Key Points
Brain involvement, although rare, can occur in HCL.
The combination of cladribine and rituximab is a highly effective treatment of HCL with brain involvement.
Introduction
Hairy cell leukemia (HCL) is a chronic indolent neoplasm of mature B cells characterized by the presence of hairy cells, which are small monocytoid lymphocytes with circumferential hairy projections. Most cases of classical HCL are associated with an activating mutation of the BRAF gene (V600E), and tumor cells are predominantly found in the bone marrow and spleen, causing pancytopenia with a small number of circulating neoplastic cells and a variable degree of splenomegaly.1,2 Neurologic complications are rarely reported in HCL patients, and most are related to infection.3-6 To date, there have been only 2 case reports of parenchymal brain involvement in HCL.7,8 Herein, we report another patient with this complication, who has achieved a complete remission after receiving a combination of rituximab and cladribine.
Case description
A 42-year-old white man presented with a 4-month history of fatigue, shortness of breath, easy bruising, and headaches. He did not have any significant past medical history, was not hypertensive, and was a nonsmoker who drank alcohol occasionally. He was not taking any medications. There was no family history of hematopoietic neoplasms. Physical examination revealed multiple petechiae and bruising. Neurologic examination revealed no focal neurologic deficits. Cervical, axillary, and inguinal lymph nodes were slightly enlarged. Abdominal examination revealed 18-cm splenomegaly and no hepatomegaly. Computed tomography scanning showed massive splenomegaly with hypoattenuating splenic lesions. Hypoattenuating hepatic lesions and lymphadenopathy in supraclavicular and portal caval lymph nodes were also found. Unenhanced computed tomography scan of the brain revealed multiple hyperdense lesions throughout both hemispheres (not shown). A lumbar puncture and blood cultures showed no evidence of infection, including acid-fast bacilli and Toxoplasma gondii. He was negative for HIV and hepatitis B and C. Magnetic resonance imaging scans of the brain demonstrated multiple small acute hemorrhages at the gray-white junction in the supratentorial and infratentorial brain in axial gradient-echo, axial fluid-attenuated inversion recovery, and sagittal T1 sequences (Figure 1A-C). Radiologic differential diagnosis included hemorrhagic metastases, septic emboli, longstanding severe hypertension, microangiopathy, and vasculitis.
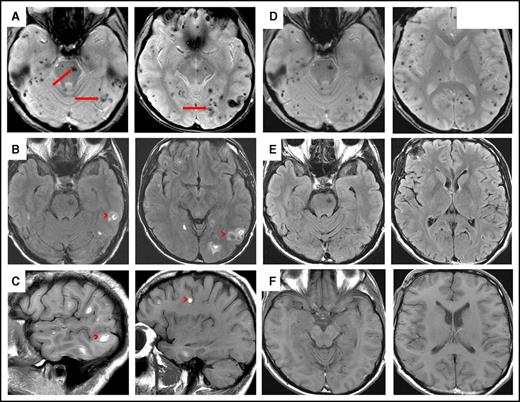
Magnetic resonance imaging of involved brain in HCL before and after chemoimmunotherapy. (A) Axial gradient-echo (GRE) sequence demonstrates innumerable microhemorrhages scattered throughout the supratentorial and infratentorial brain involving both cerebral and cerebellar hemispheres as well as the brainstem (arrows). (B) Axial fluid-attenuated inversion recovery (FLAIR) and (C) sagittal T1 sequences demonstrate multiple small acute hemorrhages at the gray-white junction in the supratentorial and infratentorial brain (these were hyperdense on unenhanced computed tomography scan; not shown) (arrowheads). (D) Axial GRE sequence after chemoimmunotherapy shows multiple microhemorrhages scattered throughout supratentorial and infratentorial brain, consistent with old blood. (E) Axial FLAIR and (F) axial T1 sequences show complete resolution of lesions after chemoimmunotherapy.
