Key Points
In vivo tracing of 13C15N-glutamine reveals that glutaminolysis is an essential contributor to RBC metabolism following HS.
Inhibition of glutaminolysis impairs transamination reactions and GSH synthesis, promoting early mortality in hemorrhaged rats.
Abstract
Red blood cells (RBCs) are the most abundant host cell in the human body and play a critical role in oxygen transport and systemic metabolic homeostasis. Hypoxic metabolic reprogramming of RBCs in response to high-altitude hypoxia or anaerobic storage in the blood bank has been extensively described. However, little is known about the RBC metabolism following hemorrhagic shock (HS), the most common preventable cause of death in trauma, the global leading cause of total life-years lost. Metabolomics analyses were performed through ultra-high pressure liquid chromatography–mass spectrometry on RBCs from Sprague-Dawley rats undergoing HS (mean arterial pressure [MAP], <30 mm Hg) in comparison with sham rats (MAP, >80 mm Hg). Steady-state measurements were accompanied by metabolic flux analysis upon tracing of in vivo–injected 13C15N-glutamine or inhibition of glutaminolysis using the anticancer drug CB-839. RBC metabolic phenotypes recapitulated the systemic metabolic reprogramming observed in plasma from the same rodent model. Results indicate that shock RBCs rely on glutamine to fuel glutathione (GSH) synthesis and pyruvate transamination, whereas abrogation of glutaminolysis conferred early mortality and exacerbated lactic acidosis and systemic accumulation of succinate, a predictor of mortality in the military and civilian critically ill populations. Glutamine is here identified as an essential amine group donor in HS RBCs, plasma, liver, and lungs, providing additional rationale for the central role glutaminolysis plays in metabolic reprogramming and survival following severe hemorrhage.
Introduction
Hemorrhagic shock (HS) is one of the main contributors to mortality by traumatic injury, the global leading cause of disability and mortality for people under the age of 441 (Centers for Disease Control and Prevention [CDC] data, June 2016). Along with traumatic brain injury, hemorrhage is the most common cause of preventable death in the trauma patient population. HS also underlies the etiology of some of the late untoward complications from traumatic injury, such as metabolic acidosis, coagulopathy, and inflammation. These complications are to some extent intertwined, such as in the case of inflammatory responses leading to the acute respiratory distress syndrome and multiple organ failure,2 processes that have been recently, in part, explained through the appreciation of novel metabolic mechanisms involving low-molecular-weight compounds, such as succinate.3 Deregulation of metabolism following HS is a long-established concept.4 Metabolic aberrations following hemorrhage can indeed predispose the body to inflammatory5-7 and coagulopathic8 complications. Understanding these mechanisms may soon provide actionable targets to improve short- and long-term outcomes in severely hemorrhaged patients in the civilian and military settings, for example, by determining whether early administration of plasma rather than normal saline can improve survival and metabolic recovery in humans, in like fashion to observations in a rodent model of profound HS.9
Appreciation of the metabolic aberrations following hemorrhage and severe HS has been recently revamped by the application of mass spectrometry (MS)-based metabolomics to the study of this clinically relevant issue. Common features of HS in humans5,6,10,11 and animal models (rodents, swine, and nonhuman primates)7,9,10,12-14 have been described, including increases in plasma glucose (also referred to as traumatic diabetes), lactic acidosis, increased lipid mobilization and lipolysis, proteolysis, amino acid catabolism, and impaired redox homeostasis. Most of these changes are a result of tissue hypoxia following depressed perfusion in hemorrhaged patients and animals, an observation relevant in that it prompts the immediate comparison to the deranged metabolism observed in response to physiological hypoxia at high altitude15 or pathological hypoxia following ischemic injury.16,17 Recent investigations have highlighted the critical role of small-molecule metabolites such as succinate (a bioactive dicarboxylic acid and metabolic intermediate in mitochondria) as both a metabolic marker of tissue hypoxia and mitochondrial uncoupling, similar to lactate, as well as a driver of ischemia reperfusion injury through mitochondrial reactive oxygen species (ROS).16,18 Succinate has been linked to coagulopathy and acute respiratory distress syndrome through its role in ischemia reperfusion injury,3,19,20 and recently has been associated with increased mortality following HS in military21 and civilian22 populations.
