Key Points
Prescribing appropriately for age and cardiovascular risk is likely to result in minimal permanent toxicity-related dasatinib cessation.
CML patients on dasatinib with pleural effusion are more likely to have achieved MR4.5 after 6-month therapy than those without effusion.
Abstract
Dasatinib has shown superiority over imatinib in achieving molecular responses (MRs) in chronic phase chronic myeloid leukemia but with a different toxicity profile, which may impact its overall benefit. Reported toxicities include pleural effusions and pulmonary hypertension, and although the incidence of these events is well described, response to therapy and impact of dose modifications on toxicity has not been comprehensively characterized in a real-world setting. We retrospectively reviewed the incidence of dasatinib adverse events in 212 chronic phase chronic myeloid leukemia patients at 17 Australian institutions. Adverse events were reported in 116 patients (55%), most commonly pleural effusions (53 patients, 25%), which was the predominant cause of permanent drug cessation. Age and dose were risk factors for pleural effusion (P < .01 and .047, respectively). Recurrence rates were higher in those who remained on 100 mg compared with those who dose reduced (P = .041); however, recurrence still occurred at 50 mg. Patients who developed pleural effusions were more likely to have achieved MR4.5 after 6 months of dasatinib than those without effusions (P = .008). Pulmonary hypertension occurred in 5% of patients, frequently in association with pleural effusion, and was reversible upon dasatinib cessation in 6 of 7 patients. Dose reductions and temporary cessations had minimal impact on MR rates. Our observations suggest that by using the lowest effective dose in older patients to minimize the effusion risk, dose modification for cytopenias, and care with concomitant antiplatelet therapy, the necessity for permanent dasatinib cessation due to toxicity is likely to be minimal in immunologically competent patients.
Introduction
Randomized studies in newly diagnosed patients with chronic myeloid leukemia in chronic phase (CML-CP) have demonstrated the superiority of the second-generation tyrosine kinase inhibitors (TKI), nilotinib and dasatinib, in terms of the rapidity and depth of achievement of major molecular response compared with imatinib.1,2 However, these advantages may be offset by other significant toxicities not seen with imatinib,3-5 and there is increasing recognition that the choice of TKI in this context needs to be individualized according to concomitant comorbidities.
Although dasatinib is generally well tolerated, dose interruptions or modifications are often required, and these requirements are dose dependent. In patients receiving dasatinib (70 mg twice daily) after imatinib resistance or intolerance, dose interruptions for toxicity and dose modification were required in 79% and 73% of patients, respectively.4 The common side effects of dasatinib are well documented, including myelosuppression, diarrhea, and pleural and pericardial effusions.6 More severe and potentially life-threatening adverse events, including pulmonary hypertension (PHTN), unusual infections, and bleeding, have also been reported, but with unclear risk predilection.7-9
Although the literature has described the incidence of these events, the predisposing factors, the resolution or otherwise of toxicities in response to dasatinib dose reduction or cessation, and the subsequent impact of these changes on the underlying CML have not been comprehensively addressed outside of a clinical trial in a “real-world” setting. This study aims to clarify these issues in a survey of Australian patients receiving dasatinib, either as first- or as subsequent-line therapy for CML-CP.
Patients and methods
This study was a snapshot survey of all eligible patients at 17 institutions who were approached to participate on the basis of known interest in CML. Deidentified patient information was collected in an Access Database (Microsoft Access 2007). Ethics approval for the retrospective collection of data was obtained at each site, and the project was classified as low to negligible risk obviating the requirement for patient consent.
Eligibility criteria included the receipt of dasatinib at any stage of therapy for CML-CP and the availability of sufficient clinical data. Each patient’s history was tracked in detail to monitor the development and outcome of any complications and consequently the response of the CML, to either cessation or dose reduction of the dasatinib. The earliest prescription of dasatinib was on 1 February 2005, and the study census date was 30 June 2015, although outcome data in enrolled patients who experienced toxicity and subsequently required drug modification close to the study end date were collected where relevant.
