Abstract
Osteonecrosis (ON) represents one of the most common and debilitating sequelae of antileukemic treatment in children and adolescents with acute lymphoblastic leukemia (ALL). Systematic screening strategies can focus on early detection and intervention to prevent ON from progressing to stages associated with pain and functional impairment. These strategies hold promise for reducing ON-associated morbidity without the risk of impairing leukemia control. Herein, we critically reviewed clinical data on pharmacological, nonpharmacological/nonsurgical, and surgical (including cellular) treatment options for ON, which are covered in the literature and/or are conceivable based on the supposed underlying ON pathophysiology. Prevention of ON progression is of paramount importance, and attempts seem to be more effective in early (precollapse) disease status than in late-stage (collapse) ON. Based on the results of ongoing prospective magnetic resonance imaging screening studies, which will hopefully identify those patients with a high risk of ON progression and debilitating sequelae, prospective interventional studies are urgently needed. Although there is still a lack of high-quality studies, based on currently available data, core decompression surgery combined with cellular therapies (eg, employing mesenchymal stem cells) appears most promising for preventing joint infraction in children at high risk of developing late-stage ON.
Background
Nowadays, >90% of children and adolescents with acute lymphoblastic leukemia (ALL) can be cured and become long-term survivors.1 However, these cure rates come at a high cost, as a substantial proportion of these children experience toxic side effects of antileukemic treatment. One of the most common and debilitating sequelae is osteonecrosis (ON), which severely impacts quality of life.2 Long, continuous exposure to corticosteroids during delayed intensification chemotherapy plays a pivotal role in the development of ON.3,4 Consequently, treatment schedules have been modified (from continuous to alternate-week dexamethasone within the delayed intensification phase), leading to a significantly reduced ON incidence in adolescents receiving alternate-week dexamethasone.3,5 However, in the CCG-1961 trial, high-risk ALL patients with ON fare better, with an event-free survival rate 17.6% greater than those without ON.3 Thus, treatment modifications aimed at reducing ON incidence must be carefully evaluated in prospective clinical trials, with strict stopping rules regarding increased risk of relapse.
Alternatively, early detection and intervention might prevent ON from progressing to stages associated with pain and functional impairment via systematic screening. This strategy holds the promise of reducing ON-associated morbidity without the risk of impairing leukemia control.
Most commonly, ON becomes apparent during delayed intensification and early maintenance.5 It can be challenging to interpret nonspecific symptoms, such as bone and muscle pain and muscle weakness, due to the very similar presentation of symptoms caused by leukemia itself, side effects of antileukemic treatment (eg, vinca alkaloids, glucocorticoids), and ON. This has an impact on recommended screening regimens, since it might be impossible to identify children with developing ON during antileukemic treatment, even when using a thorough clinical assessment.6
Magnetic resonance imaging screening and early diagnosis
The first prospective magnetic resonance imaging (MRI) screening study was reported in 1999 by Ojala et al with an overall ON incidence of 38%.7 Six out of 24 patients with MRI-detected ON remained asymptomatic at the end of the study period. To date, a total of 7 studies have reported on MRI screening for ON in children with ALL (Table 1).5,14-19 MRI time points differed widely between diagnosis and completion of therapy/follow-up in these studies. There was also significant variation in radiological and clinical classification employed, as well as the number of and time intervals between MRIs, limiting the comparability of these studies considerably.
Overview of MRI screening studies in children and adolescents with ALL
| Treatment protocol and recruitment period . | Study cohort and design . | ON assessment . | No. of pts with ON/ALL . | Outcome . | Reference . |
|---|---|---|---|---|---|
| Dexa-based DCOG-ALL98 protocol not specified | ALL not specified, multiple centers | Symptoms during treatment/within 1 y after treatment discontinuation, NCI CTCAE | 38/574 pts, CI 6.1% after 3 y | Clinical follow-up: 14/35 pts asymptomatic,14/35 pts NCI CTCAE °2, 7/35 pts NCI CTCAE °3, 7/35 pts orthop. interventions; radiological follow-up: 6/24 pts partially/completely resolved, 13/24 pts stable ON, 5/24 pts progressive ON | 5 |
| St. Jude Total Therapy Study X11 06/00-10/07 | ALL not specified, single center | After reind. I and II, at completion of therapy, NCI CTCAE | 69/364 pts, Inc.: °1 35.4%, °2-4 14.6% in first year, any ON 71.8%, °1 53.9%, °2-4 17.6% at end of therapy | 215 pts with ON NCI CTCAE °0 at first MRI: 105/215 maintained °0, 62/215 worsened to °1, 27/215 worsened to °2-4; 141 pts with ON NCI CTCAE °1 at first MRI: 14/141 resolved to °0, 82/141 maintained °1, 34/141 worsened to °2-4, 8 pts with ON NCI CTCAE °2-4 at first MRI, no outcome reported | 17 |
| St. Jude Total Therapy Study XV13 06/00-10/07 | ALL 1-18 y, single center | After reind. I and II, at completion of therapy, lesions affecting >30% of the epiphyseal surface = extensive hip osteonecrosis | 30/374 pts CI after 1 y, 17.1%; CI after 4 y, 21.7%; 19 pts with orthop. interv. | Extensive FH ON: 6.5% in all pts, 24% in pts >10 y,79% of ON within 1 y after start of therapy, all pts asymptomatic before early screening, 19/30 pts developed symptoms within 0-12 mo after ON dx.; spontaneous resolution in 4/8 hips of children <10 y, in 0/34 hips in children in >10 y; extensive lesions:40/48 joints in children >10 y, 19/40 progression to joint collapse, 0/40 improved | 18 |
| St. Jude Total Therapy XIIIA, NHL XIII12 12/91-08/94 | ALL, advanced-stage NHL <18 y, single center | Earliest MRI 1 y after ALL diagnosis Ficat classification | 17/116 pts, Inc.: 15.5% | 8/17 ON pos. pts MRI after 1y: 7/8 pts stable ON, 1/8 pts resolved ON, 15/17 ON pos. pts alive: 8/15 pts free of symptoms, 6/15 pts (occasional) pain, 1/15 pts hip replacement | 14 |
| Nordic ALL protocols9,10 07/92-12/05 | ALL 1-16 y 2 centers | At the end of therapy No established classification system | 23/97 pts, Inc.: 24% | 3/7 pts FH surgery | 15 |
| Not specified 01/90-12/03 | ALL, NHL, CML, Hodgkin, AML, not specified, retrospective study | Various clinical/research indications, according to Steinberg,84 Hernigou and Lambotte,85 and Sugano et al86 | 80 pts, Inc.: — | 66/80 pts bilateral hip ON, 23/80 pts arthroplasty (of 36 hips); median time between oncologic dx arthroplasty: 3.6 y, ON dx arthroplasty: 1.3 y | 16 |