Owing to their critical role in systemic oxygen transport and delivery, red blood cells (RBCs) are obvious yet underinvestigated players within the framework of hemorrhagic hypoxia-mediated metabolic responses. Indeed, lactic acidosis23 and succinate accumulation17 are both explained by the exacerbation of anaerobic metabolism and mitochondrial uncoupling, both phenomena being linked to scarce oxygen availability secondary to HS. Recently, we expanded on historical observations24 on the role of metabolic reprogramming of RBCs in promoting oxygen off-loading and counteracting tissue hypoxia at high altitude (>5000 m).15 These metabolic adaptations favor deoxyhemoglobin stabilization through increases in glycolysis that in turn promote the generation of adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (DPG), key allosteric modulators that promote a right shift in the oxygen-binding curve of hemoglobin and thus oxygen off-loading.25-29 Observations relevant to RBC physiological adaptations to high-altitude hypoxia have been recently exploited to propose anaerobic storage as an alternative strategy to mitigate the storage lesion of packed RBCs in the blood bank.30-33 Factors influencing RBC hypoxic metabolic reprogramming have been identified in the hypoxia-induced stabilization of deoxyhemoglobin, which competes with glycolytic enzymes for the N-terminal cytosolic domain of band 3, thereby promoting enzyme release from the membrane to the cytosol with subsequent increases in the activity of glyceraldehyde 3-phosphate dehydrogenase and phosphofructokinase, increased glycolytic fluxes, and ATP/DPG generation to reinforce this feedback loop.25-29 Of note, plasma adenosine levels, which increase significantly upon early hemorrhage even before severe HS ensues in rats,12 are important upstream contributors to these mechanisms through signaling via the RBC adenosine receptor A2B.25,26
Previous studies by Deitch and colleagues34-36 confirmed that HS adversely affects RBC morphology, probably due to oxidative stress. Despite this preliminary evidence, to the best of our knowledge, no metabolomics study of RBCs following hemorrhagic insult has been published to date. Unanswered questions remain as to whether HS reprograms RBCs similarly to what is observed in response to physiologic hypoxia or ex vivo exposure to anaerobiosis. Moreover, the accumulation of the mortality marker21 succinate in response to HS in rats is in part explained by glutaminolysis13 rather than glucose catabolism.7 This result suggested that early inhibition of glutaminolysis may decrease availability of a limiting substrate for the generation of hemorrhagic succinate,13 although late enteral supplementation of glutamine would still be beneficial in critically ill patients upon resuscitation.37,38 Conversely, observations by Deitch and colleagues would recommend caution with respect to limiting glutaminolysis, a pathway that fuels glutathione (GSH) synthesis in hypoxic RBCs31 and is already partially compromised by HS.36 In light of these considerations, here we report for the first time the effects of HS on the RBC metabolome in a well-established rodent model. Through a combination of sophisticated in vivo tracing experiments with 13C15N-glutamine and in vivo inhibition of glutaminolysis, we investigate the role of glutamine in mediating antioxidant GSH homeostasis and transamination reactions, and the effect these alterations have on the levels of lactate and succinate in RBCs, plasma, and organs of severely hemorrhaged rats.
Materials and methods
Rat model of HS
Nonlethal HS was induced in age-, sex-, and weight-matched Sprague-Dawley rats (n = 3) by controlled hemorrhage from the femoral artery until the rats achieved mean arterial pressure (MAP) equal to 30 mm Hg (MAP 30), as previously described.12 Sham rats (n = 3; no trauma; MAP, >80 mm Hg) were used as controls at matched time points. RBCs were collected before HS (baseline) and at 15 or 60 minutes after (postshock and end, respectively). Experiments performed in this study received institutional animal care and use committee approval by the University of Colorado Denver.
In vivo flux experiments
Heavy-labeled RBCs were isolated from shock/sham (n = 8 per group) rats generated as previously described.13 At the baseline time point, shock and sham rats received a 1 mL/kg IV infusion of 13C5,15N2-glutamine (Cambridge Isotope Laboratories, Inc, Tewksbury, MA) in physiological solution (35 mM) at a rate of 0.5 mL per minute. Animals remained in shock for a total of 60 minutes.
Glutaminase inhibitor treatment
Sham (n = 3) and shock (n = 5) rats were treated as described in the previous section, with or without injection of the glutaminase inhibitor CB-839 (MedChem Express, Monmouth Junction, NJ) dissolved in dimethyl sulfoxide (DMSO), at doses and volume consistent with the vendor’s instruction. Sham (n = 3) and shock (n = 5) controls only injected with equal volumes of DMSO or saline were used as well to control for the effect of injected fluid volume and DMSO alone. Plasma and RBCs were collected before HS (baseline) and at 15 or 60 minutes after (postshock and end, respectively). Liver and lungs were collected at the end time point or upon death of the animal (4 of 5 of the shock rats treated with CB-839 died before this time point).
Sample processing and metabolite extraction
Plasma and RBCs were separated through centrifugation (10 minutes at 4°C and 2500g). A total of 20 µL of plasma, 50 µL of packed RBCs, or 10 mg of liver or lung tissue were extracted in 480, 450, or 1000 µL, respectively, of ice-cold extraction solution by vortex for 30 minutes at 4°C, as reported.39,40 Insoluble proteins and lipids were pelleted by centrifugation at 4°C for 10 minutes at 10 000g and supernatants were collected and stored at −80°C until subsequent analysis.