Key data collected included sex, date of birth, start dose and duration of dasatinib therapy, sequential BCR-ABL1 results, prior CML-CP therapy, reasons for and details of any dose modifications. including temporary or permanent cessation. Toxicities were graded as per Common Terminology Criteria for Adverse Events, version 4.0,10 with the exception of suspected PHTN, which was defined as transthoracic echocardiogram evidence of pulmonary artery pressure (right ventricular systolic pressure >36 mmHg) in combination with grading according to the New York Heart Association Functional Class,11,12 without the requirement for confirmatory right heart catheterization. Specific details of the management of pulmonary toxicities were sought, including investigations (radiology, echocardiogram) and therapeutic interventions (including glucocorticosteroids, diuretics, and thoracocentesis). Pleural effusion recurrence was defined as a radiologically and clinically documented reemergence of effusions after initial resolution or progression beyond 6 weeks after the initial effusion. Information regarding less well-defined associations of dasatinib, including thrombohemorrhagic complications, infections, cytomegalovirus (CMV) reactivation, cervical lymphadenopathy, and vascular toxicities (including preexisting vascular risk factors and outcome of any medical or surgical therapies required), were specifically requested. Severe infection was defined as infection requiring intravenous antimicrobial therapy with or without radiological or operative intervention.10
Response assessment was defined by molecular responses (MR3, MR4, and MR4.5), indicating BCR-ABL1 transcript levels of ≤0.1%, ≤0.01%, and ≤0.0032% and which correspond to a 3-log, 4-log, and 4.5-log reduction from a standardized baseline, respectively.1,13,14
Statistical methods
All data were analyzed using SPSS statistics software (version 23.0; SPSS Inc, Chicago, IL). Where appropriate, continuous data were compared using the Student t test, ordinal data using the Mann-Whitney test, and categorical data using the Fisher’s exact test. The Kruskal-Wallis test was performed to evaluate any relationship between line of dasatinib as TKI therapy and the occurrence of pleural effusions. Kaplan-Meier analysis was used to assess the incidence of pleural effusion over time. A log-rank test was used to assess the difference between groups. Multiple regression was performed to evaluate the association between risk factors and pleural effusion; P values <.05 were considered significant.
Results
Demographics
Data are summarized in Table 1. The majority of patients commenced dasatinib at a dose of 100 mg daily as second-line therapy.
Patient demographics
| . | Value (range or %) |
|---|---|
| Total number of patients | 212 |
| Male/female | 106/106 |
| Median age (range) at dasatinib commencement, y | 53 (20-86) |
| Starting dose in mg, n (%) | |
| 50 | 7 (3) |
| 70 | 4 (2) |
| 80 | 1 (0.5) |
| 100 | 177 (83) |
| 140 | 21 (10) |
| Not available | 2 (1) |
| Line of therapy, n (%) | |
| First | 51 (24) |
| Second | 125 (59) |
| Third | 36 (17) |
| Median follow-up from commencement (range), mo | 27 (4-116) |
| . | Value (range or %) |
|---|---|
| Total number of patients | 212 |
| Male/female | 106/106 |
| Median age (range) at dasatinib commencement, y | 53 (20-86) |
| Starting dose in mg, n (%) | |
| 50 | 7 (3) |
| 70 | 4 (2) |
| 80 | 1 (0.5) |
| 100 | 177 (83) |
| 140 | 21 (10) |
| Not available | 2 (1) |
| Line of therapy, n (%) | |
| First | 51 (24) |
| Second | 125 (59) |
| Third | 36 (17) |
| Median follow-up from commencement (range), mo | 27 (4-116) |
Pleural effusion incidence and management
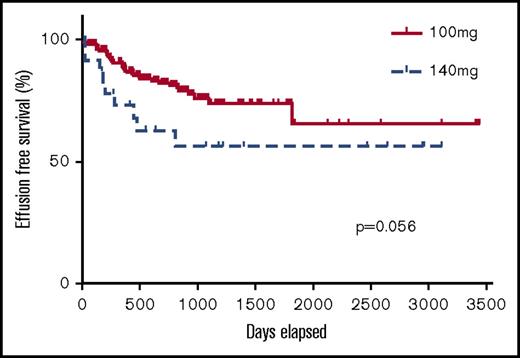
At least 1 adverse event was reported in 116 patients (55%). The most common adverse event was pleural effusion, which occurred in 25% of patients, the characteristics of whom are summarized in Table 2. Most (77%) pleural effusions were grade 1 (n = 6 patients) (asymptomatic; clinical or diagnostic observations only; intervention not indicated) or grade 2 (n = 35 patients) (symptomatic; intervention indicated, eg, diuretics or limited therapeutic thoracocentesis). Figure 1 illustrates the incidence of effusion over time according to dasatinib doses of 100 mg and 140 mg. Kaplan-Meier analysis suggests a tendency for pleural effusion to occur early with both doses, with both a higher incidence and an earlier occurrence with 140 mg than 100 mg (P = .056).