| Not specified 11/06-03/12 | ALL 3-18 y, not specified | At the end of therapy, while follow-up, no established classification system | 54/73 pts, Inc.: 57.5% at end of therapy, 16.4% at follow-up | Asympt. ON: 0/26 pts no JD; symptomatic ON: 3/47 pts JD at end of therapy, 8/47 pts JD/collapse of joint at follow-up | 19 |
| Treatment protocol and recruitment period . | Study cohort and design . | ON assessment . | No. of pts with ON/ALL . | Outcome . | Reference . |
|---|---|---|---|---|---|
| Dexa-based DCOG-ALL98 protocol not specified | ALL not specified, multiple centers | Symptoms during treatment/within 1 y after treatment discontinuation, NCI CTCAE | 38/574 pts, CI 6.1% after 3 y | Clinical follow-up: 14/35 pts asymptomatic,14/35 pts NCI CTCAE °2, 7/35 pts NCI CTCAE °3, 7/35 pts orthop. interventions; radiological follow-up: 6/24 pts partially/completely resolved, 13/24 pts stable ON, 5/24 pts progressive ON | 5 |
| St. Jude Total Therapy Study X11 06/00-10/07 | ALL not specified, single center | After reind. I and II, at completion of therapy, NCI CTCAE | 69/364 pts, Inc.: °1 35.4%, °2-4 14.6% in first year, any ON 71.8%, °1 53.9%, °2-4 17.6% at end of therapy | 215 pts with ON NCI CTCAE °0 at first MRI: 105/215 maintained °0, 62/215 worsened to °1, 27/215 worsened to °2-4; 141 pts with ON NCI CTCAE °1 at first MRI: 14/141 resolved to °0, 82/141 maintained °1, 34/141 worsened to °2-4, 8 pts with ON NCI CTCAE °2-4 at first MRI, no outcome reported | 17 |
| St. Jude Total Therapy Study XV13 06/00-10/07 | ALL 1-18 y, single center | After reind. I and II, at completion of therapy, lesions affecting >30% of the epiphyseal surface = extensive hip osteonecrosis | 30/374 pts CI after 1 y, 17.1%; CI after 4 y, 21.7%; 19 pts with orthop. interv. | Extensive FH ON: 6.5% in all pts, 24% in pts >10 y,79% of ON within 1 y after start of therapy, all pts asymptomatic before early screening, 19/30 pts developed symptoms within 0-12 mo after ON dx.; spontaneous resolution in 4/8 hips of children <10 y, in 0/34 hips in children in >10 y; extensive lesions:40/48 joints in children >10 y, 19/40 progression to joint collapse, 0/40 improved | 18 |
| St. Jude Total Therapy XIIIA, NHL XIII12 12/91-08/94 | ALL, advanced-stage NHL <18 y, single center | Earliest MRI 1 y after ALL diagnosis Ficat classification | 17/116 pts, Inc.: 15.5% | 8/17 ON pos. pts MRI after 1y: 7/8 pts stable ON, 1/8 pts resolved ON, 15/17 ON pos. pts alive: 8/15 pts free of symptoms, 6/15 pts (occasional) pain, 1/15 pts hip replacement | 14 |
| Nordic ALL protocols9,10 07/92-12/05 | ALL 1-16 y 2 centers | At the end of therapy No established classification system | 23/97 pts, Inc.: 24% | 3/7 pts FH surgery | 15 |
| Not specified 01/90-12/03 | ALL, NHL, CML, Hodgkin, AML, not specified, retrospective study | Various clinical/research indications, according to Steinberg,84 Hernigou and Lambotte,85 and Sugano et al86 | 80 pts, Inc.: — | 66/80 pts bilateral hip ON, 23/80 pts arthroplasty (of 36 hips); median time between oncologic dx arthroplasty: 3.6 y, ON dx arthroplasty: 1.3 y | 16 |
| Not specified 11/06-03/12 | ALL 3-18 y, not specified | At the end of therapy, while follow-up, no established classification system | 54/73 pts, Inc.: 57.5% at end of therapy, 16.4% at follow-up | Asympt. ON: 0/26 pts no JD; symptomatic ON: 3/47 pts JD at end of therapy, 8/47 pts JD/collapse of joint at follow-up | 19 |
°, degree of severity of the toxicity/disease; add., additional; asympt., asymptomatic; CI, cumulative incidence; CTCAE, Common Terminology Criteria for Adverse Events; Dexa, dexamethasone; dx, diagnosis; examin., examination; exclud., excluded; FH, femoral head; Inc., incidence; incl., included; interv., intervention; JD, joint deformation; NCI, National Cancer Institute; orthop., orthopedic; reind., reinduction; pts, patients; sympt., symptomatic.
One of the 3 largest studies included only children presenting with symptoms (thus not representing a truly unbiased screening approach), while another reported only extensive osteonecrotic lesions of the hips, and the third examined only follow-up of early-stage ON.5,17,18 As a result, there is a significant difference across all studies between the overall incidences of radiologically detected (asymptomatic) and clinically symptomatic ON.5,7,18 In the study by Kawedia et al,17 the majority of ON (65%) diagnosed as stage I at first screening MRI (graded using the National Cancer Institute Common Terminology Criteria for Adverse Events) remained unchanged during follow-up, while 10% resolved and ∼25% worsened to stage II to IV. The outcome of initially stage II to IV ON was not reported. Regarding outcome, 25% of the ON lesions in symptomatic patients reported by te Winkel et al5 regressed, ∼50% remained stable, and ∼20% progressed. No data were provided about the characteristics that could potentially indicate which lesions are more likely to progress. However, 60% of the patients remained symptomatic at the end of the study. Kaste et al18 reported that extensive ON of the femoral head (FH) was more likely in adolescents >10 years than in younger children. While some of these extensive lesions resolved spontaneously in younger children, this was not the case in any of the adolescent patients. Instead, half of these adolescents subsequently required total hip arthroplasty.
Thus, at this time, identifying patients at risk of functional impairment and debilitating progressive joint disease still remains an elusive goal; neither initial radiologic manifestation nor clinical presentation allows for sufficiently precise prediction of the further course of ON. Moreover, the use of comprehensive radiological ON classification, including extent of involvement, is essential to improving the treatment of patients with ON. Specifying the location and extent of ON is crucial for clinical approaches to the disease. A radiological classification of ON in children with ALL was first made available in 2015, enabling the classification of ON regardless of the site of the lesion.20 Also, in 2016, the Ponte di Legno Toxicity Working Group published a consensus definition of ON, which is partly MRI based but likewise accounts for clinical symptoms.21
Clearly, data are urgently needed on the proportion of children who can be diagnosed with early-stage asymptomatic ON by MRI and subsequently develop symptomatic ON and joint infraction. In addition, identification of the critical points in the timeline of ON development and knowledge of the long-term outcome of asymptomatic ON lesions identified only by MRI are necessary in order to enable future evaluation of preventive or therapeutic interventions.