UHPLC-MS metabolomics
Analyses were performed using a Vanquish ultra-high-pressure liquid chromatography (UHPLC) system (Thermo Fisher Scientific, San Jose, CA) coupled online to a Q Exactive mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). Samples were resolved over a Kinetex C18 column (2.1 × 150 mm, 1.7 µm; Phenomenex, Torrance, CA) equipped with a guard column (SecurityGuard Ultracartridge; Phenomenex) at 25°C using a 3-minute isocratic condition of 5% acetonitrile, 95% water, and 0.1% formic acid flowed at 250 µL per minute as reported,39 or through a 9-minute gradient at 400 µL per minute from 5% to 95% B (A: water, 0.1% formic acid; B: acetonitrile, 0.1% formic acid).41 The mass spectrometer was operated independently in positive or negative ion mode, scanning in full MS mode (2 μscans) from 60 to 900 m/z at 70 000 resolution, with 4-kV spray voltage, 15 sheath gas, 5 auxiliary gas. Calibration was performed prior to analysis using Pierce Positive and Negative Ion Calibration Solutions (Thermo Fisher Scientific). Acquired data were then converted from .raw to .mzXML file format using Mass Matrix (Cleveland, OH). Metabolite assignments, isotopologue distributions, and correction for expected natural abundances of 13C and 15N isotopes were performed using MAVEN (Princeton, NJ).42
Graphs, pathway analyses, heat maps, and statistical analyses (either Student t test, analysis of variance [ANOVA], or partial least squares discriminant analysis [PLS-DA]) were prepared with GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA), GENE-E (Broad Institute, Cambridge, MA), and MetaboAnalyst 3.0.43
Results
HS affects RBC amino acid metabolism, transamination, and GSH homeostasis, and mirrors metabolic derangements previously observed in plasma
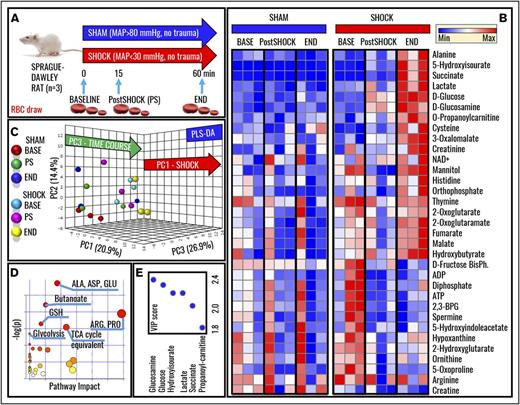
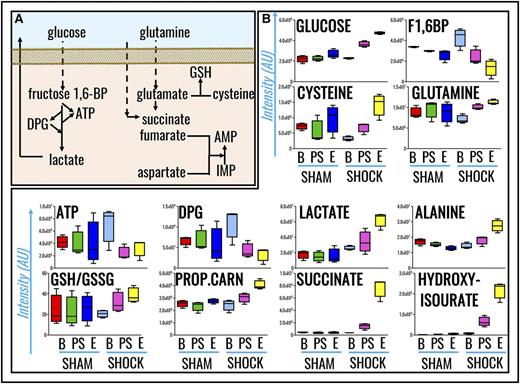
Metabolomics analyses were performed on RBCs from Sprague-Dawley rats undergoing HS to MAP <30 mm Hg for 0 minutes (baseline), 15 minutes (postshock), and 60 minutes (end), and results were compared with nonhemorrhaged shams (MAP, >80 mm Hg; Figure 1A). Raw data are reported in supplemental Table 1. Hierarchical clustering and heat-map elaborations of the data are extensively provided in supplemental Figure 2; Figure 1B highlights the most significant changes (repeated measures ANOVA; P < .05). Of note, only 5 metabolites significantly (P < .05, 2-tailed Student t test) increased (cysteine, serotonin, aspartate, indole-acetate; fold change, >2) or decreased (α-ketoglutarate; fold change, <0.5) in shock baseline animals in comparison with controls, a caveat either related to biological variability across animals from the sham and shock group, or resulting from the time necessary to hemorrhage rats and the stress response generated by the procedure. PLS-DA correctly classified samples on the basis of metabolic phenotypes, with principal component 1 (PC1) explaining 20.9% of the variance and mostly describing the effects of HS, PC3 (26.9%) accounting for time-course effects, and PC2 (14.4%) mostly explaining biological variability across samples (Figure 1C). Pathway analysis based on these metabolites revealed that amino acid metabolism (especially transamination and redox homeostasis-related metabolites), GSH homeostasis, glycolysis, and Krebs cycle carboxylic acid equivalents were significantly increased by HS RBCs (Figure 1D). Sorting metabolites on the basis of loading weights and variable importance in projection (VIP) scores further confirms these observations, showing that, in like fashion to previous metabolic observations on plasma from rats undergoing hemorrhage,12 metabolites with the highest fold changes and lowest P values (repeated measures ANOVA) include glucose, lactate, succinate, and purine catabolism/oxidation byproduct hydroxyisourate (Figure 1E). A pathway view of the main significant changes observed here is provided in Figure 2A. Briefly, increased glucose and lactate generation were here associated with unexpected decreases in the levels of glycolytic intermediates fructose 1,6-bisphosphate, ATP, and DPG (Figure 2B). Pyruvate transamination product alanine increased in the shock but not in the sham group. Analogously, propanoyl-carnitine, succinate, hydroxyisourate, cysteine, glutamine, reduced GSH, and the reduced fraction of the GSH pool (GSH/GSH disulfide [GSSG]) followed similar trends (Figure 2B).
