Key Points
This article describes the first reports of Zika and chikungunya infections in HSCT recipients and oncohematological patients.
Fever and exanthema should prompt arbovirus diagnosis, especially in areas at risk or in patients returning from endemic or epidemic regions.
Abstract
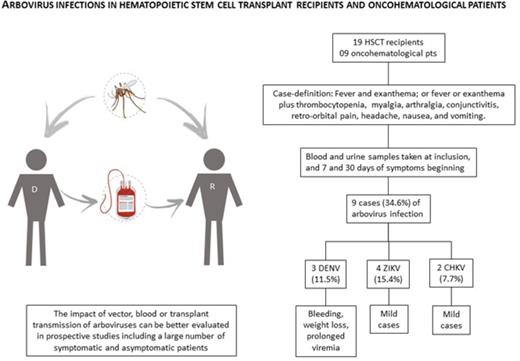
Aedes mosquitoes are well adapted in domestic environments and widespread in tropical regions. Since 2015, Brazil has been experiencing a triple epidemic of dengue (DENV), chikungunya (CHKV), and Zika (ZIKV) viruses. The last 2 viruses are likely following the path of DENV, which has been endemic in most parts of the country since the 1980s. Given this triple epidemic, we proposed a prospective and collaborative study to assess the prevalence, morbidity, and mortality of DENV, CHKV, and ZIKV infections in hematopoietic stem cell transplant (HSCT) recipients and oncohematological patients. A case definition strategy (fever and rash) was used to prompt diagnostic investigation of DENV, ZIKV, and CHKV, which was accomplished by real-time polymerase chain reaction with plasma and urine samples. Clinical follow-up was performed 7 and 30 days after symptom onset. We report here the first cases of ZIKV and CHKV infections diagnosed in this ongoing study. From February to May 2016, 9 of the 26 patients (34.6%) fulfilling case definition criteria were diagnosed with DENV (3 cases), ZIKV (4 cases), or CHKV (2 cases) infections. Prolonged viremia and viruria were observed in dengue and Zika fever cases, respectively. Thrombocytopenia was the most frequent complication. Delayed engraftment was noted in 1 patient who acquired ZIKV 25 days before HSCT. All patients survived without sequelae. With the geographic expansion of arboviruses, donor and recipient screening may become mandatory. Patients living in areas where these viruses are not endemic are also at risk, since these viruses can be transmitted by blood as well as organ or tissue transplantation.
Introduction
Aedes mosquitoes are the vectors of several arbovirus infections, such as dengue virus (DENV), West Nile virus, Japanese encephalitis, chikungunya virus (CHKV), and Zika virus (ZIKV). Located in tropical and subtropical regions greatly infested by Aedes, Brazil is a populous country that is ranked second in terms of the absolute number of kidney and liver transplants, with 5,556 kidney, 1,809 liver, and 2,137 hematopoietic stem cell transplant (HSCT) procedures performed in 2015.1 Consequently, transplant recipients living in Brazil are at risk of mosquito-transmitted infections. Brazil is currently experiencing a triple epidemic of DENV, CHKV, and ZIKV,2 the latter two following the path of DENV, which has been endemic in the country since the 1980s.3
Few retrospective studies have addressed the question of DENV in transplant recipients, reporting both mild4,5 and severe cases of dengue hemorrhagic fever and dengue shock syndrome.6-8 Less information is available in the case of CHKV and ZIKV infections. One case of travel-associated CHKV infection has been reported in an HIV-infected kidney transplant recipient,9 and CHKV infection of corneal grafts has also been documented.10 No cases of CHKV have been reported in HSCT recipients. ZIKV infection in a breast cancer patient11 and a probable case of transfusion-transmitted (TT) ZIKV to a liver transplant recipient have been recently reported.12 Another 4 cases of putative mosquito-transmitted ZIKV infection have recently been described in solid organ transplant recipients (2 renal and 2 liver), and no patient died or developed neurological symptoms.13
Methods
Given the triple epidemic in Brazil, we proposed a prospective study to assess the morbidity and mortality of DENV, CHKV, and ZIKV infections in symptomatic HSCT recipients and oncohematological patients. A case-definition approach was used to prompt diagnostic investigation, which was done by real-time polymerase chain reaction (PCR) in plasma and urine samples, at the Virology Laboratory of Institute of Tropical Medicine.14-17 A suspected case was defined by (1) fever and exanthema or (2) fever or exanthema plus one of the following symptoms: thrombocytopenia, myalgia, arthralgia, conjunctivitis, retro-orbital pain, headache, nausea, and vomiting. Clinical information was obtained at inclusion and 7 and 30 days thereafter. Patients who tested positive at inclusion had blood samples taken in the following visits.