Characteristics of patients who developed pleural effusions
| . | Value (range or %) |
|---|---|
| No. of patients (% of total study population) | 53 (25) |
| Sex | |
| Male/female | 26/27 |
| Median age at effusion onset (range), y | 65 (41-86) |
| Median duration of dasatinib therapy (range) at time of effusion onset, y | 11 (0.5-59) |
| Grade effusion, n (% of all patients with effusion) | |
| 1-2 | 41 (77) |
| 3-4 | 10 (19) |
| No grade provided | 2 |
| Patients per line of therapy, n (% patients receiving this line with effusion) | |
| First | 7 (14) |
| Second | 40 (32) |
| Third | 6 (17) |
| Dasatinib dose at onset, mg, n (% patients receiving this dose with effusion) | |
| 50 | 2* |
| 70 | 4* |
| 80 | 1* |
| 100 | 37 (21) |
| 140 | 9 (43) |
| MR at effusion onset, log reduction, n (% of all patients with effusion) | |
| 0 | 4 (8) |
| MR1 | 7 (13) |
| MR2 | 2 (4) |
| MR3 | 5 (9) |
| MR4 | 7 (13) |
| MR4.5 | 27 (51) |
| No data available | 1 |
| . | Value (range or %) |
|---|---|
| No. of patients (% of total study population) | 53 (25) |
| Sex | |
| Male/female | 26/27 |
| Median age at effusion onset (range), y | 65 (41-86) |
| Median duration of dasatinib therapy (range) at time of effusion onset, y | 11 (0.5-59) |
| Grade effusion, n (% of all patients with effusion) | |
| 1-2 | 41 (77) |
| 3-4 | 10 (19) |
| No grade provided | 2 |
| Patients per line of therapy, n (% patients receiving this line with effusion) | |
| First | 7 (14) |
| Second | 40 (32) |
| Third | 6 (17) |
| Dasatinib dose at onset, mg, n (% patients receiving this dose with effusion) | |
| 50 | 2* |
| 70 | 4* |
| 80 | 1* |
| 100 | 37 (21) |
| 140 | 9 (43) |
| MR at effusion onset, log reduction, n (% of all patients with effusion) | |
| 0 | 4 (8) |
| MR1 | 7 (13) |
| MR2 | 2 (4) |
| MR3 | 5 (9) |
| MR4 | 7 (13) |
| MR4.5 | 27 (51) |
| No data available | 1 |
The percentage of patients with effusion/patients not calculated for starting doses <100 mg due to many subsequent dose fluctuations in these patients.
Kaplan-Meier curve demonstrating the incidence of pleural effusion in patients taking 100 mg and 140 mg dasatinib.
Kaplan-Meier curve demonstrating the incidence of pleural effusion in patients taking 100 mg and 140 mg dasatinib.
At a dose of 100 mg, effusions occurred in 21% of patients, most commonly in older patients (≥60 years): 23/69 (33%) vs 14/108 (13%) aged <60 years. Pleural effusions occurred in 43% of patients who received 140 mg. Again, age (as a continuous variable) and dose were independent risk factors for effusion (P values <.01 and .047, respectively), but sex (P = .54) and line of therapy (P = .22) were not.
Management of pleural effusions varied between institutions. Twenty-three patients (43% of those with effusions) underwent thoracocentesis; 25 (47%) received prednisolone, and 42 (79%) received diuretics.
Figure 2 examines the group of 37 patients who experienced pleural effusion while taking 100 mg dasatinib. Five different treatment approaches were identified: (1) immediate drug cessation, (2) continuation of 100 mg dasatinib, (3) temporarily withholding the drug and recommencing 100 mg within 6 weeks, (4) continuation at a reduced dose, and (5) temporarily withholding the drug and then resumption at a lower dose. With the exception of those patients who ceased dasatinib immediately, effusions recurred in patients across the remaining 4 categories (after median 20 weeks; range 3-157 weeks). All 4 patients who continued taking 100 mg without cessation experienced recurrence, including 2 patients who received steroids for acute effusion management. The risk of recurrence was significantly increased in those patients who did not cease the drug immediately (n = 29) compared with those who did (n = 8) (P = .0006). In those who continued the drug, the risk of recurrence was higher in those patients who remained on 100 mg (n = 12) compared with those who had a dose reduction (n = 17) (P = .041). Of the 37 patients who experienced pleural effusion while on 100 mg, 19 (51%) ultimately ceased dasatinib due to recalcitrant or recurrent pleural effusion.
Of the 53 patients with pleural effusion, 25 (47%) received prednisolone; all received a minimum of 25 mg daily (range 25-50 mg) in the acute phase followed by a tapered course with a median duration 4 weeks (range 3 days to 6 weeks). However, 7/25 continued with a longer-term maintenance dose (defined as beyond 6 weeks after acute effusion episode), with median duration 12 months (range 4-24 months). All 7 of these patients temporarily withheld dasatinib, with recommencement after resolution of the effusion in conjunction with ongoing maintenance prednisolone: 3/7 at the original dasatinib dose, 4/7 dose reduced. Two of 7 experienced recurrence. Both had been dose reduced: 100 mg to 80 mg, recurrence occurred after 17 months of maintenance prednisolone, on a dose of 5 mg alternate days; and 80 mg to 40 mg, recurrence after 9 months of maintenance prednisolone, on a dose of 5 mg daily. Three of 7 patients who received maintenance steroids to prevent effusion recurrence had concurrent autoimmune disorders, and it is possible that the indication for steroids in these patients was mixed.