Metabolomics of RBCs in a rat model of HS. (A) A well-established rodent model of HS in Sprague-Dawley rats was used to investigate the effect of severe HS (MAP, <30 mm Hg; no trauma) on RBC metabolism at baseline (BASE), 15, or 60 minutes through shock (Post-Shock and END, respectively). Results were compared against sham controls (no trauma; MAP, >80 mm Hg). (B) Heat-map and hierarchical-clustering analysis of metabolites was performed (B; an extended and vectorial version is provided in supplemental Figure 1). (C) PLS-DA was performed, revealing that metabolic phenotypes could discriminate samples on the basis of grouping (sham vs shock, PC1; explaining 20.9% of the variance) and time-course progression (PC3, 26.9%), whereas PC2 mostly explained biological variability across rats (14.4%). (D) Metabolites informing hierarchical clustering analysis and PLS-DA discrimination (ie, metabolites with the highest loading weights from the analysis in panel C) were used to elaborate pathway analyses of HS RBCs. (E) The top 5 metabolites are ranked on the basis of VIP scores from the PLS-DA analysis. 2,3-BPG, 2,3-bisphosphoglyceric acid; ADP, adenosine 5′-diphosphate; BisPh, bisphosphoglycerate; Max, maximum; Min, minimum; TCA, tricarboxylic acid.
Metabolomics of RBCs in a rat model of HS. (A) A well-established rodent model of HS in Sprague-Dawley rats was used to investigate the effect of severe HS (MAP, <30 mm Hg; no trauma) on RBC metabolism at baseline (BASE), 15, or 60 minutes through shock (Post-Shock and END, respectively). Results were compared against sham controls (no trauma; MAP, >80 mm Hg). (B) Heat-map and hierarchical-clustering analysis of metabolites was performed (B; an extended and vectorial version is provided in supplemental Figure 1). (C) PLS-DA was performed, revealing that metabolic phenotypes could discriminate samples on the basis of grouping (sham vs shock, PC1; explaining 20.9% of the variance) and time-course progression (PC3, 26.9%), whereas PC2 mostly explained biological variability across rats (14.4%). (D) Metabolites informing hierarchical clustering analysis and PLS-DA discrimination (ie, metabolites with the highest loading weights from the analysis in panel C) were used to elaborate pathway analyses of HS RBCs. (E) The top 5 metabolites are ranked on the basis of VIP scores from the PLS-DA analysis. 2,3-BPG, 2,3-bisphosphoglyceric acid; ADP, adenosine 5′-diphosphate; BisPh, bisphosphoglycerate; Max, maximum; Min, minimum; TCA, tricarboxylic acid.
RBC metabolic pathways affected by HS. (A) A schematic overview of these pathways. (B) Single box-and-whisker plots are shown for metabolites with the highest fold change and lowest P values when comparing sham vs shock RBCs (all of the results shown have P values <.01; ANOVA). From left to right, the following color code was used: (sham group) red, baseline; green, postshock; blue, end; (shock group) light blue, shock baseline; purple, postshock; yellow, shock end. 1,6-BP, 1,6-bisphosphate; AMP, adenosine monophosphate; AU, arbitrary unit; B, baseline; E, end; IMP, inosine monophosphate; PROP.CARN, propanoyl-carnitine; PS, postshock.
RBC metabolic pathways affected by HS. (A) A schematic overview of these pathways. (B) Single box-and-whisker plots are shown for metabolites with the highest fold change and lowest P values when comparing sham vs shock RBCs (all of the results shown have P values <.01; ANOVA). From left to right, the following color code was used: (sham group) red, baseline; green, postshock; blue, end; (shock group) light blue, shock baseline; purple, postshock; yellow, shock end. 1,6-BP, 1,6-bisphosphate; AMP, adenosine monophosphate; AU, arbitrary unit; B, baseline; E, end; IMP, inosine monophosphate; PROP.CARN, propanoyl-carnitine; PS, postshock.
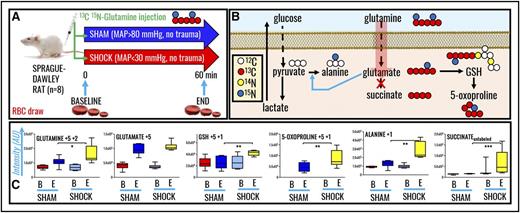
In vivo tracing of heavy glutamine metabolism reveals a role for alanine synthesis through pyruvate transamination and de novo GSH synthesis in hemorrhaged rat RBCs
To confirm and expand upon steady-state observations, we performed in vivo tracing experiments with 13C15N-glutamine to monitor isotopologue distribution in glutamine-derived metabolites. A summary of the experimental design (n = 8 each for both sham and shock groups) is provided in Figure 3A. A preview of the expected heavy isotopologues derived from glutamine labeling is shown in Figure 3B. Shock increased RBC glutamine uptake (M+5+2). Glutamine uptake resulted in similar heavy glutamate accumulation in both groups, though only shock rats were characterized by significantly higher levels of de novo synthesized reduced GSH and 5-oxoproline (Figure 3C). Glutamine-derived glutamate can serve as amine group donor for transamination reactions generating aspartate (M+1) and alanine (M+1) from oxaloacetate and pyruvate, respectively. Although these reactions occur mostly in the liver, we recently noted through proteomics technologies and metabolic flux analysis experiments that RBCs hold the protein machinery to catalyze such reactions.44 Here, we did not observe any significant accumulation of heavy-labeled aspartate, whereas 15N-alanine was observed to significantly accumulate in shock RBCs only (Figure 3C). No labeling in RBC succinate was observed, confirming that RBCs mostly uptake succinate from plasma but a fraction of that succinate is labeled from glutamine.