Results
The main clinical findings are described in Table 1 (full case reports are described in supplemental Results).
Clinical findings of DENV, CHKV, and ZIKV infections in HSCT recipients and oncohematological patients
| Patient group . | UD . | Age (y) . | Sex . | Days to diagnosis* . | Symptoms . | Platelets (×109/L) . | Virus . | Sample . | Viremia/viruria duration . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|
| HSCT MRD | AML | 30 | M | 1 | Fever, exanthema, nausea, vomiting, myalgia, arthralgia | 168 000 | CHKV | P | <30 d | None |
| HSCT MUD | ALL | 4 | F | 4 | Fever, headache, diarrhea, myalgia, arthralgia, GI bleeding | 14 000 | DENV2 | P | <30 d | Enterorrhagia |
| HSCT MRD | ALL | 46 | F | >30 | Fever, headache, nausea, vomiting myalgia, abdominal pain | 19 000 | DENV | P | Unknown | None |
| HSCT AUTO | SS | 48 | M | 18 | Fever, headache, exanthema, myalgia, arthralgia | 114 000 | ZIKV | U | <7 d | None |
| HSCT MRD | ALL | 34 | F | 7 | Exanthema, myalgia, somnolence | 245 000 | ZIKV | P+U | 7 d (U) | None |
| Oncohematological | Testicular tumor | 17 | M | 3 | Fever, exanthema, headache, myalgia, arthralgia, lethargy | 133 000 | CHKV | P | 3 d (P) | None |
| Onco hematological | NHL | M | 18 | Fever, headache, nausea, lethargy, somnolence | 16 000 | DENV1 | P | ≥30 d (P + U) | Weight loss, persistent leukopenia | |
| Oncohematological | AML | 15 | F | 7 | Fever, nausea, exanthema | 15 200 | ZIKV | P, U | 7 d (P + U) | Delayed engraftment |
| Oncohematological | ALL | 7 | F | 7 | Fever, exanthema, conjunctivitis, petechiae | 16 000 | ZIKV | P, U | 7 d (U) | None |
| Patient group . | UD . | Age (y) . | Sex . | Days to diagnosis* . | Symptoms . | Platelets (×109/L) . | Virus . | Sample . | Viremia/viruria duration . | Complications . |
|---|---|---|---|---|---|---|---|---|---|---|
| HSCT MRD | AML | 30 | M | 1 | Fever, exanthema, nausea, vomiting, myalgia, arthralgia | 168 000 | CHKV | P | <30 d | None |
| HSCT MUD | ALL | 4 | F | 4 | Fever, headache, diarrhea, myalgia, arthralgia, GI bleeding | 14 000 | DENV2 | P | <30 d | Enterorrhagia |
| HSCT MRD | ALL | 46 | F | >30 | Fever, headache, nausea, vomiting myalgia, abdominal pain | 19 000 | DENV | P | Unknown | None |
| HSCT AUTO | SS | 48 | M | 18 | Fever, headache, exanthema, myalgia, arthralgia | 114 000 | ZIKV | U | <7 d | None |
| HSCT MRD | ALL | 34 | F | 7 | Exanthema, myalgia, somnolence | 245 000 | ZIKV | P+U | 7 d (U) | None |
| Oncohematological | Testicular tumor | 17 | M | 3 | Fever, exanthema, headache, myalgia, arthralgia, lethargy | 133 000 | CHKV | P | 3 d (P) | None |
| Onco hematological | NHL | M | 18 | Fever, headache, nausea, lethargy, somnolence | 16 000 | DENV1 | P | ≥30 d (P + U) | Weight loss, persistent leukopenia | |
| Oncohematological | AML | 15 | F | 7 | Fever, nausea, exanthema | 15 200 | ZIKV | P, U | 7 d (P + U) | Delayed engraftment |
| Oncohematological | ALL | 7 | F | 7 | Fever, exanthema, conjunctivitis, petechiae | 16 000 | ZIKV | P, U | 7 d (U) | None |
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; F, female; GI, gastrointestinal; M, male; MRD, matched related donor; MUD, matched unrelated donor; P, plasma; SS, systemic sclerosis; U, urine; UD, underlying disease.