Magnetic resonance imaging of involved brain in HCL before and after chemoimmunotherapy. (A) Axial gradient-echo (GRE) sequence demonstrates innumerable microhemorrhages scattered throughout the supratentorial and infratentorial brain involving both cerebral and cerebellar hemispheres as well as the brainstem (arrows). (B) Axial fluid-attenuated inversion recovery (FLAIR) and (C) sagittal T1 sequences demonstrate multiple small acute hemorrhages at the gray-white junction in the supratentorial and infratentorial brain (these were hyperdense on unenhanced computed tomography scan; not shown) (arrowheads). (D) Axial GRE sequence after chemoimmunotherapy shows multiple microhemorrhages scattered throughout supratentorial and infratentorial brain, consistent with old blood. (E) Axial FLAIR and (F) axial T1 sequences show complete resolution of lesions after chemoimmunotherapy.
Methods
Formalin-fixed paraffin-embedded sections were processed using an automated immunostainer and stained with antibodies against CD5, CD20, CD43, CD123, BCL1, BCL2, BCL6, and DBA.44. Molecular studies for BRAF V600E mutation and FISH studies for t(11;14) and del(17p) were done at the outside institutions per their established protocols. Computed tomography and magnetic resonance imaging scans were performed per standard protocols. Patient's medical chart was reviewed to obtain clinical history and laboratory data. This case report was reviewed and approved by the Health Research Ethics Board at the University of Manitoba, Winnipeg, MB, Canada.
Results and discussion
Peripheral blood and bone marrow findings
The patient was anemic with hemoglobin 96 g/L (normal, 140-180 g/L) and platelets 43 × 109/L (normal, 140-440 × 109/L). He had marked leukocytosis with white blood cell count 37.7 × 109/L (normal, 4.5-11 × 109/L), lymphocytes 35.4 × 109/L (normal, 1.3-3.2 × 109/L), and normal monocytes 0.64 × 109/L (normal, 0.3-0.8 × 109/L). Peripheral blood smear showed numerous medium-size lymphoid cells with oval to slightly irregular nuclei, homogenous ground-glass chromatin, and abundant pale blue cytoplasm with hairy projections (Figure 2A). Bone marrow aspiration was a dry tap, and the core biopsy demonstrated extensive interstitial infiltration by lymphoid cells with slightly irregular nuclei and abundant cytoplasm (Figure 2B). Bone marrow showed significant fibrosis. Flow cytometry on the peripheral blood detected a λ light chain-restricted B-cell population with expression of CD19, CD20 (bright), CD11c, CD25, and CD103. Molecular studies were positive for the BRAF-V600E mutation, consistent with classical HCL. Fluorescence in situ hybridization studies were negative for del(17p).
HCL involving blood, bone marrow, and brain. (A) Hairy cells in the peripheral blood showing oval to slightly irregular nuclei, ground-glass chromatin, and abundant pale blue cytoplasm with hairy projections (Wright-Giemsa stain, oil immersion lens, original magnification ×1000). (B) Bone marrow shows extensive interstitial infiltration by HCL (hematoxylin and eosin [H&E] stain, original magnification ×200). (C) Brain biopsy showing fragments of brain parenchyma (right) and area of atypical lymphoid cells (HCL infiltrate) with focal area of necrosis (left) (H&E stain, original magnification ×50). (D) Atypical lymphoid cells involving the brain are small, with slightly irregular nuclei and inconspicuous nucleoli; scattered hemosiderin-laden macrophages are admixed (H&E stain, original magnification ×400). The HCL infiltrate is positive for (E) CD20 and (F) BCL1 (immunohistochemical stains, original magnification ×200).