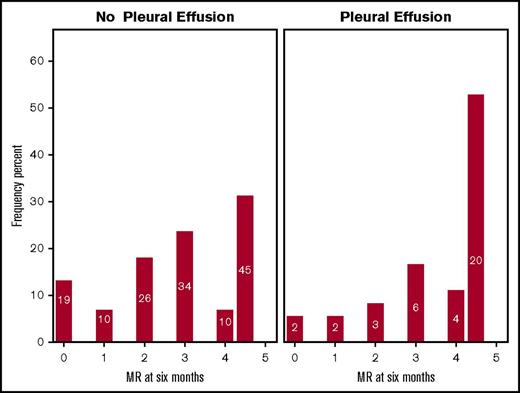
Molecular responses and pleural effusions
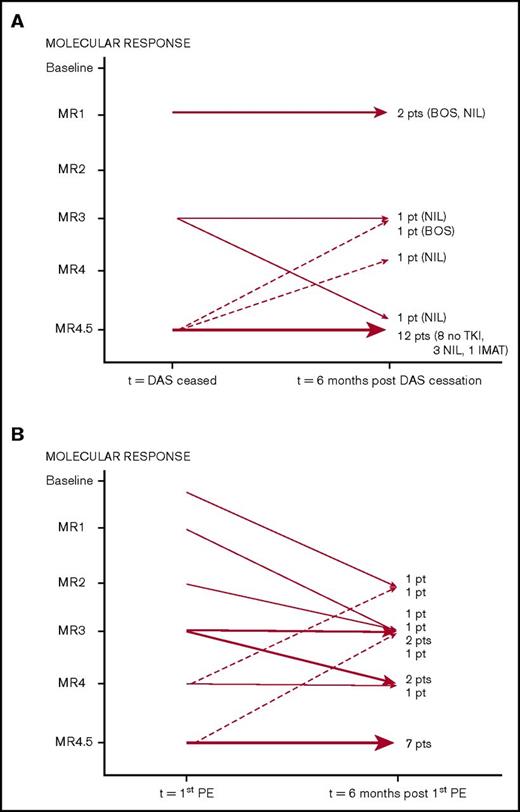
Patients on 100 mg dasatinib who developed pleural effusions were more likely to have achieved MR4.5 after 6 months of therapy than those who did not (P = .008) (Figure 3); this was independent of line of therapy (P = .390). Figure 4 documents MR status 6 months after the diagnosis of pleural effusions, focusing on 35 evaluable patients receiving 100 mg dasatinib. At the time of effusion, 22 were in MR4.5. Figure 4A documents the outcome in the 18 patients who ceased dasatinib permanently within the 6 months following effusion. Ten of these patients changed to an alternative TKI within 5 weeks of dasatinib cessation. Eight patients, all in MR4.5 at the time of effusion, remained off TKI therapy without molecular progression. Data were not available on the duration of MR4.5 prior to the onset of effusion in these patients. Figure 4B documents changes in MR in 17 patients who continued dasatinib with a median dose of 70 mg (range 25-120 mg) at 6 months. Fifteen (88%) of these patients had a stable or improved MR at 6 months.
Bar graph demonstrating percentage of patients per group (y-axis) achieving MR between 0 and 4.5 log reduction (x-axis) after 6 months of 100 mg dasatinib treatment. Patients who did not (A; n = 144) and did (B; n = 37) develop effusion (P = .008) are shown. Numbers within bars reflect the number of patients achieving each MR.
Bar graph demonstrating percentage of patients per group (y-axis) achieving MR between 0 and 4.5 log reduction (x-axis) after 6 months of 100 mg dasatinib treatment. Patients who did not (A; n = 144) and did (B; n = 37) develop effusion (P = .008) are shown. Numbers within bars reflect the number of patients achieving each MR.
Sequential (6 month) molecular response assessment in patients who developed pleural effusions. (A) Patients who ceased dasatinib (n = 18): switched TKI (n = 10), ceased TKI (n = 8). (B) Patients who continued dasatinib (n = 17). BOS, bosutinib; DAS, dasatinib; IMAT, imatinib; NIL, nilotinib; PE, pleural effusion.
Sequential (6 month) molecular response assessment in patients who developed pleural effusions. (A) Patients who ceased dasatinib (n = 18): switched TKI (n = 10), ceased TKI (n = 8). (B) Patients who continued dasatinib (n = 17). BOS, bosutinib; DAS, dasatinib; IMAT, imatinib; NIL, nilotinib; PE, pleural effusion.
Other adverse events
Table 3 indicates other adverse events reported in >5% of patients. Echocardiography was not routinely performed in patients and only undertaken at physician discretion, usually for investigation of unexplained dyspnea. Ten (5%) patients, on a median dose of 100 mg (range 100-140 mg) and all on dasatinib as second- or third-line therapy, had PHTN. All cases were diagnosed with echocardiography; none proceeded to right heart catheterization. All of these patients described dyspnea, although notably 8 had pleural effusions either concurrently or within 6 months of PHTN diagnosis. An additional 3 patients had mildly elevated pulmonary pressures that did not meet diagnostic criteria for PHTN. Of the 7 PHTN patients with available follow-up echocardiographic data, right ventricular systolic pressure normalized in 6 after dasatinib cessation. The remaining patient had persistently mildly elevated pressures (but ≤36 mmHg) after drug cessation and remained symptomatic.