Interval from symptom onset to diagnostic sampling.
From February to May 2016, 26 patients (19 HSCT recipients and 7 patients with hematological disorders) fulfilling the case definition criteria were included. Median age was 37 and 15 years in HSCT recipients and oncohematological patients, respectively. A total of 9 cases (34.6%) of arbovirus infection were identified: 3 cases of DENV (11.5%), 2 cases of CHKV (7.7%), and 4 cases of ZIKV (15.4%) (Table 1). Vector transmission was considered in these cases detected during the rainy season, when Aedes infestation is higher.
Fever and exanthema are good markers of arbovirus infection, as laboratory-confirmed cases were found in ∼35% of included patients. However, it is important to highlight the similarity of symptoms in patients with and without proven arboviral infection, as shown in Table 2. Consequently, a high index of suspicion should be kept in these populations presenting such symptoms; otherwise, the opportunity of arboviral diagnosis will be missed.18 Thrombocytopenia, a hallmark of dengue infection, occurred in 55.5% of the infected patients and in 37.5% of the noninfected patients (P = .67). Conjunctivitis, more frequently seen in Zika infection, occurred in 1 of the 9 (11.1%) infected patients in comparison with 1 of the 16 (6.25%) noninfected patients (P = not significant). In comparison with the 50% rate in the immunocompetent population, conjunctivitis was less frequent, occurring in 25% of cases.19 Morbidity of CHKV and ZIKV infections was mild to moderate, likely similar to the immunocompetent population. So far, the most severe cases observed in the ongoing study were the dengue cases, all with thrombocytopenia (<20 000/mm3), 1 with intestinal bleeding, and another case with extreme weight loss and persistent viremia (>1 month). In the 2 patients with proven CHKV infections, the duration of fever was <2 days, and the most significant symptom was joint pain that resolved within a few days. It appears that thrombocytopenia is a well-characterized DENV event but is not as evident in CHKV or ZIKV infections. In the present series, HSCT recipients with ZIKV or CHKV did not develop thrombocytopenia. Low platelet counts were observed only in oncohematological patients with ZIKV or CHKV infections who were receiving chemotherapy and were therefore likely due to the treatment of the underlying disease. Other authors have observed thrombocytopenia in solid organ transplant recipients with ZIKV infection.13
Frequency of symptoms in 25 patients with and without arbovirus infections
| Symptoms . | Frequency (%) . | P . | |
|---|---|---|---|
| Arboviral infection (n = 9) . | No arboviral infection (n = 16)* . | ||
| Fever | 8 (88.8) | 11 (68.7) | .36 |
| Exanthema | 6 (66.6) | 12 (75) | .67 |
| Headache | 5 (55.5) | 6 (37.5) | .43 |
| Myalgia | 5 (55.5) | 8 (50) | 1.0 |
| Thrombocytopenia | 5 (55.5) | 6 (37.5) | .67 |
| Nausea | 4 (44.4) | 6 (37.5) | 1.0 |
| Arthralgia | 4 (44.4) | 5 (31.2) | .67 |
| Somnolence | 3 (33.3) | 1 (6.25) | .11 |
| Diarrhea | 2 (22.2) | 2 (12.5) | .60 |
| Vomit | 2 (22.2) | 2 (12.5) | .60 |
| Abdominal pain | 1 (11.1) | 3 (18.7) | 1.0 |
| Conjunctivitis | 1 (11.1) | 1 (6.25) | 1.0 |
| Bleeding | 1 (11.1) | 3 (18.7) | 1.0 |
| Agitation | 0 (0) | 2 (12.5) | .52 |
| Symptoms . | Frequency (%) . | P . | |
|---|---|---|---|
| Arboviral infection (n = 9) . | No arboviral infection (n = 16)* . | ||
| Fever | 8 (88.8) | 11 (68.7) | .36 |
| Exanthema | 6 (66.6) | 12 (75) | .67 |
| Headache | 5 (55.5) | 6 (37.5) | .43 |
| Myalgia | 5 (55.5) | 8 (50) | 1.0 |
| Thrombocytopenia | 5 (55.5) | 6 (37.5) | .67 |
| Nausea | 4 (44.4) | 6 (37.5) | 1.0 |
| Arthralgia | 4 (44.4) | 5 (31.2) | .67 |
| Somnolence | 3 (33.3) | 1 (6.25) | .11 |
| Diarrhea | 2 (22.2) | 2 (12.5) | .60 |
| Vomit | 2 (22.2) | 2 (12.5) | .60 |
| Abdominal pain | 1 (11.1) | 3 (18.7) | 1.0 |
| Conjunctivitis | 1 (11.1) | 1 (6.25) | 1.0 |
| Bleeding | 1 (11.1) | 3 (18.7) | 1.0 |
| Agitation | 0 (0) | 2 (12.5) | .52 |
All clinical data were not available in 1 case.