In vivo tracing of 13C5 15N2-glutamine in RBCs from rats undergoing HS. (A) Samples were collected at baseline (B) or end (E) (60 minutes through shock) time points in sham (MAP, >80 mm Hg; no trauma) and shock (MAP, <30 mm Hg; no trauma) Sprague-Dawley rats (n = 8). (B) An overview of the expected labeling scheme in downstream metabolites to glutamine catabolism in mitochondria-devoid RBCs. (C) Box-and-whisker plots of key heavy isotopologues of glutamine and its catabolites.
In vivo tracing of 13C5 15N2-glutamine in RBCs from rats undergoing HS. (A) Samples were collected at baseline (B) or end (E) (60 minutes through shock) time points in sham (MAP, >80 mm Hg; no trauma) and shock (MAP, <30 mm Hg; no trauma) Sprague-Dawley rats (n = 8). (B) An overview of the expected labeling scheme in downstream metabolites to glutamine catabolism in mitochondria-devoid RBCs. (C) Box-and-whisker plots of key heavy isotopologues of glutamine and its catabolites.
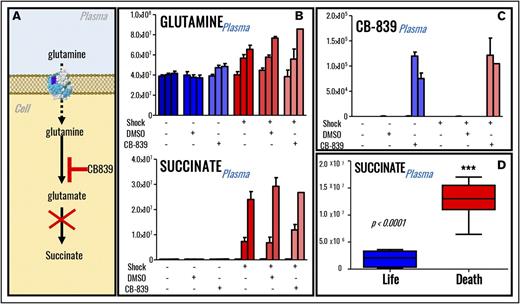
Inhibition of glutaminolysis through the glutaminase inhibitor CB-839 increases mortality of hemorrhaged rats but does not decrease plasma levels of succinate
In previous studies, HS-induced synthesis of succinate, a marker of increased mortality in military21 and civilian22 critically ill patients, is in part explained by glutaminolysis.13 In this view, we provocatively hypothesized that suppression of glutaminolysis in vivo should limit availability of a rate-limiting substrate for the synthesis of hemorrhagic succinate (n = 5; Figure 4A). Of note, 80% shock rats receiving the glutaminase inhibitor and anticancer drug CB-83945 died within 45 minutes from the treatment, suggesting that glutamine availability is indispensable for the survival of hemorrhaged rats (supplemental Figure 2A). Specific inhibition of glutaminase activity through CB-839 significantly increased plasma glutamine levels in sham rats and only minimally but significantly (P < .05) in shock rats (Figure 4B). However, no significant effect was observed on plasma succinate (Figure 4B), whereas minor decreases in the plasma levels of glucose and other tricarboxylic acid cycle intermediates were observed only in the lone rat surviving CB-839 treatment after shock at the end time point (60 minutes through shock; supplemental Figure 3). The absence of effects on the levels of succinate was not attributable to the lack of drug bioavailability, which was indeed identified (based on accurate intact mass and isotope fingerprint) through UHPLC-MS in plasma in the treated sham and shock rats (Figure 4C). Of note, succinate levels positively correlated with mortality in rats undergoing HS when merging data from the current and our previous7,9,12,13 studies on this very same rodent model of HS (Figure 4D).
Inhibition of glutaminolysis does not decrease plasma succinate levels in rats (n = 5) undergoing severe HS. (A) An overview of glutamine metabolism and inhibition of glutaminase by CB839. (B) Inhibition of glutaminolysis by administration of glutaminase inhibitor CB-839 in vivo promotes accumulation of plasma glutamine but not accumulation of plasma succinate. (C) The drug could be detected through MS only in the plasma from rats receiving its administration, confirming its systemic bioavailability after the treatment. Labeling scheme in panels B and C is based on the presence (+) or absence (−) of shock, DMSO (control for vehicle to the drug CB-839), and glutaminase inhibitor. Three columns are graphed per each group, indicating baseline, postshock, and end-of-shock values. However, plasma levels of succinate significantly correlated with mortality in rats (D), and only 1 shock rat receiving glutaminase inhibitor survived (supplemental Figure 3).
Inhibition of glutaminolysis does not decrease plasma succinate levels in rats (n = 5) undergoing severe HS. (A) An overview of glutamine metabolism and inhibition of glutaminase by CB839. (B) Inhibition of glutaminolysis by administration of glutaminase inhibitor CB-839 in vivo promotes accumulation of plasma glutamine but not accumulation of plasma succinate. (C) The drug could be detected through MS only in the plasma from rats receiving its administration, confirming its systemic bioavailability after the treatment. Labeling scheme in panels B and C is based on the presence (+) or absence (−) of shock, DMSO (control for vehicle to the drug CB-839), and glutaminase inhibitor. Three columns are graphed per each group, indicating baseline, postshock, and end-of-shock values. However, plasma levels of succinate significantly correlated with mortality in rats (D), and only 1 shock rat receiving glutaminase inhibitor survived (supplemental Figure 3).