Adverse events in >5% patients
| Adverse events . | No. of patients (%) . |
|---|---|
| Elevated pulmonary artery pressure | |
| Elevated but not meeting PHTN criteria | 3 (1) |
| Grade 1-2 | 6 (3) |
| Grade 3-4 | 4 (2) |
| Total | 13 (6) |
| Thrombocytopenia (grade 3-4) | 11 (5) |
| Infection | 12 (6) |
| Fatigue | 12 (6) |
| Adverse events . | No. of patients (%) . |
|---|---|
| Elevated pulmonary artery pressure | |
| Elevated but not meeting PHTN criteria | 3 (1) |
| Grade 1-2 | 6 (3) |
| Grade 3-4 | 4 (2) |
| Total | 13 (6) |
| Thrombocytopenia (grade 3-4) | 11 (5) |
| Infection | 12 (6) |
| Fatigue | 12 (6) |
Grade 3 or 4 thrombocytopenia occurred in 11 patients (5%). There were 7 reported cases of grade 3 or 4 bleeding; in 3/7, the platelet count was <50 × 109/L, and 1 patient had a normal platelet count but was on aspirin (Table 4). Two patients had clinically significant bleeding in the absence of thrombocytopenia or concomitant antiplatelet therapy: a 77-year-old woman with a spontaneous fatal intracerebral hemorrhage without clear etiology or risk factors; and a 44-year-old woman with a significant decline in hemoglobin 2 weeks after commencing dasatinib, and endoscopy revealed gastric antral vascular ectasia, which was managed and dasatinib was resumed without further bleeding events.
Grade 3 or 4 bleeding
| Age/sex . | Bleeding complication . | Platelet count, ×109/L . | Concomitant antiplatelet agents . |
|---|---|---|---|
| 45/F | Heavy PV bleeding; concomitant fibroids | 33 | No |
| 75/F* | PR bleed | 13 | No |
| 77/F* | Subdural hemorrhage | 81 | No |
| 59/F | GI blood loss | 13 | No |
| 44/F | Gastric antral vascular ectasia | 263 | No |
| 51/M | GI bleeding | 409 | Aspirin |
| 77/F | Fatal intracerebral hemorrhage | 157 | No |
| Age/sex . | Bleeding complication . | Platelet count, ×109/L . | Concomitant antiplatelet agents . |
|---|---|---|---|
| 45/F | Heavy PV bleeding; concomitant fibroids | 33 | No |
| 75/F* | PR bleed | 13 | No |
| 77/F* | Subdural hemorrhage | 81 | No |
| 59/F | GI blood loss | 13 | No |
| 44/F | Gastric antral vascular ectasia | 263 | No |
| 51/M | GI bleeding | 409 | Aspirin |
| 77/F | Fatal intracerebral hemorrhage | 157 | No |
F, female; GI, gastrointestinal; M, male; PR, per rectal; PV, per vaginal.
Same patient with 2 severe bleeding events, in 2005 at age 75 and in 2007 at age 77.
There were 12 episodes of clinically significant infection, predominately respiratory tract. Only 1 episode was associated with neutropenia: an episode of culture-negative febrile neutropenia. There was 1 atypical infection, Salmonella empyema in a patient with a pleural effusion, and a single infective death in an 84-year-old male patient who died of nonneutropenic sepsis without an identified pathogenic organism. Investigators were specifically asked about CMV infection; no cases were reported. No cases of hepatitis B virus reactivation were reported.
Cardiac and vascular events were uncommon. The details of the events, including previously identified cardiovascular risk factors and prior nilotinib use, are listed in Table 5. There were 2 cases of peripheral vascular disease requiring intervention, both in patients who had prior nilotinib exposure. One patient had 4 established cardiovascular risk factors and developed cardiac ischemia perivascular surgery, and a subsequent coronary angiogram revealed diffuse small vessel cardiac disease. The event occurred 6 months after switching from nilotinib (duration of therapy 30 months) to dasatinib. The second patient, with no vascular risk factors, received nilotinib for 12 months, and 4 months after switching from nilotinib to dasatinib required both right and left femoral-popliteal bypass surgeries 3 months apart.
