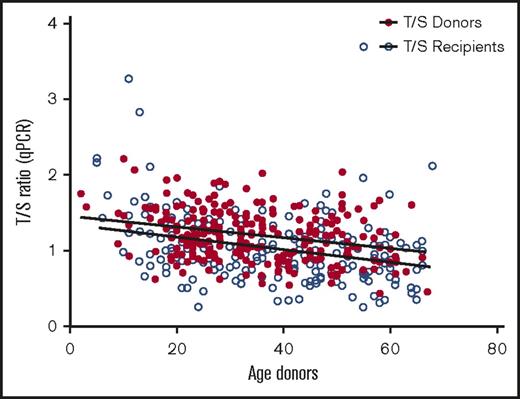
Telomere length was measured in 178 patients and respective donors, who served as controls to build the standard curve. Telomeres were shorter in patients in comparison with their sibling donors (Figure 1). Pretransplantation telomere length in the patients’ samples was adjusted for age according to the telomere attrition observed with aging in the donor group. As the distribution of telomere length was linear from 10 to 60 years of age in donors, we determined the linear curve of telomere length as a function of age (Figure 2). The patient population was dichotomized into those with short telomeres (lowest age-adjusted telomere length quartile) and longer telomeres (upper 3 quartiles), as previously described.9
Telomere length distribution between sibling donors (left) and recipients (right).
Telomere length distribution between sibling donors (left) and recipients (right).
Linear curve representation of telomere-length distribution according to age for donors and recipients.
Linear curve representation of telomere-length distribution according to age for donors and recipients.
Factors associated with TRM
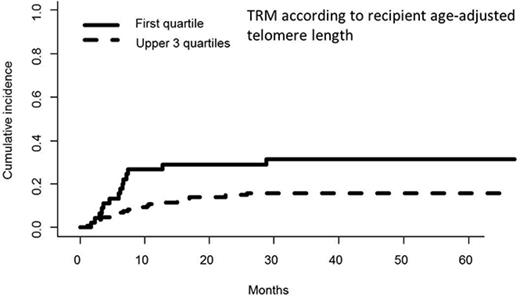
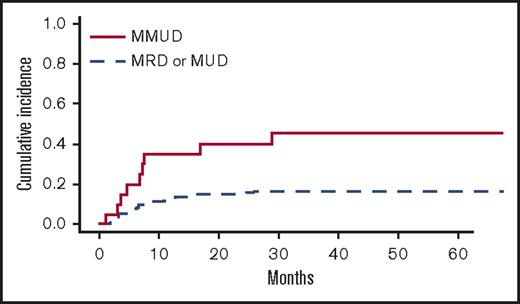
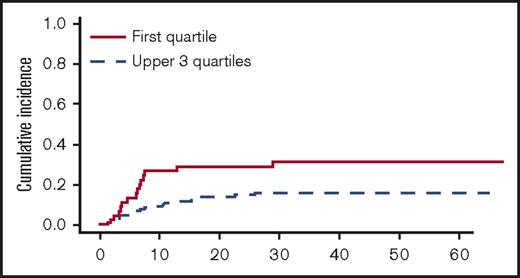
In univariate analyses, the factors significantly associated with TRM were the following: recipient age, unrelated vs related donor (P = .00056), HLA matching (MMUD vs MUD vs MRD, P = .0005) (Figure 3), donor and recipient CMV status (recipient positive/donor negative R+/D− vs others, P = .044), and age-adjusted recipient telomere length (shorter vs longer telomeres, P = .022) (Figure 4). Ferritin, CRP, and HCT-CI were not associated with TRM.12
Cumulative incidence of TRM (univariate analysis) by HLA-matching (MMUD vs others).
Cumulative incidence of TRM (univariate analysis) by HLA-matching (MMUD vs others).
Cumulative incidence of TRM (univariate analysis) by telomere length (first quartile vs upper 3 quartiles).
Cumulative incidence of TRM (univariate analysis) by telomere length (first quartile vs upper 3 quartiles).
In multivariate analysis, MMUD (HR, 2.79; 95% CI, 1.19-6.58; P = .019) and short recipient telomere length (HR, 2.17; 95% CI, 1.03-4.57; P = .041) were the only pretransplant factors independently associated with higher TRM.
Discussion
In this study, we prospectively investigated the aggregate ability of pretransplant factors to predict TRM after allo-HSCT. When comorbidity index scores were examined together with clinical and biological markers, MMUD and age-adjusted recipient telomere length were the only predictors of TRM after allo-HSCT.
Previously, we found that shorter pretransplant recipient telomere length correlated with higher TRM, but the study was limited to patients transplanted from HLA-identical siblings after a myeloablative conditioning regimen.9 This time, we broadened our analysis to a more diverse, independent population, in which recipient telomere length was confirmed as a predictor of TRM. Moreover, others have reported that longer donor telomere length correlates with survival, but only in patients transplanted for aplastic anemia.10 We failed to find a correlation between donor telomere length and TRM or OS (data not shown), suggesting that such influences on transplant outcomes may only occur in aplastic anemia patients. As telomere erosion may be etiologic in aplastic anemia and is genetically determined by mutations in telomere-associated genes, donor telomere length may act as a surrogate for the donor’s silent genetic status, which may result in a limited stem cell reserve. Besides genetic predisposition, aging, environment (reactive oxidative stress), and iatrogenic interventions (chemotherapy and radiation) may also cause telomere loss. Telomere shortening in our population may result from pretransplant exposure to multiple chemotherapy courses.9 Equivalent chemotherapy/radiation doses may not have equivalent consequences on each patient because of different medical practices or patient responses to drug metabolism and toxicity. Cumulative DNA damage and subsequent accelerated physiological “aging” may be seen more clearly in telomere length than medical history.
Our work has strengths and limitations. Comorbidities were coded prospectively for all patients by a single observer, which may make comorbidity scoring more consistent.12 However, our findings should be interpreted with caution, as good-quality DNA to assess telomere length was available for only 178 patients, limiting the size of our cohort. These preliminary results need to be confirmed in a larger, multicentric prospective study.
In conclusion, age-adjusted recipient pretransplant telomere length and MMUDs were identified as independent predictors of TRM after allo-HSCT. Predicting major transplantation-related complications is critical to transplantation decisions. Pre-HSCT telomere length thus appears to be a useful new predictor of TRM in the setting of HSCT. However, these results need to be confirmed by further studies involving a larger allo-HSCT patient cohort.
Acknowledgment
The authors thank David Williams for proofreading and helping to edit the final manuscript.
Authorship
Contribution: A.X., M.B., G.S., R.T.C., and R.P.d.L. conceived and designed the study; A.X., R.C., M.B., M.R., N.D., T.C., A.C.-H., F.S.d.F., D.M., G.S., R.T.C., and R.P.d.L. provided study material and patients; A.X., R.C., M.B., R.T.C., and R.P.d.L. collected and assembled data; A.X., M.B., G.S., R.T.C., and R.P.d.L. analyzed and interpreted data; A.X., R.T.C., and R.P.d.L. wrote the manuscript; and A.X. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Aliénor Xhaard, Service d’hématologie-greffe, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, 75010 Paris, France; e-mail: alienor.xhaard@aphp.fr.
References
Author notes
R.T.C. and R.P.d.L. contributed equally to this study.