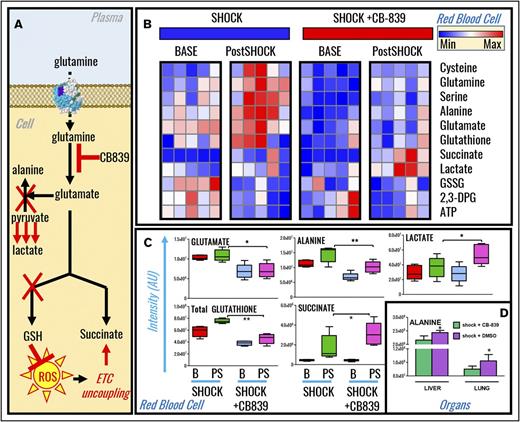
Increased mortality in hemorrhaged rats following glutaminase inhibition correlates with depression of redox homeostasis and pyruvate transamination in RBCs, liver, and lungs
Metabolomics analyses of RBCs from sham and shock rats undergoing glutaminase inhibition with CB-839 were performed (supplemental Table 1) and results were plotted as hierarchical clustering analyses (supplemental Figure 4). In light of steady-state and tracing experiment observations, we hypothesized that inhibition of glutamine metabolism would limit glutamate availability, a rate-limiting substrate to fuel GSH biosynthesis and pyruvate transamination reactions (Figure 5A). Results confirmed this hypothesis (Figure 5B). Specifically, CB-839 decreases baseline and postshock (15 minutes through shock) RBC levels of glutamate, alanine, and the marker of redox homeostasis GSH (Figure 5C). Increases in lactate and succinate in CB-839–treated end-shock rats were observed (Figure 5C). Lactate-to-pyruvate ratios, a marker of the ratio between nicotinamide adenine dinucleotide (NAD+) and its reduced form (NADH)46 according to the formula in supplemental Figure 2B, were increased in CB-839–treated postshock RBCs (supplemental Figure 2C). Levels of liver and lung alanine in postshock rats were lower than those measured in untreated postshock rats (Figure 5D), consistent with observations in RBCs. Inhibition of glutaminolysis in HS rats increased aspartate consumption (supplemental Figure 2D).
Inhibition of glutaminolysis prevented GSH synthesis and pyruvate transamination in shock rat RBCs, liver, and lung. (A) An overview of the proposed mechanism. (B) Metabolomics analyses revealed that, upon treatment with the glutaminase inhibitor CB-839, baseline and 15-minute postshock rats undergoing severe HS had significant impairments in GSH synthesis and alanine transamination that correlated with exacerbation of RBC accumulation of lactate and succinate (shock vs shock+CB-839). (C-D) A detailed overview of significant changes in glutamate, alanine, lactate, GSH, succinate in RBCs (C), and alanine in liver and lungs (D) is shown in the form of box-and-whisker plots. ETC, electron transport chain.
Inhibition of glutaminolysis prevented GSH synthesis and pyruvate transamination in shock rat RBCs, liver, and lung. (A) An overview of the proposed mechanism. (B) Metabolomics analyses revealed that, upon treatment with the glutaminase inhibitor CB-839, baseline and 15-minute postshock rats undergoing severe HS had significant impairments in GSH synthesis and alanine transamination that correlated with exacerbation of RBC accumulation of lactate and succinate (shock vs shock+CB-839). (C-D) A detailed overview of significant changes in glutamate, alanine, lactate, GSH, succinate in RBCs (C), and alanine in liver and lungs (D) is shown in the form of box-and-whisker plots. ETC, electron transport chain.