Vascular adverse events
| Age/sex . | Adverse event . | CV risk factors . | Previous nilotinib? . |
|---|---|---|---|
| 62/F | Newly diagnosed IHD | HTN, dyslipidemia, ex-smoker | No |
| 63/M | PVD requiring surgery | None | Yes |
| 61/M | Ischemic CVA, pericardial effusion | HTN, DM, ex-smoker | Yes |
| 64/F | Elective AAA repair | Previous IHD, ex-smoker | No |
| 65/M | AF, pericardial effusion (in setting of sepsis) | Ex-smoker | Yes |
| 47/F | PVD requiring surgery, complicated by NSTEMI | HTN, DM, dyslipidemia, ex-smoker | Yes |
| 63/F | Ischemic CVA | DM, HTN, ex-smoker | No |
| 65/M | Newly diagnosed IHD | DM, HTN | No |
| Age/sex . | Adverse event . | CV risk factors . | Previous nilotinib? . |
|---|---|---|---|
| 62/F | Newly diagnosed IHD | HTN, dyslipidemia, ex-smoker | No |
| 63/M | PVD requiring surgery | None | Yes |
| 61/M | Ischemic CVA, pericardial effusion | HTN, DM, ex-smoker | Yes |
| 64/F | Elective AAA repair | Previous IHD, ex-smoker | No |
| 65/M | AF, pericardial effusion (in setting of sepsis) | Ex-smoker | Yes |
| 47/F | PVD requiring surgery, complicated by NSTEMI | HTN, DM, dyslipidemia, ex-smoker | Yes |
| 63/F | Ischemic CVA | DM, HTN, ex-smoker | No |
| 65/M | Newly diagnosed IHD | DM, HTN | No |
AAA, aortoabdominal aneurysm; AF, atrial fibrillation; CV, cardiovascular; CVA, cerebrovascular accident; DM, diabetes mellitus; HTN, hypertension; IHD, ischemic heart disease; NSTEMI, non-ST elevated myocardial infarction; PVD, peripheral vascular disease.
Investigators were specifically asked about the presence of benign cervical lymphadenopathy and this condition was reported in 2 male patients; both cases resolved spontaneously without interruption of dasatinib.
Drug cessation due to adverse effects
Table 6 summarizes the incidence and predominant reason for drug cessation per line of therapy. Of the 79 patients (37% of study population) who ceased the drug, 51 (24% study population) ceased because of adverse events. For the cohort who ceased because of adverse events, the median age was 65 years (range 29-87 years); median duration of dasatinib therapy was 16 months (range 0.1-55 months), and the dasatinib dose at time of drug cessation was 40 mg (n = 4), 50 mg (n = 5), 70 mg (n = 10), 80 mg (n = 2), 100 mg (n = 28), and 140 mg (n = 2). Twenty-one of these patients (10% of total cohort) experienced adverse events of sufficient severity to cease the drug immediately.
Drug cessation by line of therapy
| Line of therapy . | No. patients ceased drug/no. on therapy (%) . | Median time to drug cessation (mo) (range 0.1-55 mo) . | Reason for drug cessation . |
|---|---|---|---|
| First | 9/51 (18) | 14.4 | Adverse events, n = 6 |
| Progression/treatment failure, n = 3 | |||
| Second | 59/125 (47) | 15.5 | Adverse events, n = 40 |
| Progression/treatment failure, n = 13 | |||
| Pregnancy, n = 1 | |||
| Good CML response, n = 1 | |||
| Death, n = 4 | |||
| Third | 11/36 (30) | 13.5 | Adverse events, n = 5 |
| Progression/treatment failure, n = 1 | |||
| Death, n = 5 |
| Line of therapy . | No. patients ceased drug/no. on therapy (%) . | Median time to drug cessation (mo) (range 0.1-55 mo) . | Reason for drug cessation . |
|---|---|---|---|
| First | 9/51 (18) | 14.4 | Adverse events, n = 6 |
| Progression/treatment failure, n = 3 | |||
| Second | 59/125 (47) | 15.5 | Adverse events, n = 40 |
| Progression/treatment failure, n = 13 | |||
| Pregnancy, n = 1 | |||
| Good CML response, n = 1 | |||
| Death, n = 4 | |||
| Third | 11/36 (30) | 13.5 | Adverse events, n = 5 |
| Progression/treatment failure, n = 1 | |||
| Death, n = 5 |
Pleural effusions were the most common reason for drug cessation (n = 25) with a further 6 patients ceasing dasatinib because of concomitant pleural effusions and PHTN. Other reasons included rash (n = 4), gastrointestinal including nausea and abdominal pain (n = 4), myelosuppression (n = 2), PHTN alone (n = 2), fluid retention (n = 2), and 1 patient each for cough, constitutional symptoms, subdural hemorrhage in the setting of moderate thrombocytopenia, fatigue, and headache. A further patient experienced acute pulmonary edema in the setting of hypoalbuminemia due to nephrotic range proteinuria, with renal biopsy demonstrating drug-induced lupus nephritis, positive antinuclear antibody, and anti-double-stranded DNA antibodies. Dasatinib, as the presumed causative agent, was ceased with subsequent complete resolution of the proteinuria.
Overview of dasatinib dose reduction due to adverse events
Of the 51 patients who had to cease the drug permanently because of adverse events, 41 had previous dose reductions and/or temporary cessations prior to eventual cessation. An additional 35 patients (16%) had dose reductions for adverse events; 12 patients (5%) had the drug temporarily ceased, and a further 12 (5%) had both measures taken.