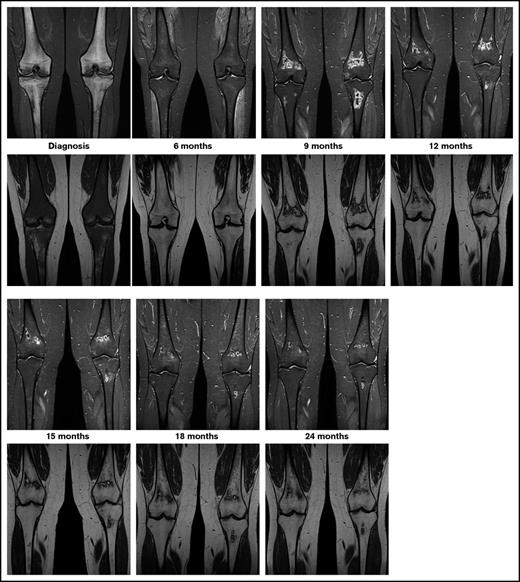
The currently ongoing OPAL trial (osteonecrosis in pediatric patients with acute lymphoblastic leukemia and lymphoblastic lymphoma)6 is addressing these questions via serial MRI screening of adolescents with newly diagnosed ALL or lymphoblastic lymphoma. Preliminary results of this trial highlight the unpredictable course of asymptomatic MRI lesions, ranging from spontaneous regression via subclinical persistence to rapid progression. Epiphyseal disease tends to progress rapidly. In contrast to this, as seen in Figure 1, metaphyseal/diaphyseal disease does not commonly cause any harm, and it may even disappear, since it is far away from the joint line. Thus, ON studies in children with ALL should focus on the lesions located in the epiphyseal areas near the joint line, where the risk of joint collapse increases.
Exemplary patient from the OPAL trial. On coronal images of knees, short tau inversion recovery images (top row) show diffuse hyperintense inhomogeneous signals, and T1-weighted images (bottom row) show diffuse hypointense signals revealing extensive leukemic infiltration of bone at diagnosis. At the 6-month follow-up examination, the hyperintense signal in short tau inversion recovery decreased and the hypointense signal in T1 increased toward normal appearance. At 9 months into treatment, MRI shows asymptomatic lesions in the metaphyses, which shows spontaneous regression beginning at 12 months into treatment.
Exemplary patient from the OPAL trial. On coronal images of knees, short tau inversion recovery images (top row) show diffuse hyperintense inhomogeneous signals, and T1-weighted images (bottom row) show diffuse hypointense signals revealing extensive leukemic infiltration of bone at diagnosis. At the 6-month follow-up examination, the hyperintense signal in short tau inversion recovery decreased and the hypointense signal in T1 increased toward normal appearance. At 9 months into treatment, MRI shows asymptomatic lesions in the metaphyses, which shows spontaneous regression beginning at 12 months into treatment.
Treatment options
Principally, the type of ON treatment should be chosen according to the presumed disease etiology. However, based on the lack of knowledge in this field, neither an evidence-based guideline nor a consensus on management of osteonecrosis in children with ALL exists. Thus, management and treatment vary widely and often depend on local preferences and available therapeutic options. In an evidence assessment of published intervention studies on ON in children with ALL, the authors concluded in 2014 that good-quality studies are lacking and, thus, treatment recommendations for ON in children with ALL still cannot be made.22
However, a large number of medical and surgical (including cellular) ON treatment options of various etiologies are covered in the literature or are additionally conceivable based on the supposed underlying ON pathophysiology but yet not clinically evaluated. Assuming that prevention of ON progression is dependent on but also most effective when taking disease status (early-stage/precollapse disease versus late-stage/collapse disease) into account, a discussion of treatment options should consider this.
With regard to previously published data, osteoedema (corresponding to stage I) is a frequent MRI finding in children with ALL but only progresses to osteonecrosis (stage II or higher) in ∼25% of cases.17 Thus, in children presenting with asymptomatic epiphyseal osteoedema in weight-bearing joints, short-term follow-up MRI seems to be most reasonable, and might be combined with supportive measures, such as correction of coagulopathies and hyperlipidemia as presumed risk factors for development of ON. The additional correction of bone metabolism (eg, vitamin D deficiency) might be considered as well. However, while adequate vitamin D and calcium intake and good nutritional status are important for bone health in children with leukemia, there are no data suggesting that vitamin D deficiency is a risk factor for the development of ON or that vitamin D supplementation decreases the risk of ON.
Previous MRI studies reported that a substantial number of children with ALL show (a)symptomatic ON stage II in screening MR images.6,17 The outcome of these ON is, to date, highly variable and thus entirely unpredictable. Thus, close monitoring by clinical evaluation and MRI is highly recommended to recognize disease progression in a timely manner. These children might particularly benefit from treatment interventions aimed at prevention of further ON progression and preservation of joint integrity. However, based on the unknown but clearly substantial proportion of those children not progressing to symptomatic ON and/or later stages, such interventions require an exceedingly good safety profile if they are to provide an overall favorable risk/benefit ratio.
Literature search
The database used for the literature search was PubMed/Medline. To identify studies to be included or considered for this review, the following MESH terms were used: osteonecrosis, children, treatment, and leukemia. Additionally, we screened the reference lists of the retrieved articles. Only English-language articles were included.
Based on the profound heterogeneity of published studies with regard to indication for ON-directed intervention and reported outcome parameters, a formal meta-analysis could not be performed. In order to summarize the available data in a systematic fashion, Tables 1, 2, and 3 list specific study parameters and reported outcomes according to the type of intervention.
