Introduction
von Willebrand disease (VWD) is the most common inherited bleeding disorder, affecting 1% of the population.1,2 VWD is caused by defective or deficient von Willebrand factor (VWF), a large multimeric protein that promotes primary hemostasis by binding to collagen in the blood vessel wall, platelets, and factor VIII (FVIII). VWF deficiency results in mucosal bleeding, and, in women, excess reproductive tract bleeding.3,4 Postpartum hemorrhage (PPH), defined as 500 cm3 or greater blood loss in the 24 hours after vaginal delivery, is more common in women with VWD than healthy controls5,6 and associated with blood loss anemia, red cell transfusion, and prolonged hospitalization. Yet, despite treatment with VWF concentrate at delivery, women with VWD have lower VWF levels and greater blood loss at delivery than pregnant controls without VWD7,8 (Figure 1A-B). Why current treatment fails is unknown, and the optimal dose and duration of VWF concentrate at delivery and in the postpartum period remains elusive. The purpose of this paper is to review peripartum changes in VWF levels and blood volume, postpartum blood loss, potential problems with current treatment, and the potential role of blood-volume based VWF dosing to prevent PPH in women with VWD. This novel approach is proposed as an alternative to antifibrinolytic therapy which is discussed in the accompanying “Point” commentary.
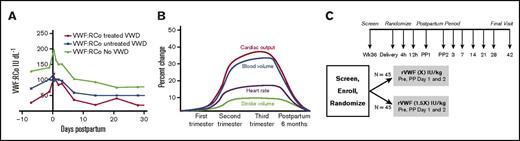
Changes in VWF and blood volume during pregnancy, and proposed clinical trial. (A) VWF:RCo level during pregnancy. von Willebrand ristocetin cofactor activity in VWD treated patients (in red), in VWD untreated patients, and in pregnant controls. Adapted from James et al.7 (B) Blood volume during pregnancy. Blood volume (blue line) increases 1.5-fold by the eighth month of pregnancy, compared with baseline. Adapted from Liu and Arany.18 (C) PREVENT PPH in VWD trial schema. This is the schema for Prevent Postpartum Hemorrhage in von Willebrand Disease, or PREVENT PPH in VWD, a prospective, randomized, controlled trial in women with VWD. Adapted from Machin et al.25
Changes in VWF and blood volume during pregnancy, and proposed clinical trial. (A) VWF:RCo level during pregnancy. von Willebrand ristocetin cofactor activity in VWD treated patients (in red), in VWD untreated patients, and in pregnant controls. Adapted from James et al.7 (B) Blood volume during pregnancy. Blood volume (blue line) increases 1.5-fold by the eighth month of pregnancy, compared with baseline. Adapted from Liu and Arany.18 (C) PREVENT PPH in VWD trial schema. This is the schema for Prevent Postpartum Hemorrhage in von Willebrand Disease, or PREVENT PPH in VWD, a prospective, randomized, controlled trial in women with VWD. Adapted from Machin et al.25
Changes in VWF during pregnancy and postpartum
In normal pregnancy, VWF ristocetin activity (VWF:RCo), VWF antigen (VWF:Ag), and FVIII activity (FVIII:C) increase progressively, peaking at the time of delivery, then gradually falling, and returning to prepregnancy levels by 3 weeks postpartum.9-11 In women with VWD, a recent prospective study indicates the trajectories of VWF:RCo, VWF:Ag, and FVIII:C, although parallel to those in control women without VWD, do not increase to the same degree and fall more rapidly than controls.7 VWF:RCo levels peak at 250% of baseline earlier in women with VWD, at 4 hours postpartum, as compared with 12 hours postpartum in controls, and then fall rapidly to baseline prepregnancy levels over the 3 weeks, with lower levels at each time point, despite VWF concentrate replacement7 (Table 1; Figure 1A). FVIII levels peak in the third trimester at 40% to 50% of baseline, but fall more rapidly in those with VWD than controls, reaching 20% of baseline at 24 hours postpartum and declining to prepregnancy levels over 3 weeks, with lower levels at each time point.7
VWF levels in women with VWD
| Groups . | Reference . | Controls, N = 40 . | VWD, N = 17 . | VWD treated, N = 15 . |
|---|---|---|---|---|
| Blood loss, cm3 | ||||
| Postpartum | 7 | 427 [346, 529] | 448 [379, 517] | 615 [473, 758] |
| VWF:RCo, IU/mL | ||||
| Prepregnancy | 7 | 0.77 | 0.49 | — |
| Third trimester | 1.29 | 0.73 | 0.34 | |
| Postpartum | 1.99 | 1.02 | — | |
| Blood volume, cm3 | ||||
| Prepregnancy | 18 | 2699 | — | — |
| Third trimester | 3689 | — | — |
| Groups . | Reference . | Controls, N = 40 . | VWD, N = 17 . | VWD treated, N = 15 . |
|---|---|---|---|---|
| Blood loss, cm3 | ||||
| Postpartum | 7 | 427 [346, 529] | 448 [379, 517] | 615 [473, 758] |
| VWF:RCo, IU/mL | ||||
| Prepregnancy | 7 | 0.77 | 0.49 | — |
| Third trimester | 1.29 | 0.73 | 0.34 | |
| Postpartum | 1.99 | 1.02 | — | |
| Blood volume, cm3 | ||||
| Prepregnancy | 18 | 2699 | — | — |
| Third trimester | 3689 | — | — |
Bracketed numbers are the 95% confidence intervals.