HCL involving blood, bone marrow, and brain. (A) Hairy cells in the peripheral blood showing oval to slightly irregular nuclei, ground-glass chromatin, and abundant pale blue cytoplasm with hairy projections (Wright-Giemsa stain, oil immersion lens, original magnification ×1000). (B) Bone marrow shows extensive interstitial infiltration by HCL (hematoxylin and eosin [H&E] stain, original magnification ×200). (C) Brain biopsy showing fragments of brain parenchyma (right) and area of atypical lymphoid cells (HCL infiltrate) with focal area of necrosis (left) (H&E stain, original magnification ×50). (D) Atypical lymphoid cells involving the brain are small, with slightly irregular nuclei and inconspicuous nucleoli; scattered hemosiderin-laden macrophages are admixed (H&E stain, original magnification ×400). The HCL infiltrate is positive for (E) CD20 and (F) BCL1 (immunohistochemical stains, original magnification ×200).
Brain biopsy findings
A brain biopsy revealed fragments of brain parenchyma and a partially necrotic area with atypical lymphoid cells (Figure 2C). These cells were small, with slightly irregular nuclei and inconspicuous nucleoli, admixed with hemosiderin-laden macrophages (evidence of hemorrhage; Figure 2D). Blood vessels in the brain parenchyma and in the lymphoid infiltrate appeared morphologically intact. By immunohistochemistry, lymphoid cells were positive for CD20 (Figure 2E), BCL1 (partial staining; Figure 2F), CD123, DBA.44, and BCL2 and negative for CD5, BCL6, and CD43. Moreover, molecular studies, performed on the brain tissue, detected the BRAF-V600E mutation. Findings were consistent with brain involvement by HCL. Fluorescence in situ hybridization study for t(11;14)(q13;q32) was attempted, but results were inconclusive because of suboptimal tissue quality and necrosis.
Clinical follow-up and discussion
Because of the aggressive and unusual clinical presentation with lymphadenopathy, massive splenomegaly, high lymphocyte count, anemia, normal monocyte count, and central nervous system (CNS) involvement, the patient was initially thought to have atypical HCL and was treated with a combination of cladribine (0.14 mg/m2 once per day for 5 days) and rituximab (375 mg/m2 once per week for 8 weeks) per previously published recommendations.9-11 After completion of his treatment, his symptoms, splenomegaly, and lymphadenopathy resolved. The white blood cell count was 4.52 × 109/L; hemoglobin, 128 g/L; platelets, 158 × 109/L; lymphocytes, 0.57 × 109/L; monocytes, 0.39 × 109/L; and neutrophils, 1.48 × 109/L. Six months after completing treatment, the patient was clinically well, and the blood counts and physical examination were normal with the exception of lymphocytopenia (lymphocytes, 0.54 × 109/L); thus, follow-up bone marrow examination was not performed, but flow cytometry of the peripheral blood showed 0.02% cells expressing a hairy cell phenotype. Repeat magnetic resonance imaging scans performed 9 months after completion of therapy showed complete resolution of acute brain lesions in axial fluid-attenuated inversion recovery and axial T1 sequences (Figure 1E-F). Axial gradient-echo sequencing demonstrated multiple microhemorrhages scattered throughout the supratentorial and infratentorial brain, consistent with old blood (Figure 1D).
Lymphocytosis is mainly associated with a HCL variant that is typically BRAF negative12 and is rarely reported in cases with classical HCL phenotype.13 This case demonstrates that BRAF-mutated classical HCL can also have lymphocytosis and atypical clinical presentation.
The differential diagnosis of multiple hemorrhagic CNS lesions on imaging has not traditionally included HCL. In HCL patients, most described CNS lesions have been secondary to infection,3-6 with only 2 previously reported cases of brain involvement by hairy cells.7,8 The first reported patient was a 35-year-old man with a rapidly progressive clinical course who died 5 months after the diagnosis of HCL. Brain involvement was detected at autopsy.7 The second reported patient was treated with high-dose steroids, high-dose methotrexate (because it crosses the blood-brain barrier), and 7 days of cladribine. During the course of treatment, the patient developed anemia, bleeding, and neutropenia with sepsis, which was successfully treated. He also developed gastrointestinal bleeding, became hemodynamically unstable, and died 66 days after initial presentation. Interestingly, this patient, similar to our patient, also presented with a marked leukocytosis (60 × 109/L).8
Here we report a patient who presented with the clinical features of atypical HCL (lymphadenopathy/splenomegaly, high lymphocyte count, normal monocyte count) and CNS involvement, but morphologic, immunophenotypic, and molecular features that were consistent with classical HCL. This patient shows why brain biopsy is important when there are cerebral lesions in HCL. Although the radiologic differential diagnosis included brain microhemorrhages as a result of several nonneoplastic causes, the tissue biopsy was consistent with parenchymal brain involvement by HCL and associated hemorrhage. Moreover, the patient did not have a clinical history of hypertension or evidence of other diseases that could cause brain microhemorrhages.