Discussion
Understanding the role RBCs play in health and disease is an extremely relevant question in biology and medicine. Indeed, >80% of the total host cells in the human body (25 trillion of 30 trillion) are RBCs.47 The RBC role in oxygen transport and metabolic homeostasis under hemorrhagic hypoxemia is intuitively anticipated to be critical in preserving systemic metabolic homeostasis. Inversely, HS has been shown to promote RBC hemolysis and morphological lesions, owing to exacerbation of oxidative stress. Here, we used an innovative approach, combining in vivo metabolic flux analysis with 13C15N-glutamine with specific glutaminase inhibition during HS to further our understanding of the role glutamine plays in hemorrhagic succinate accumulation, transamination, and redox homeostasis in rat RBCs, plasma, and organs. The data presented question the hypothesis that inhibition of glutamine catabolism would limit succinate generation by constraining the availability of a rate-limiting substrate (glutamine-derived glutamate). This is relevant in the light of the role of succinate as a marker of mortality in critically ill patients in the military21 and civilian population22 , and the increasingly appreciated role succinate plays in (postshock) inflammatory responses3 and trauma-induced coagulopathy.8 Appreciation of the limited contribution of glutaminolysis to hemorrhagic RBC succinate in this model suggests that, analogously to previous observations in ischemic rodents,16 other substrates may fuel hemorrhagic succinate accumulation. For example, increased proteolysis results in increased availability of circulating free amino acids that can be metabolized to succinate through alternative reactions, such as glutamate, isoleucine, valine, methionine, threonine. Catabolism of proteolysis-derived free amino acid would thus make inhibition of glutaminolysis insufficient to prevent succinate accumulation. Alternatively, activation of other transamination enzymes, such as phosphoserine aminotransferase, may bypass the blockade induced by CB-839 and result in the generation of glutamate and glutamate-derived succinate in the absence of glutaminase activity. Of note, we recently identified phosphoserine aminotransferase with high confidence in mature human RBCs (47% sequence coverage) through high-pH reversed-phase fractionation of the RBC cytosolic proteome.22
Importantly, propanoyl-carnitine was one of the most significant metabolic changes observed. These results suggest that fatty acid catabolism may represent a potential metabolic target for follow-up mechanistic studies on the phenomenon of hemorrhagic succinate accumulation in cell types other than mitochondria-devoid mature erythrocytes. Although sustained lipid mobilization and increases in circulating levels of fatty acids have been noted in response to hemorrhage in rats,12 pigs,48 and humans,5 it is worth considering that fatty acid catabolism is more demanding in terms of oxygen consumption and may thus be constrained by oxygen availability in response to hemorrhagic hypoxia. Further studies with stable isotope tracers (eg, 13C-palmitate) in models like the one investigated here should be performed.
Finally, if mechanisms of hemorrhagic hypoxemia mirror in part events occurring under ischemic hypoxia, purine salvage reactions may fuel hypoxic accumulation of succinate through aspartate deamination feeding into the dicarboxylate reservoirs (as previously described by Chouchani et al16 ). To further support this hypothesis, we previously noted that the earliest systemic responses to mild hemorrhage (prior to sustained HS) involve the accumulation of plasma adenosine,12 a metabolite that triggers metabolic cascades (through its A2B receptor) in circulating RBCs to promote oxygen off-loading in response to (high-altitude) hypoxia,25,26 suggesting that extremes of this physiological adaptation to systemic hypoxemia may contribute to early responses to hemorrhagic hypoxia.
The presented data suggest a role for glutamine metabolism in supporting de novo GSH synthesis and thus redox homeostasis in hemorrhaged rats, providing a rationale to support a beneficial role of early glutaminolysis upon hemorrhage in like fashion to previously hypothesized benefits of late enteral supplementation of glutamine in critically ill patients upon resuscitation.37,38,49 This novel role of glutamine is relevant in that impaired redox homeostasis would contribute to the accumulation of ROS, a phenomenon that would in turn further promote (instead of preventing) mitochondrial uncoupling and succinate accumulation (model summarized in Figure 5A), as our data here seem to suggest.
Previous observations of RBC metabolic responses to hypoxia in vitro, ex vivo, and in vivo have highlighted an increase in glycolytic rates to sustain ATP and DPG synthesis, which would promote stabilization of deoxyhemoglobin and oxygen off-loading to counteract tissue hypoxia.15,25,26,28,30,33,50 Surprisingly, decreases in ATP and DPG were observed in shock RBCs, suggesting that shock acidosis rather than high-energy phosphate compounds may be the main driver of oxygen off-loading through the Bohr effect in hemorrhaged rats. Increased intracellular acidification in shock RBCs is mirrored by increased NADH/NAD+ ratios, which would in turn fuel antioxidant enzymes such as methemoglobin reductase to counter oxidative stress. Notably, steady-state analyses and tracing experiments clearly showed that availability of glutamine-derived glutamate fuels transamination of pyruvate to alanine in HS RBCs. This phenomenon, also observed in matched plasma, liver, and lung of hemorrhaged rats, may be instrumental in fueling RBC methemoglobin reductase by limiting substrate availability for pyruvate to lactate (and concomitant NADH to NAD+) conversion by lactate dehydrogenase, as previously observed in RBCs from glucose 6-phosphate dehydrogenase–deficient patients.51 Increased glutamine uptake by postshock RBCs was observed in tracing experiments in vivo. Interestingly, mature RBCs have been suggested to rely on glutamine uptake to preserve GSH synthesis and intracellular redox homeostasis.52 Glutamine transport in mammalian cells is dependent on Na+ and/or proton gradients through symport/antiport-mediated mechanisms, respectively.52 Though merely a speculation at this stage, it is fascinating to suggest that intracellular acidification itself may promote increases in glutamine uptake to promote transamination of pyruvate and compensate excess lactate accumulation. Inhibition of glutaminolysis resulted in compensatory aspartate consumption (an alternative amine group donor for transamination reactions when glutamate is availability is constrained) and increased lactate accumulation, a phenomenon consistent with what is observed in highly glycolytic cancer cells when lactate export is constrained by deregulation of lactate transporter expression or correct translocation to the membrane.53 Inhibition of glutaminolysis resulted in increased mortality in rats, in part explained by increased metabolic acidosis (increases in intracellular lactate) and potentially by the limited availability of a critical energy substrate to mitigate myocardial hypoxia/ischemia.