Pharmacological interventions on nontraumatic osteonecrosis
| Treatment . | Indication . | Study cohort . | Dose . | Study design . | ON classification . | Follow-up . | Conclusions . | Reference . |
|---|---|---|---|---|---|---|---|---|
| LMW heparin | ||||||||
| LMW heparin | ON and thrombophilia and/or hypofibrinolysis | No full text | Enoxaparin 60 mg/d for 12 wk | n.s. | Ficat | n.s. | May prevent progression | 29 |
| Prostacyclin analog | ||||||||
| Iloprost | BME/AVN of any etiology including leukemia | 50 pts (11-76 y) | 0, 5-1, 0 ng/kg/min 5 d (6 h) | Single center | ARCO | 6 mo | In BME and early AVN: significant improvement in pain, functional, and radiological outcome stages | 31 |
| Statins | ||||||||
| Statin | high dose GCS | 284 pts (27-71 y) | ≥1 statins | n.s. | MRI ON verifying | 5-11 y | Protection against osteoporosis and osteonecrosis | 35 |
| Lovastatin | Steroid treated rabbits | 54 rabbits (28-32 wk) | Lovastatin 5 mg/kg/d | Laboratory | Histopathology | 12 wk after steroids | Prevention of development of steroid-induced ON in rabbits by inhibiting adipogenesis | 47 |
| Bisphosphonate | ||||||||
| Alendronate | ALL/NHL | 10 pts (3, 6-14, 6 y) | Alendronate 30-70 mg/week, calcium daily over 6 mo | n.s. | Osteopenia monitoring | 6 mo | Accretion of bone mineral | 42 |
| Alendronate | ON after BMT (ALCL/ALL relapse) | 2 pts (10-12 y) | 5-10 mg/d po for 11-12 mo | Case report | None | 6, 5-7 y | Pain reduction, improvement motor function, MRI improvement, densitometry improvement | 38 |
| Pamidronate/alendronate | ALL (16 HR) | 17 pts (5-16 y) | 6 pts (later 3 pts) alendronate 70 mg/week | Single center | Volume affected 0 to >200 cm3 | 0-21 mo | Pamidronate: pain reduction Improvement motor function of affected joints | 37 |
| 3 pts (later 6 pts) pamidronate 16, 8-65 mg/m2 | ||||||||
| Pamidronate | ALL (SR/HR) | 17 pts (2, 9-19, 4 y) | 14 pts pamidronate (dose unknown) | Single center | Articular surface affected <15% to >50% | 6-72 mo | Pain reduction, improvement motor function | 40 |
| Zoledronic acid | ALL/AML/NHL/HL/ benign hematologic disorder | 20 pts (7, 8-14, 5 y) | Number of doses 2-8; 5-25 mo | n.s. | ARCO | 24 mo | Controlling pain, slowed process of progressive joint destruction in knee joints | 39 |
| Treatment . | Indication . | Study cohort . | Dose . | Study design . | ON classification . | Follow-up . | Conclusions . | Reference . |
|---|---|---|---|---|---|---|---|---|
| LMW heparin | ||||||||
| LMW heparin | ON and thrombophilia and/or hypofibrinolysis | No full text | Enoxaparin 60 mg/d for 12 wk | n.s. | Ficat | n.s. | May prevent progression | 29 |
| Prostacyclin analog | ||||||||
| Iloprost | BME/AVN of any etiology including leukemia | 50 pts (11-76 y) | 0, 5-1, 0 ng/kg/min 5 d (6 h) | Single center | ARCO | 6 mo | In BME and early AVN: significant improvement in pain, functional, and radiological outcome stages | 31 |
| Statins | ||||||||
| Statin | high dose GCS | 284 pts (27-71 y) | ≥1 statins | n.s. | MRI ON verifying | 5-11 y | Protection against osteoporosis and osteonecrosis | 35 |
| Lovastatin | Steroid treated rabbits | 54 rabbits (28-32 wk) | Lovastatin 5 mg/kg/d | Laboratory | Histopathology | 12 wk after steroids | Prevention of development of steroid-induced ON in rabbits by inhibiting adipogenesis | 47 |
| Bisphosphonate | ||||||||
| Alendronate | ALL/NHL | 10 pts (3, 6-14, 6 y) | Alendronate 30-70 mg/week, calcium daily over 6 mo | n.s. | Osteopenia monitoring | 6 mo | Accretion of bone mineral | 42 |
| Alendronate | ON after BMT (ALCL/ALL relapse) | 2 pts (10-12 y) | 5-10 mg/d po for 11-12 mo | Case report | None | 6, 5-7 y | Pain reduction, improvement motor function, MRI improvement, densitometry improvement | 38 |
| Pamidronate/alendronate | ALL (16 HR) | 17 pts (5-16 y) | 6 pts (later 3 pts) alendronate 70 mg/week | Single center | Volume affected 0 to >200 cm3 | 0-21 mo | Pamidronate: pain reduction Improvement motor function of affected joints | 37 |
| 3 pts (later 6 pts) pamidronate 16, 8-65 mg/m2 | ||||||||
| Pamidronate | ALL (SR/HR) | 17 pts (2, 9-19, 4 y) | 14 pts pamidronate (dose unknown) | Single center | Articular surface affected <15% to >50% | 6-72 mo | Pain reduction, improvement motor function | 40 |
| Zoledronic acid | ALL/AML/NHL/HL/ benign hematologic disorder | 20 pts (7, 8-14, 5 y) | Number of doses 2-8; 5-25 mo | n.s. | ARCO | 24 mo | Controlling pain, slowed process of progressive joint destruction in knee joints | 39 |
ACTH, adrenocorticotropic hormone; ALCL, anaplastic large cell lymphoma; AML, acute myeloid leukemia; ARCO, association research circulation osseous; AVN, avascular necrosis; BME, bone marrow edema; BMT, bone marrow transplantation; GCS, glucocorticosteroids; HL, Hodgkin lymphoma; HR, high risk; LMW, low molecular weight; NHL, non-Hodgkin lymphoma; n.s., not specified; ON, osteonecrosis; po, by mouth; pts, patients; SR, standard risk.