Deaths
Sixteen patients who received dasatinib died; 11 were on the drug at the time of or within 4 months of death. Causes of death included blast crisis CML (n = 5), and 1 each of sepsis, suicide, intracerebral hemorrhage, and a cardiac event. A 73-year-old died in the setting of a febrile illness; further details were not available. The cause of death is unknown in 2 patients aged 73 and 89 years, who died in locations other than their primary health care institution.
Discussion
Although dasatinib is generally regarded as a well-tolerated drug, our “real-world” study found that more than half of patients commenced on dasatinib experienced adverse events that resulted in permanent drug cessation or required dose modification and/or temporary cessation.
The most common adverse event was pleural effusions, reported in 25% patients (21% of patients receiving the most common daily dose of 100 mg), comparable with the 28% rate observed in the 5-year follow-up of the DASISION study.1 We confirmed previous observations that significant risk factors for effusions included age and dasatinib dose.15 Autoimmune disorders are also a reported risk factor16 ; we did not specifically request information in this respect. Of note, 3 of the 7 patients prescribed maintenance steroids had concurrent active autoimmune disorders. Our observation that effusions were associated with both early and deep molecular response as well as improved CML outcomes is consistent with the literature and are likely indicative of potency of the TKI effect.17,18
Management of effusions varied substantially between institutions reflecting a lack of a consensus approach to this adverse event. Approaches included dasatinib dose reduction, temporary or permanent cessation, steroids acutely and as maintenance, and diuretics. Recurrence of effusions was high if the same dasatinib dose was continued or resumed, even after a drug holiday of up to 6 weeks. Lowering the dose reduced but did not abolish the risk of recurrence. Our findings supported observations of others in that dose reductions and temporary drug cessations have minimal impact on CML response rate and patient survival.19,20 Unfortunately, we did not have data correlating maintenance of MR4.5 with prior duration of MR4.5, but our observations suggest that in patients with effusions and sustained excellent molecular responses, a trial of ongoing cessation of TKI therapy in patients with prolonged deep molecular responses may be warranted provided close molecular monitoring is available.21 However, if dasatinib is recommenced, our data suggest this should be at a substantially lower dose, perhaps in conjunction with measuring drug levels if available.22,23 Data from the OPTIM study (published thus far in abstract form only) suggest a personalized dasatinib dosing schedule (with median dose of 50-60 mg/d) may lower the risk of effusions without compromising the depth of molecular response.23
Prednisolone was used in several patients for the acute management of pleural effusions; however, because this generally occurred in conjunction with drug cessation and/or dose reduction, we cannot reliably determine whether this reduced the length of time to resolution of effusion symptoms or signs. Similarly, the benefit of diuretics cannot be directly assessed. Maintenance prednisolone was also used in some patients to prevent effusion recurrence; although the numbers were small, this did appear to be a potentially effective strategy.
We suggest that a reasonable approach to the management of symptomatic effusions includes temporary drug cessation and gentle diuretic therapy (albeit of uncertain benefit) until complete resolution. Steroid therapy (eg, prednisolone 25-50 mg daily) during the acute effusion phase may aid effusion resolution, and low-dose maintenance therapy may reduce the risk of recurrence. Diagnostic and therapeutic thoracocentesis is not usually necessary and should only be performed if required for symptomatic support.
Other serious side effects were uncommon. PHTN diagnosed by echocardiography (and hence not by the “gold standard” of right heart catheterization) was observed in 5% of patients, comparable with the DASISION data.1 Numbers were small, and so no predilection according to sex or age was able to be demonstrated. Consistent with the literature, all cases were associated with dasatinib as second- or later-line TKI therapy,24 and there was a strong association with concurrent effusions or effusions within 6 months of the diagnosis of PHTN.1,24 PHTN has been reported to be partially or completely reversible upon drug cessation, as occurred in 6 of the 7 applicable patients in this study.24 The temporal relationship of the resolution of symptoms and echocardiogram findings after drug cessation makes it extremely likely PHTN was due to dasatinib. We concur with recently published suggestions that a baseline transthoracic echocardiogram, particularly in the elderly and those with risk factors for PHTN (age >60, smoking history, diabetes mellitus, hypertension, known cardiovascular disease), should be considered when initiating dasatinib therapy to exclude preexisting PHTN.24,25 In view of the association between PHTN and pleural effusion, a transthoracic echocardiogram should also be performed in patients presenting with pleural effusion.
There have been reports of an association between dasatinib and atypical infections, including Epstein-Barr virus–positive mucosal leucoplakia, Pneumocystis jirovecii pneumonia,26 and CMV reactivation, thought to be due to the drug’s immunosuppressive effects on T cells and natural killer cells (observed in patients receiving 140 mg dasatinib daily).9 An atypical infection was reported in just 1 patient in our study, and there were no reported cases of CMV reactivation despite investigators being specifically asked about this complication, although monitoring for CMV viremia was not routinely performed, so minimally symptomatic low-level viremia may not have been detected. There was only 1 case of febrile neutropenia, suggesting that the rates of clinically significant opportunistic infections in otherwise immunologically competent patients are very low.