Inhibition of glutamine catabolism here resulted in impaired GSH synthesis. These results support and expand on previous observations by Deitch’s group, suggesting a role for oxidative stress in morphological lesions and hemolysis of HS RBCs.34-36 GSH synthesis, an ATP-dependent process, was previously observed in hypoxic RBCs under physiological conditions in vivo and proportionally to hypoxia in vitro in human and mouse RBCs.26,31 On the other hand, several groups26,28,29,50 including ours15,32 have shown that hypoxia limits RBC antioxidant capacity by limiting metabolic fluxes through the pentose phosphate pathway, which generates reducing equivalents (NAD phosphate) for the reduction of oxidized GSH/recycling of antioxidant enzymes (eg, GSH reductase). Even though only steady-state metabolism was monitored with respect to this pathway and future glucose tracing experiments may be necessary to further confirm these observations, no significant changes were observed in shock animals with respect to ribose phosphate (and isobaric pentose phosphate compounds) in common to sham animals, whereas significant decreases in pentose phosphate pathway intermediates were noted (eg, 6-phosphogluconate, erythrose phosphate, and sedoheptulose phosphate). Consistently, cysteine accumulation (a marker of RBC aging in vivo54,55 ) was here observed in hemorrhaged rats.
Despite the novelty and originality of the approach, the proposed study holds some limitations as well. The in vivo tracing experiments performed here do not allow for the disentanglement of the relative contribution of RBC-specific reactions vs the extracellular milieu in the absence of analysis of matched plasma from the same animals. Future studies will be designed accordingly, potentially including the selective inhibition of specific transporters (eg, amino acid, mono- or di-/tri-carboxylate transporters), in order to understand the relative contribution of cellular components (not necessarily just circulating RBCs) to alterations to the plasma metabolome.
In conclusion, in this study, we provide the first metabolomics characterization of RBCs from HS rats. Our observations are relevant in that they indicate that metabolic changes in RBCs following hemorrhagic hypoxia mirror those previously reported in plasma in the same rodent model.12 In addition, these observations pave the way for a deeper understanding of pathological hypoxic metabolic reprogramming in RBCs, suggesting that metabolic acidosis rather than availability of high-energy phosphate compounds may drive oxygen off-loading in RBCs following HS. We also report that RBCs from HS rats rely on glutamine to fuel de novo GSH synthesis, confirming previous observations on RBCs exposed to high-altitude hypoxia in vivo15 or ex vivo.31 These observations also confirm and expand on previous reports about the role of oxidative stress as a potential etiological factor of RBC morphological lesions and hemolysis in response to HS.34-36 In addition, we show that glutamine-derived glutamate is necessary to fuel pyruvate transamination to alanine, a phenomenon we observed in RBCs, plasma, liver, and lungs of HS rats. By contributing to pyruvate-to-alanine ratios, glutaminolysis indirectly influences NADH-to-NAD+ ratios and thus influences ongoing glycolysis (lactate acidosis) and potentially the activities of enzymes relying on reducing cofactors, such as methemoglobin reductase, in analogy to what was observed in RBCs from glucose 6-phosphate dehydrogenase–deficient donors. Finally, we conclude that inhibition of glutaminolysis does not limit the generation of succi-nate as we would have anticipated from recent tracing experiments13 and rather results in significant increases in mortality in rats. Altogether, these results suggest that other substrates such as aspartate (via purine salvage reactions) or fatty acids may fuel hemorrhagic succinate accumulation, a predictor of mortality in the military21 and civilian22 critically ill population. Finally, our results support the practice of glutamine supplementation in critically ill patients, suggesting that this compound plays a critical role as an amine group donor following HS.
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by funds from the National Blood Foundation Early Career grant 2016 (A.D.), the Boettcher Webb-Waring Foundation Early Career Award 2017 (A.D.), the Shared Instrument grant by the National Institutes of Health (Office of the Director S10OD021641) (K.C.H.), the University of Colorado Comprehensive Cancer Center Core Support (National Cancer Institute P30 CA046934-17), and the National Institute of General Medical Sciences under award numbers P50GM049222 and T32GM008315, and grant P50 GM049222 (A.B., E.E.M., C.C.S., K.C.H., and A.D.). A.D. is a Boettcher Investigator.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.A.R., R.C.-H., and A.D. performed metabolomics analyses and prepared figures and tables; A.L.S., M.F., and E.D.P. performed animal experiments; A.L.S., J.A.R., E.E.M., C.C.S., E.D.P., K.C.H., A.B., and A.D. designed the experiments and supervised the analyses; A.D. wrote the first draft of the manuscript; and all authors critically revised it and contributed to its finalization.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angelo D’Alessandro, Department of Biochemistry and Molecular Genetics, University of Colorado Health Sciences Center, 12801 East 17th Ave, Aurora, CO 80045; e-mail: angelo.dalessandro@ucdenver.edu.
References
Author notes
J.A.R. and A.L.S. contributed equally and share first authorship.