Nonpharmacological, nonsurgical interventions for nontraumatic osteonecrosis
| Treatment . | Indication . | Study cohort . | Dose . | Study design . | ON classification . | Follow-up . | Conclusions . | Reference . |
|---|---|---|---|---|---|---|---|---|
| HBO | ON ALL/ NHL | 19 pts (0, 7-15, 6 y) | 13-80 treatments | Single center | Number of lesions (MRI) | 12 mo | Pain reduction | 52 |
| ESWT | >30 y and ARCO I-III | 19 pts (30 hips) | A (1000 shocks/session, EFD per shock 0.12 mJ/mm2) | Single center | ARCO | 6 mo | VAS, Harris Hip Score, and Osteoarthritis Index improved | 53 |
| B (1000 shocks/ session, EFD per shock 0.32 mJ/mm2) | ||||||||
| ESWT | n.s. | 40 pts (31.9-54 y) | Group A, group B: prostacyclin and bisphosphonate | Single center, prospective, randomized | Bone marrow edema | >12 mo | Improvement in pain and function of affected knees; complete regression in all pts of group A | 59 |
| (S)PEMF | In vitro cell culture, in vivo mice | 2 humans/ 18 mice | Mice: 1 Tesla, 30 pulses/d | Single center | Mice: histopathology | 4 wk | Bone repair, neovascularization, and cell growth in necrotic bone in mice | 57 |
| Treatment . | Indication . | Study cohort . | Dose . | Study design . | ON classification . | Follow-up . | Conclusions . | Reference . |
|---|---|---|---|---|---|---|---|---|
| HBO | ON ALL/ NHL | 19 pts (0, 7-15, 6 y) | 13-80 treatments | Single center | Number of lesions (MRI) | 12 mo | Pain reduction | 52 |
| ESWT | >30 y and ARCO I-III | 19 pts (30 hips) | A (1000 shocks/session, EFD per shock 0.12 mJ/mm2) | Single center | ARCO | 6 mo | VAS, Harris Hip Score, and Osteoarthritis Index improved | 53 |
| B (1000 shocks/ session, EFD per shock 0.32 mJ/mm2) | ||||||||
| ESWT | n.s. | 40 pts (31.9-54 y) | Group A, group B: prostacyclin and bisphosphonate | Single center, prospective, randomized | Bone marrow edema | >12 mo | Improvement in pain and function of affected knees; complete regression in all pts of group A | 59 |
| (S)PEMF | In vitro cell culture, in vivo mice | 2 humans/ 18 mice | Mice: 1 Tesla, 30 pulses/d | Single center | Mice: histopathology | 4 wk | Bone repair, neovascularization, and cell growth in necrotic bone in mice | 57 |
ARCO, association research circulation osseous; EFD, energy flux density; HBO, hyperbaric oxygenation; ESWT, extracorporeal shock wave therapy; HSCT, hematopoietic stem cell transplantation; NHL, non-Hodgkin lymphoma; n.s., not specified; (S)PEMF, (single) pulsed electromagnetic fields; VAS, visual analog scale
Non–weight-bearing therapy
The initial treatment of ON generally includes non–weight-bearing therapy for at least 6 weeks, with the possible addition of a rehabilitation and training program. Whereas this proved useful in a variety of other orthopedic diseases, no advantage could be shown for non–weight-bearing therapy as the only treatment, even in early-stage nontraumatic ON of etiologies other than ALL.23-25 In children with ALL, it has not been evaluated or proven to be beneficial for the treatment of ON.
Pharmacological interventions
Intravascular clotting, increased marrow pressure, direct blood vessel injury, and direct toxic effects of chemotherapy on blood vessels, osteoblasts, and osteocytes are all considered to contribute to the development of ON.26-28 While the pathophysiology of ON is still not fully understood, many presumed disease mechanisms assume that the bone vasculature is a critical component.
Thus, pharmacological interventions addressing these pathophysiological mechanisms are conceivable, either alone or in combination with, for example, surgical interventions. Low-molecular-weight (LMW) heparin might positively affect intravascular clotting, especially in patients with a prothrombotic underlying disease.29,30 Prostacyclin analogs have antiedema, anti-inflammatory, vasodilatory, and antiaggregant effects.31-34 To reduce the adverse effects of steroids on lipid metabolism and lipocyte proliferation resulting in increased marrow pressure, lipid-clearing agents such as statins can reduce lipid levels in blood and tissues during high-dose glucocorticoid therapy.35 Bisphosphonates reduce osteoclast activity and prevent osteocyte and osteoblast apoptosis and thus might reduce the direct toxic effects of chemotherapy on bone cells.36-42 Adrenocorticotropic hormone stimulates vascular endothelial growth factor, which enhances osteoblast activity and revascularization of necrotic bone.43,44
The aforementioned pharmacological interventions might be of most significant impact when applied concomitant with antileukemic treatment associated with hypercoagulopathy and hyperlipidemia to prevent the occurrence of stage I ON and the progression to stage II ON. However, they will most likely fail to impede progression of symptomatic ON stage II or higher, as the circulatory damage is irreversibly set at this stage.
According to the data reported by Glueck et al,29,30 LMW heparin holds promise to prevent ON progression from early- to late-stage ON in primary ON associated with thrombophilia or hypofibrinolysis, but not in secondary ON caused by glucocorticoid treatment. The latter is thought to represent the major pathogenic mechanism in the development of ON in adolescents with ALL. In addition, one has to consider that LMW heparin leads to decreased bone density and increases the risk of bleeding.
While prostacyclin analog infusions have been shown to improve clinical outcome (pain, functional, and, partly, radiological outcome) in early-stage ON of other etiologies in adults,31-34 particularly in osteoedema, no effect on progression in adolescents with ALL has been delineated. In addition, some of the patients reported in these studies underwent core decompression prior to the iloprost treatment, thus challenging any assessment of the specific effect of iloprost on ON outcome.
Statins proved to efficiently reduce glucocorticoid-induced increased lipid-levels in blood and tissues in adults. Pritchett et al35 reported that the preemptive long-term use of statin drugs reduces the later development of ON in adults receiving steroids compared with published incidences of adults receiving high-dose steroids. Mogensen et al45 recently reported hyperlipidemia as a significant risk factor for the development of ON in children and adolescents with ALL, while Bhojwani et al46 reported no association between very elevated triglycerides and the development of ON. Until now, no data on the safety and efficacy of statin use for prevention and/or treatment of early-stage ON in children with ALL have been available.47 Moreover, no clinical data on the role of drug-drug interactions with statins and vincristine/glucocorticoids are available. Beyond that, the use of statins seems to be most effective when given preemptively and not as a pharmacological intervention in manifest ON.
Some authors reported improved pain scores and functional parameters in ON in children and adolescents with ALL as an effect of bisphosphonates administration but no favorable effect on ON progression and radiological outcome.36,37 There is still controversy surrounding the use of bisphosphonates in a growing skeletal system, since they remain in the bone tissue for years and might impair bone remodeling for prolonged time periods.
Another potentially promising therapy is receptor activator of nuclear factor-κB ligand (RANKL) inhibitors (eg, denosumab).48 The RANKL/osteoprotegerin system plays an important role in the regulation of bone resorption. RANKL/RANK signaling regulates the formation of osteoclasts from their precursors, as well as their activation and survival in normal bone.49,50 A recent study indicates that the expression of osteoprotegerin, RANK, and RANKL genes plays a crucial role in the progression of ON of the FH.51
Taken together, currently available data on pharmacological interventions in children and adolescents with ON during or following ALL therapy are sparse, and none of these approaches have convincingly demonstrated a substantial impact on the progression of ON to symptomatic/debilitating stages or irreversible joint damage. A detailed summary of pharmacological intervention studies and their outcomes is presented in Table 2.29,31,35,37-40,42,47
Prospective clinical trials are needed to elucidate potential toxicities and thus to facilitate an accurate risk/benefit analysis of these pharmacological interventions.