CHKV viremia was not detected 30 days after diagnosis. ZIKV viremia persisted for at least 7 days in 1 oncohematological patient. In the remaining cases, ZIKV viremia was not detected 1 week after symptom onset. Viruria lasted longer and should be the preferred sample for diagnosing ZIKV in suspected cases with >7 days of symptoms, as observed by other authors.20 Interestingly, we observed that the oncohematological patient who had ZIKV immediately before transplantation had delayed neutrophil engraftment (27 days). These observations must be interpreted with caution, because we herein report preliminary data from a small number of confirmed cases. A better understanding of this scenario will come as more cases are reported. Similarly, longer follow-up is necessary to evaluate the occurrence of any neurological disability in ZIKV cases.
Discussion
In the face of the expansion of autochthonous cases of DENV, CHKV, and ZIKV infections in a growing number of countries in regions of the Americas, there are other reasons for concern. Aside from vector transmission, blood transmission has been well documented. Therefore, transmission by tissue, cell, and organ transplantation may also occur. During the 2006 epidemic on Reunion Island, CHKV genome was identified in 1 of 250 donated platelet units screened by PCR, and one-third of eligible corneas from asymptomatic donors were infected with CHKV.10 According to some authors, cornea donation should be banned in areas where CHKV circulates, unless systematic CHKV screening of donors is made.10 TT dengue has also been demonstrated in some studies.21,22 The largest study of TT dengue was conducted in Brazil and included 39 134 blood donors. The TT rate was 37.5%, significantly higher than the viremia rate in non-exposed recipients (0.93%).22 During the 2013-2014 ZIKV outbreak in French Polynesia, 2.8% of blood donations tested positive by PCR.23 Recently, a case of probable TT ZIKV infection to a liver transplant recipient was reported in Brazil, and sequencing confirmed ZIKV in the donor and patient samples.12
The impact of vector, blood, or transplant transmission of arboviruses can be better evaluated in prospective studies that include a large number of symptomatic and asymptomatic patients. The Brazilian Agency of Health Surveillance stipulated a 30-day period after proven or suspected ZIKV infection or sexual contact with a person with proven or suspected ZIKV infection to define the eligibility of donor cells, organs, or tissues. Except for unrelated stem cell donors, no recommendation was made concerning systematic screening of blood or organ donors by PCR.24 Diagnostic tests for DENV, ZIKV, and CHKV infections should be added to the laboratory portfolio for the differential diagnosis of febrile transplant recipients living in endemic countries and in those returning from regions with known circulation of arboviruses.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all health professionals who assisted the patients included in this study.
This study was supported in part by the São Paulo Research Foundation (research grant 2015/06947-5) (C.M.M.) and by the Virology Laboratory of Institute of Tropical Medicine, University of São Paulo.
Authorship
Contribution: C.M.M. designed research, performed research, analyzed and interpreted data, and wrote the manuscript; B.B.d.S.P. collected data, performed research, analyzed and interpreted data, and wrote the manuscript; A.C.F. performed research, analyzed and interpreted data, and contributed analytical tools; M.C.O., L.G.D., and E.J.d.A.P. collected data, performed research, and wrote the manuscript; and M.P.d.S., F.N., V.R.C., and B.P.S. collected data, performed research, and analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clarisse Martins Machado, Virology Laboratory, Institute of Tropical Medicine, Av Dr Eneas de Carvalho Aguiar, 470, São Paulo SP 05403-000, Brazil; e-mail: clarimm@usp.br.