Postpartum blood loss in VWD
At delivery, the mean estimated blood loss in women with VWD is 1.4-fold greater than in controls, 615 vs 448 mL, despite VWF concentrate treatment, and continues over the next 6 weeks postpartum, accompanied by a significantly lower hematocrit, 28.6%7 (Figure 1B). These findings, confirmed in retrospective studies,8-11 remain poorly understood. Why do women with VWD experience excessive postpartum blood loss despite treatment? Although women receiving VWF concentrate at delivery are more severe with lower third trimester VWF:RCo levels, <0.50 IU/mL, this suggests the dose or dose frequency may be inadequate to prevent excess postpartum blood loss.
Current treatment recommendations
The treatment regimen to prevent PPH in women with VWD is based on expert opinion, grade 3, level C, and not clinical evidence as no randomized trials have been done in this rare disease population. The regimen recommended for women with third trimester VWF levels <0.50 IU/mL, is 50 IU/kg VWF concentrate at delivery (or epidural), continued for 3 to 5 days.12-15 Although this dosing regimen is recommended by experts from 3 international groups, it is lower than regimens recommended for surgery, 60 to 80 IU/kg.12,13,15 Some attribute this difference in dosing to fear of thrombosis in the peripartum period, considered a transient hypercoagulable state (see “Thrombosis risk in VWD with VWF concentrate”). Whether alternate dosing regimens will reduce PPH is a topic of current debate.
Blood volume and VWF factor dosing
During normal pregnancy, there is a progressive 1.4- to 1.5-fold increase in blood volume by the third trimester16-18 (Figure 1B). The 1.4-fold greater postpartum blood loss and 1.4-fold lower postpartum VWF levels in women with VWD, as compared with controls,7 suggests the possibility of dosing VWF concentrate at delivery based on blood volume rather than body weight. Although this strategy remains untested, there is precedent for adjusting factor concentrate based on blood volume. In children with hemophilia, factor dosing is 20% higher than in adults to compensate for their greater blood volume.19,20 In obese individuals with hemophilia, factor dosing is based on blood volume rather than body weight, as fatty tissue contains little blood.21 In pregnant women, pharmacokinetic studies suggest that antihypertensive therapy and other medications should be dosed based on blood volume.22-24 These facts support the hypothesis that VWF concentrate dosing based on blood volume rather than body weight will improve VWF levels and reduce PPH. A recent survey of hemophilia treatment center (HTC) physicians indicates they prescribe 50 to 80 IU/kg of VWF concentrate at delivery for women with VWD25 but, given the greater postpartum blood loss in women with VWD, are willing to participate in a clinical trial of blood-volume-based VWF concentrate dosing to reduce PPH in VWD (M.V.R., manuscript submitted January 2017).