Nonpharmacological and nonsurgical interventions
Hyperbaric oxygenation (HBO) therapy increases the oxygen content of blood mostly independent of blood flow and hemoglobin levels and has antiedematous effects.52 Extracorporeal shock wave therapy (ESWT) upregulates factors such as cell proliferation, vascular endothelial growth factor, alkaline phosphatase, bone morphogenic protein 2, and runt-related transcription factor 2. ESWT is also suggested to promote angiogenesis and bone remodeling, as well as a regenerative effect through the induction of the nitric oxide pathway.53-55 Single pulsed electromagnetic fields ((S)PEMF) might accelerate osteogenic differentiation of bone marrow mesenchymal stem cells and enhance bone repair, neovascularization, and cell growth in necrotic bone.56,57
However, the single available study on HBO therapy in children with ALL and ON including osteoedema reported no difference in pain outcome or the need for surgery between patients with and without HBO therapy.52 ESWT is increasingly used with favorable outcome in pain and functional scores in adults with FH ON stage I to III of various etiologies.54,55,58,59 Moreover, some data indicate that it is superior to core decompression. However, no data on the efficacy, safety, or tolerability in children with ALL are available. In contrast, while the efficiency of (S)PEMF has been investigated primarily in steroid-induced ON, thus far, these data are exclusively derived from animal models, and there is still a lack of studies on (S)PEMF in humans. A summary of nonpharmacological, nonsurgical intervention studies is presented in Table 3.
The latter therapeutic approaches are not only of limited availability but also continue to be of experimental character. They are also associated with extraordinary (time) efforts hardly compatible with antileukemic treatment. As a result, those approaches are restricted to single patients with symptomatic ON stage II to III, applied on an individual basis.
Surgical interventions
Core decompression (CD) surgery by retrograde drilling of 1 larger or several smaller holes into the necrotic bone is the most widely used method for delaying the progress of ON lesions of different etiologies destroying the FH.24 The proposed mechanism of action of CD surgery includes, among other things, the direct reduction of the increased intraosseous pressure resulting from edema, malperfusion, and inflammation. This mechanism also induces limited tissue damage to promote healing processes that involve vascular sprouting and opening blood vessels to foster angiogenesis, restoring sufficient blood supply.
In early-stage FH necrosis, CD might lead to significant postoperative pain reduction.24,60,61 However, the efficacy of CD treatment and, hence, the final clinical outcome is critically dependent on the size and location of the necrotic lesion and the extent to which the entire necrotic segment can be removed.24,60,62,63 Moreover, reconstruction and repair after CD alone are usually incomplete (as has been shown in postoperative MRI and pathomorphological studies) and, thus, are only likely to delay, but not prevent, the progress of joint destruction.64
Currently available data on the outcome of CD surgery for ON in children and adolescents with ALL demonstrate that it is necessary to combine surgery with approaches that harbor regenerative potential to address the pathogenic mechanisms of ON (ie, local inflammation, circulatory compromise, and impaired local osteoregenerative potential). In late-stage osteonecrosis, total hip arthroplasty currently seems to be the best treatment with good functional restoration.65,66 Another surgical option is transtrochanteric rotational osteotomy, which shows variable rates of success.67-69
Cellular therapies
Osteonecrotic lesions comprise bradytrophic tissue areas with low concentrations of oxygen and nutrients and severely impaired osteogenic potential for repair often associated with an inflammatory microenvironment.70 To date, multipotent mesenchymal stromal/stem cells (MSCs) are considered to be the most potent of the osteogenic cells in the marrow with the principal capacity for self-renewal and multilineage differentiation. Moreover, MSCs have been shown to promote endogenous repair of various tissues via secretion of paracrine factors, and they provide strong anti-inflammatory and angiogenic stimuli, thus addressing many of the proposed pathophysiologic mechanisms of ON.
Previous studies by Hernigou et al71,72 and Gangji et al73-75 have demonstrated the feasibility, tolerability, and preliminary efficacy of autologous bone marrow cell (sorted and concentrated to mononuclear cells) implantation into the FH during early-stage nontraumatic ON of various etiologies in combination with CD. However, the reconstructive repair process appears to be particularly slow and thus highlights the need for both early intervention and long-term follow-up to improve and accurately determine the efficacy of such interventions, respectively. Importantly, unseparated total bone marrow cell grafts are composed of substantially variable proportions of hematopoietic, vasculogenic, and osteogenic progenitor cells, which may critically impair the consistent efficacy of this approach.
In a randomized study comparing CD of the FH alone vs CD plus autologous MSCs as a more homogeneous cellular therapeutic, Zhao et al76 demonstrated the feasibility, safety, and preliminary efficacy of the combined approach in delaying or avoiding FH collapse. A faster reconstructive repair process (within 60 months) might be promoted by the implantation of a high number of MSCs.76 All in all, the combination of CD and MSCs in ON of the FH seems to be favorable compared with CD alone, when patients are treated in an earlier ON/precollapse stage and receive a greater cell number.77-81 Further details on studies on cellular therapies are given in Table 4.