Because of the unusually aggressive clinical presentation, our patient was treated with a combination of cladribine and rituximab because both agents penetrate the blood brain-barrier and are frequently used as first-line therapy for variant HCL.9-11,14,15 We speculate that rituximab played a significant role in the excellent clinical response of our patient, because this response was not observed in the previously reported patient treated with cladribine and methotrexate.8 Moreover, a recent report described a patient with extensive leptomeningeal involvement in HCL who was initially treated with high-dose cytarabine, systemic rituximab, and intrathecal methotrexate, but who progressed despite treatment. He was then given vemurafenib, a BRAF inhibitor approved in the second-line setting for HCL, and he achieved complete remission.16 The role of vemurafenib in treatment of these patients needs to be further investigated because some studies indicate that it does not cross the blood-brain barrier.17
Authorship
Contribution: A.M.P. collected the data, reviewed the case, and wrote the manuscript; K.M., V.W., and M.A. reviewed the case and collected data; D.H. managed patient consent forms and collected data; J.B.J. collected data; V.B. collected data and wrote the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Versha Banerji, Section of Hematology/Oncology, Department of Internal Medicine, University of Manitoba and CancerCare Manitoba, 675 McDermot Ave, Winnipeg, MB R3E 0V9, Canada; e-mail: vbanerji1@cancercare.mb.ca.

![Figure 2. HCL involving blood, bone marrow, and brain. (A) Hairy cells in the peripheral blood showing oval to slightly irregular nuclei, ground-glass chromatin, and abundant pale blue cytoplasm with hairy projections (Wright-Giemsa stain, oil immersion lens, original magnification ×1000). (B) Bone marrow shows extensive interstitial infiltration by HCL (hematoxylin and eosin [H&E] stain, original magnification ×200). (C) Brain biopsy showing fragments of brain parenchyma (right) and area of atypical lymphoid cells (HCL infiltrate) with focal area of necrosis (left) (H&E stain, original magnification ×50). (D) Atypical lymphoid cells involving the brain are small, with slightly irregular nuclei and inconspicuous nucleoli; scattered hemosiderin-laden macrophages are admixed (H&E stain, original magnification ×400). The HCL infiltrate is positive for (E) CD20 and (F) BCL1 (immunohistochemical stains, original magnification ×200).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/1/14/10.1182_bloodadvances.2017004697/3/m_advances004697f2.jpeg?Expires=1765910076&Signature=WFLyDgB35oSOXRaZllpCizp4V3XQNHTYPx3fKC31JWw~ttVwkmd3NH1OMsKyL1V2gFLJykpDA~wG3KuLsELh8U7uUxIFk~kfHf7X5IsIjuyabj0iOh8vSgUpM0Hhgx1GNJxCOzuvhcCQkWsgOPMW9~jkbmo3Xz7N4fcpQNHc06DtTX1-4C3scRegFGEhBAnRG5otAKP8H1Sgkv8l6RX9KSxtp18Gvw7j~x0A5-FGRxPt~Ehz~VUJUB1DtBTIjApGBKHFcCzstJoAFnGeE~3p8KqHdNxFTK~LeOxgK9-~GF4orIcFUVteLQHQEcdlXnCHODjS9tHDCXWyxKEyLwRNNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)