Thrombosis risk in VWD with VWF concentrate
VWF dosing at delivery is lower than for major surgery, 60 to 80 IU/kg,12,13,15 a practice attributed to potential thrombosis risk. What is the basis for concern for thrombosis with VWF concentrate in pregnancy? In normal pregnancy, the physiologic increase in both VWF and FVIII levels may increase thrombosis risk,26 particularly in those at risk (ie, with thrombophilia or past venous thromboembolism). Several studies, however, suggest individuals with VWD are at lower risk for cardiovascular disease and thrombosis. In a cross-sectional study of the Atherosclerosis Risk in Communities study, subjects with VWF:Ag <0.50 IU/mL appear to be protected from cardiovascular risk with lower odds ratios for cardiovascular events.27 Similarly, a recent analysis of the National Inpatient Sample, a 20% sample of US hospital discharges, found risk factors for cardiovascular disease were reduced in individuals with VWD,28 and another study found that arterial thrombosis is reduced.29 What is the thrombotic risk associated with VWF concentrate? It appears to be uncommon: in 474 individuals with VWD participating in 13 published safety, efficacy, or pharmacokinetic studies of plasma-derived VWF (pdVWF) concentrate or recombinant VWF (rVWF) concentrate up to doses of 220 IU/kg, the rate of thrombosis was 0.4%.25 The latter included 2 individuals receiving pdVWF, of whom 1 had a catheter-related thrombosis and one of whom who had a remote venous thromboembolism after orthopedic surgery.25 This rate is comparable to the 0.1% per patient per year thrombosis rate reported by the manufacturer (Henry Meade, CSL Behring Inc., e-mail, 5 January 2017). Although none of the study subjects in these reports was known to be pregnant, and fewer data are available for rVWF, these findings suggest that thrombosis risk with VWF concentrate is low. Clearly, more data are needed to confirm this is the case in pregnant women with VWD.
rVWF concentrate to prevent PPH
rVWF concentrate, Vonvendi, is a US Food and Drug Administration–approved clotting concentrate for treatment of VWD. It has comparable safety and efficacy to pdVWF concentrate as demonstrated in clinical trials.30,31 Compared with pdVWF concentrate, rVWF concentrate has higher purity and specific activity, with more hemostatically active VWF ultralarge multimer proteins, which account for its stronger binding to collagen, FVIII, and platelets, with no thrombosis.30-32 rWWF also has a 1.4-fold longer half-life than pdVWF,30,31 suggesting it might be dosed less frequently than pdVWF. Because it is a high-purity factor, rVWF contains no FVIII:C but rather stimulates FVIII synthesis within 3 to 4 hours of infusion. For this reason, in patients with FVIII:C <0.40 IU/dL requiring immediate hemostasis (eg, as in trauma or surgery), a dose of FVIII concentrate should be given in addition to rVWF.31 If hemostasis is not required immediately, no FVIII concentrate is required. Whether that is the case at delivery has not been studied. An advantage of the longer half-life with rVWF is the potential for longer-lived hemostatic levels and/or the possibility of every-other-day dosing postpartum.
Targeting women with VWD at risk for PPH
Which women with VWD should be treated to prevent PPH? Although current recommendations indicate women with VWF:RCo levels lower than 0.50 IU/mL in the eighth month of pregnancy should be treated at delivery,12-15 other risks may be important. In a recent small single-center study, prepregnancy VWF:RCo <0.50 IU/dL and weight were predictive of PPH, and prepregnancy bleeding score correlated directly with PPH risk.25 These findings should be replicated in future larger studies. To determine the safety and efficacy of a 1.4- to 1.5-fold increased VWF dose at delivery in women with VWD will require a prospective, randomized, multicenter phase 3 clinical trial, the PREVENT PPH Trial (Figure 1C), with pharmacokinetic monitoring of VWF:RCo, VWF:Ag, and F:VIII:C; blood loss monitoring for up to 6 weeks postpartum; and vigilance for thromboembolism. To accomplish the proposed trial will require the collaboration of hematologists, obstetricians, and, as VWD is a rare disease, up to 14 willing HTC sites with sufficient subjects.
Conclusion
Women with VWD experience excess postpartum bleeding as compared with controls without VWD, despite treatment. rVWF concentrate is safe and effective in patients with VWD, and contains hemostatically active ultralarge multimers with prolonged half-life that if dosed based on blood volume, may provide better postpartum hemostasis than with current treatment in those at risk.
Authorship
Contribution: M.V.R. wrote the manuscript.
Conflict-of-interest disclosure: M.V.R. has received research funding from Baxalta (Shire, Baxter) and CSL Behring and has served on advisory boards for Baxalta (Shire, Baxter).
Correspondence: Margaret V. Ragni, Hemophilia Center of Western PA, 3636 Blvd of the Allies, Pittsburgh, PA 15213-4306; e-mail: ragni@dom.pitt.edu.