Cellular therapies in patients with osteonecrosis
| Intervention/cell source . | Indication . | Study cohort . | Cell numbers . | Study design . | Classification . | Follow-up . | Outcome . | Reference . |
|---|---|---|---|---|---|---|---|---|
| CD and volume-reduced autologous bone marrow mononuclear cells | ON (steroids, alcohol, sickle cell disease) of the hip | 534 hips in 342 pts (16-61 y) | Average 24 × 103 CFUs | Single center, single arm | Steinberg stage I-II | 8-18 y | Stage I and II: total hip replacement 94/534 hips | 72 |
| CD ± bone marrow cells | Nontraumatic ON of the hip | 24 hips in 19 pts | CFU-F 92.6 ± 22.4 × 107/cells | Single center, randomized, blinded | ARCO I-II | 60 mo | Significant difference in the time to failure between CD alone and CD with bone marrow cells; significant reduction in pain and joint symptoms and reduced incidence of fractural stages in pts with CD with bone marrow cell implantation | 75 |
| CD ± autologous bone marrow mononuclear cells | Traumatic/nontraumatic (steroids, alcohol, idiopathic, pregnancy, Cushing disease) ON of the hip | 51 hips in 40 pts | 5 × 108 MNC | Single center, randomized | ARCO I-II, Mitchell | 3, 6, 12, and 24 mo | Significant improvement in clinical outcome (HHS); significantly better clinical score and mean hip survival with BMNCs), especially in pts with adverse prognostic features at initial presentation | 79 |
| CD and bone marrow concentrate and backfilling Calcium sulfate/calcium phosphate bioceramic | ON of the hip | 37 hips in 31 pts, (24–56 y) | 15-20 cm3 bone marrow concentrate (not further specified) | Single center, single arm | Steinberg I or II or with early segmental collapse, Steinberg IIIa | 12-32 mo | Increase in mean HHS from 68 to 86 points; 78.4% of the hips improved or had no further collapse; overall clinical success rate of 86.5% with three conversions to total hip arthroplasty | 80 |
| CD and autologous bone marrow derived MSCs | Corticosteroid induced ON of the knee | 6 pts (9-21 y), thereof 2 with ALL /NHL, 2 children | 31 × 106 to 240 × 106 MSCs | Single center, single arm | n.s. | Median 16 mo | Reduced pain, ADL improved | 82 |
| CD ± autologous bone marrow–derived MSCs | ONFH and risk factors (trauma, corticosteroids, alcohol, Caisson disease, idiopathic) | 100 pts (18-55 y) | 2 × 106 /kg MSCs | Single center, randomized | ARCO IC-IIC | 6, 12, 24, and 60 mo | 2/53 MSCs treated hips progressed;10/44 hips with CD alone progressed; significant improvement of the HHS and decreased volume of femoral head low signal intensity zone of the hips in the MSC treated group | 76 |
| CD ± autologous bone marrow aspirate concentrate | Nontraumatic femoral head ON | 25 hips in 24 pts (>18 y) | 50.0 ± 15.9 CFUs | Single center, randomized | ARCO II | 12 and 24 mo | No difference between the groups regarding pain and function, head survival rate, and interval to THA | 81 |
| Intervention/cell source . | Indication . | Study cohort . | Cell numbers . | Study design . | Classification . | Follow-up . | Outcome . | Reference . |
|---|---|---|---|---|---|---|---|---|
| CD and volume-reduced autologous bone marrow mononuclear cells | ON (steroids, alcohol, sickle cell disease) of the hip | 534 hips in 342 pts (16-61 y) | Average 24 × 103 CFUs | Single center, single arm | Steinberg stage I-II | 8-18 y | Stage I and II: total hip replacement 94/534 hips | 72 |
| CD ± bone marrow cells | Nontraumatic ON of the hip | 24 hips in 19 pts | CFU-F 92.6 ± 22.4 × 107/cells | Single center, randomized, blinded | ARCO I-II | 60 mo | Significant difference in the time to failure between CD alone and CD with bone marrow cells; significant reduction in pain and joint symptoms and reduced incidence of fractural stages in pts with CD with bone marrow cell implantation | 75 |
| CD ± autologous bone marrow mononuclear cells | Traumatic/nontraumatic (steroids, alcohol, idiopathic, pregnancy, Cushing disease) ON of the hip | 51 hips in 40 pts | 5 × 108 MNC | Single center, randomized | ARCO I-II, Mitchell | 3, 6, 12, and 24 mo | Significant improvement in clinical outcome (HHS); significantly better clinical score and mean hip survival with BMNCs), especially in pts with adverse prognostic features at initial presentation | 79 |
| CD and bone marrow concentrate and backfilling Calcium sulfate/calcium phosphate bioceramic | ON of the hip | 37 hips in 31 pts, (24–56 y) | 15-20 cm3 bone marrow concentrate (not further specified) | Single center, single arm | Steinberg I or II or with early segmental collapse, Steinberg IIIa | 12-32 mo | Increase in mean HHS from 68 to 86 points; 78.4% of the hips improved or had no further collapse; overall clinical success rate of 86.5% with three conversions to total hip arthroplasty | 80 |
| CD and autologous bone marrow derived MSCs | Corticosteroid induced ON of the knee | 6 pts (9-21 y), thereof 2 with ALL /NHL, 2 children | 31 × 106 to 240 × 106 MSCs | Single center, single arm | n.s. | Median 16 mo | Reduced pain, ADL improved | 82 |
| CD ± autologous bone marrow–derived MSCs | ONFH and risk factors (trauma, corticosteroids, alcohol, Caisson disease, idiopathic) | 100 pts (18-55 y) | 2 × 106 /kg MSCs | Single center, randomized | ARCO IC-IIC | 6, 12, 24, and 60 mo | 2/53 MSCs treated hips progressed;10/44 hips with CD alone progressed; significant improvement of the HHS and decreased volume of femoral head low signal intensity zone of the hips in the MSC treated group | 76 |
| CD ± autologous bone marrow aspirate concentrate | Nontraumatic femoral head ON | 25 hips in 24 pts (>18 y) | 50.0 ± 15.9 CFUs | Single center, randomized | ARCO II | 12 and 24 mo | No difference between the groups regarding pain and function, head survival rate, and interval to THA | 81 |
BMNCs, bone marrow mononuclear cells; CD, core decompression; CFU, colony-forming units; HHS, Harris Hip Score; n.s., not specified; ONFH, osteonecrosis of the femoral head; pts, patients.
Unfortunately, only adults with ON of an etiology other than ALL were included in these 2 aforementioned studies, which limits the transferability of the results to children with ALL. Furthermore, in all these studies, only patients with ON of the FH were evaluated, but a substantial number of children with ALL present with ON of the knees. Müller et al demonstrated the feasibility and safety of CD combined with MSCs in the treatment of ON of the femoral condyles and/or tibial plateau, including 2 children with corticosteroid-induced ON.82 These studies provide the most promising data on successful intervention in symptomatic ON and thus are worth further prospective evaluation. However, it remains challenging to precisely identify adolescents who require such an approach and to define the most effective point in time for this intervention, so these elements are subject to prospective clinical evaluation.
To date, under the search terms “osteonecrosis,” “children,” and “mesenchymal stem cells,” only 2 studies are listed at www.clinicaltrials.gov (#NCT00813267 and #NCT02065167). The status of one study is listed as recruiting, but it includes only patients between 18 and 65 years, and the status of the second study, which includes patients 12 to 60 years, is unknown.
Of note, interventions employing isolated and in vitro–expanded autologous MSCs necessitate a 2-step approach, beginning with bone marrow harvesting from the iliac crest, followed by a surgical intervention 3 to 4 weeks later, involving injection of MSCs through the CD tract into the area of necrosis. There is a treatment delay associated with in vitro expansion of autologous MSCs, which must be carefully considered, since it may prove critical in the setting of rapidly progressing ON. In addition, potential contamination with submicroscopically present leukemia cells must be taken into account in order to minimize the risk of reimplantation of malignant cells with the autologous MSC graft. Notably, genetic data indicate that variants in MSCs may play a role in the development of osteonecrosis.83 However, the immediate implications of this finding in therapy using autologous MSCs are unclear. As an alternative strategy, the use of banked cryopreserved allogeneic MSCs derived from healthy volunteer bone marrow donors may also be considered. When comparing autologous vs allogeneic MSC treatment approaches in the setting of ON in children with ALL, the potential benefits and drawbacks have to be carefully considered, and prospective clinical trials with both types of MSCs are thus urgently needed.