Dasatinib has also been associated with a risk of greater than or equal to grade 3 bleeding (∼3%) in CML-CP patients,27 due to either thrombocytopenia- and/or dasatinib-induced platelet dysfunction (impaired arachidonic acid and adrenaline-induced aggregation).27 In our cohort, 4 of 7 clinically significant bleeding events occurred in association with at least moderate thrombocytopenia (≤50 × 109/L) and in 1 other patient in the presence of aspirin. There were 2 bleeding events in patients with normal platelet counts and no concomitant antithrombotic medications, including a fatal intracerebral hemorrhage. Although we concur with recommendations to closely monitor blood counts and use concomitant anticoagulant and antiplatelet therapy with caution during dasatinib therapy, particularly in elderly patients or those with an underlying anatomical predisposition to bleeding,3 the overall bleeding risk in the absence of these factors is likely to be very low.
Benign cervical lymphadenopathy is another relatively recent reported association with dasatinib, albeit uncommon (0.7%).28,29 This entity was reported in 2 patients (1%) in our cohort and spontaneously resolved despite dasatinib continuation, suggesting this is a self-limiting phenomenon that should not impact patient or disease management.
Ischemic vascular events were rare, with 6 of the 7 cases occurring in patients with vascular risk factors, 4 of whom had previously received nilotinib, with an overall incidence of 3%. This finding is comparable to the vascular event rate of 4% observed in the DASISION study.1 Overall, patients treated with dasatinib alone did not appear to be at an increased risk of vascular events compared with the general population.30
There are case reports of renal impairment with dasatinib therapy,31,32 including 1 patient who developed nephrotic syndrome secondary to biopsy-proven chronic thrombomicroangiopathy, who experienced rapid resolution of proteinuria upon dasatinib cessation.33 One patient in our cohort also developed nephrotic syndrome, due to a presumed drug-induced lupus nephritis, which resolved upon dasatinib cessation. Renal complications of dasatinib therapy appear very rare, along with other complications such as colitis, pancreatitis, and deranged liver function tests.
Our study is subject to the usual caveats of a retrospective “real-world” analysis of medical records, and it is possible that events, such as mild asymptomatic cytopenias, were overlooked and thus underreported. Nevertheless, the key data have been interrogated for accuracy and completeness, and the major observations regarding the important complication of pleural effusions are valid, in particular, the high recurrence rate, association with age and dasatinib dose, and the rationale for a trial of TKI cessation (with vigilant monitoring) in patients with significant effusions and a concomitant deep molecular response.
Dasatinib is an effective drug in the treatment of CML-CP and optimizing its use to diminish the risk of side effects leading to its cessation is important. Prior to initiation of therapy, a screening echocardiogram may be useful in identifying early PHTN, which is probably an indication not to use this drug, and there should be a low threshold for repeat echocardiogram in patients who develop dyspnea. Pleural effusion, however, is the commonest side effect leading to drug discontinuation with risk factors being age and dose. Using lower doses in older patients, perhaps with adjustments according to molecular response, may lower the incidence of this complication. In our study, the rates of serious bleeding and infection were low, but caution is warranted when prescribing concurrent antiplatelet agents. With these approaches, we suggest that the necessity for permanent dasatinib cessation due to toxicity is likely to be minimal in immunologically competent patients.
Acknowledgments
The authors acknowledge the following people who participated in data collection at their institutions: Lisa Carne (Royal Adelaide Hospital), Michele Gambrill (Calvary Mater Hospital), Jennifer Devos (Concord Hospital, Sydney, Australia), Zar Nwe (Royal Hobart Hospital), and Kimberley Bury (Townsville Hospital, Townsville, Australia).
Authorship
Contribution: This project was conceptualized by A.G., who also interpreted data and edited the manuscript; data collection was performed by L.C.F., K.D.C., D.Y., K.B., J.S., S.F., A.S., R.C., C.F., M.T., O.M., A.M., R.H., A.H., S.R., K.-L.C., W.-H.H., A.A., and F.P.; data were collated, analyzed, and interpreted by L.C.F., who also wrote the manuscript; statistical analysis and manuscript review were performed by B.C.; the database was managed by F.P.; and the manuscript was reviewed and approved by all authors.
Conflict-of-interest disclosure: J.S. and D.Y. have received honoraria from Novartis and Bristol-Myers Squibb (BMS) and research funding from BMS and participated in Advisory Boards for Novartis. S.F. has received honoraria from BMS and research funding from BMS and participated in Advisory Boards for Ariad. C.F. has received a travel grant from BMS. A.G. has participated in Advisory Boards for Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Andrew Grigg, Haematology Department, Austin Hospital, PO Box 5555, Heidelberg, VIC 3084, Australia; e-mail andrew.grigg@austin.org.au.