Based on (1) the substantial sequelae of children with ALL suffering from symptomatic ON, which is often associated with lifelong debilitating consequences and immobility, and (2) the growing clinical experience documenting the overall safety of MSC administration in a variety of clinical settings, prospective studies on MSC administration are clearly justified and eagerly awaited.
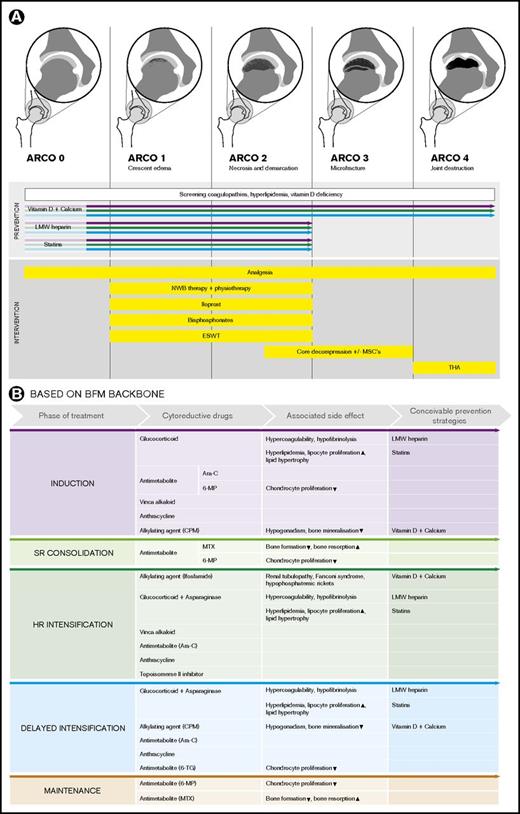
An overview of conceivable pharmacological, nonpharmacological, nonsurgical, and surgical (combined with cellular therapies) prevention and intervention options in children and adolescents with ALL, depending on ON stage (exemplarily depicted according to ARCO classification for the hip joints), is given in Figure 2. Notably, the presence of ON during ongoing therapy (mainly during delayed intensification and maintenance) might likewise suggest antileukemic therapy adjustments (eg, cessation of ongoing glucocorticoids). However, there is not enough evidence in the literature to support advising adjustments, such as ceasing treatment or limiting doses of corticosteroids or other antileukemic drugs.22 Instead, antileukemic therapy adjustments for symptomatic ON must be carefully addressed in future prospective clinical trials.
Overview of conceivable pharmacological, nonpharmacological, nonsurgical, and surgical (combined with cellular therapies) potential prevention and intervention options in children and adolescents with ALL. (A) The illustrations depict examples according to the association research circulation osseous (ARCO) classification for the hip joints. Screening of underlying coagulopathies and hyperlipidemia to prevent progression of early-stage ON and eventual compensation of vitamin D deficiency may be considered. The colored arrows indicate in which treatment phases and ON stages the depicted prevention strategies might be effective. (B) Overview of conceivable preventive pharmacological interventions in children and adolescents with ALL assigned to the different phases of the Berlin Frankfurt Muenster (BFM) therapy backbone and the cytostatic drugs and the treatment-related metabolic side effects. CPM, cyclophosphamide; HR, high risk; MP, mercaptopurine; MTX, methotrexate; NWB, non–weight-bearing; SR, standard risk; TG, thioguanine; THA, total hip arthroplasty.
Overview of conceivable pharmacological, nonpharmacological, nonsurgical, and surgical (combined with cellular therapies) potential prevention and intervention options in children and adolescents with ALL. (A) The illustrations depict examples according to the association research circulation osseous (ARCO) classification for the hip joints. Screening of underlying coagulopathies and hyperlipidemia to prevent progression of early-stage ON and eventual compensation of vitamin D deficiency may be considered. The colored arrows indicate in which treatment phases and ON stages the depicted prevention strategies might be effective. (B) Overview of conceivable preventive pharmacological interventions in children and adolescents with ALL assigned to the different phases of the Berlin Frankfurt Muenster (BFM) therapy backbone and the cytostatic drugs and the treatment-related metabolic side effects. CPM, cyclophosphamide; HR, high risk; MP, mercaptopurine; MTX, methotrexate; NWB, non–weight-bearing; SR, standard risk; TG, thioguanine; THA, total hip arthroplasty.
Outlook: translation of genetic risk factors into treatment
Three genome-wide association studies have been done to identify genetic risk factors for ON.17,27,83 Although these studies have not directly translated into treatment strategies or identified robust predictors of ON, they have implicated various pathways (glutamate receptor pathway, adipogenesis pathways, enhancers active in MSCs, bone morphogenic protein) that could potentially be targeted in the future. However, one must keep in mind that the studies also demonstrated that genetic risk factors significantly depend on the patient’s age.
Conclusions
Due to the incomplete understanding of disease pathophysiology and the paucity of prospective clinical studies, treatment of osteonecrosis in children with ALL remains a significant challenge. Accurate risk-prediction models must be developed, incorporating the results of ongoing early screening MRI and other factors, such as genetic data. These will hopefully identify those patients with a high risk of ON progression and debilitating sequelae. Prospective interventional studies based on such prediction models are urgently needed. Core decompression surgery combined with cellular therapies (eg, employing MSCs) appears promising in children at high risk of developing late-stage ON with joint infraction.
Acknowledgments
This work was supported by the German Childhood Cancer Foundation (DKS 2011.11). The authors thank Janina Klasen-Sansone for providing the magnetic resonance images and Jessica I. Hoell for critical reading of the manuscript. The authors thank Stewart Boden for providing English editing.
Authorship
Contribution: M. Kuhlen designed and supervised the project and wrote the manuscript; M. Kunstreich and K.K. screened the literature, collected the data, and compiled the tables; M. Kunstreich drafted Figure 2; and R.M. and A.B. critically revised the manuscript for important intellectual content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michaela Kuhlen, Department of Pediatric Oncology, Hematology and Clinical Immunology, Center for Child and Adolescent Health, Moorenstr 5, 40225 Duesseldorf, Germany; e-mail: michaela.kuhlen@med.uni-duesseldorf.de.
References
Author notes
R.M. and A.B. contributed equally to this study.