TO THE EDITOR:
With improvements in medical care and the resultant positive impact on life expectancy over the past few decades, the proportion of the population aged >65 years has seen significant increase, with the very elderly (age >80 years) expressed as a percentage of those aged >65 years almost doubling since 1950 (13.3% in 1950 increasing to 25.3% in 2015).1 Like most cancers, the probability of acquiring non-Hodgkin lymphoma (NHL) increases with age (0.2% to 0.3% in those aged <50 years and 1.4% to 1.8% in those aged >69 years).2 Moreover, recent statistics also suggest an increase in the incidence of NHL, especially among men.2 Because the mean age at diagnosis of diffuse large B-cell lymphoma (DLBCL) is 70 years,3 there has been a number of studies focusing on the elderly with DLBCL, more so after the introduction of rituximab (R), the monoclonal antibody directed against CD-20 on B cell.4 However, a majority of patients in these studies were in the age group of 60 to 80 years,5-7 and the very elderly (age >80 years) were mostly not part of these studies due to their multiple comorbidities, poor performance status, or concerns about chemotherapy-induced toxicities that preclude optimizing therapy.8-10 Hence, there is a lack of definite consensus regarding treatment of DLBCL in the 80+ age group. However, due to the increase in life expectancy and increase in incidence of NHL, especially DLBCL, among octogenarians, there have been a few recent studies, mostly retrospective, dealing with patients in this age group.11-13 Some of these studies have shown improved survival outcomes when standard therapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is used rather than observation or suboptimal therapy, whereas others showed better survival and tolerability with less toxic non-anthracycline-based therapy or dose reduction in various components of R-CHOP.14,15 However, there are little data on DLBCL on those aged >90 years.16
In this context, we conducted a population-based study utilizing the National Institutes of Health National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 18 database to analyze the survival outcomes in 2 groups of very elderly patients: those aged between 80 and 89 years and 90 years and above, diagnosed with DLBCL prior to and after the approval of R.17 The SEER program of the National Cancer Institute publishes cancer incidence and survival data from 18 population-based cancer registries covering 28% of the US population.
From the SEER 18 database, we identified cases diagnosed with DLBCL between 1983 and 2013 using the International Classification of Diseases for Oncology, third edition codes 9680/3 and 9684/3, limiting the selection to patients aged 80 years and above at the time of diagnosis and with single primary malignancy. The data were split into 3 cohorts: cohort 1 comprised cases diagnosed between 1983 and 1996, with a study cutoff of December 1996; cohort 2 comprised cases diagnosed between 1997 and 2005, with a study cutoff of December 2005; and cohort 3 comprised cases diagnosed between 2006 and 2013. These study periods were selected because R was approved for treatment of relapsed or refractory, low-grade or follicular, B-cell NHL in 1997, and its approval was extended to treatment of DLBCL in 2006. Each cohort was further divided into 2 groups: group A aged between 80 and 89 years and group B aged 90 years and above (Table 1 describes characteristics of the study group).
Characteristics of the study population
| . | Cohort 1 . | Cohort 2 . | Cohort 3 . | |||
|---|---|---|---|---|---|---|
| . | Group A . | Group B . | Group A . | Group B . | Group A . | Group B . |
| Number of cases | 2239 | 339 | 4436 | 695 | 5740 | 937 |
| Sex | ||||||
| Male | 875 | 114 | 1858 | 228 | 2540 | 359 |
| Female | 1364 | 225 | 2578 | 467 | 3200 | 578 |
| Race | ||||||
| White | 2058 | 314 | 3979 | 619 | 5030 | 817 |
| Black | 54 | 4 | 125 | 25 | 180 | 20 |
| Other | 126 | 20 | 326 | 46 | 493 | 95 |
| Unknown | 1 | 1 | 6 | 5 | 37 | 5 |
| Ann Arbor stage | ||||||
| Stage I | 677 | 114 | 1282 | 236 | 1404 | 303 |
| Stage II | 409 | 64 | 855 | 128 | 1075 | 190 |
| Stage III | 273 | 20 | 619 | 64 | 994 | 98 |
| Stage IV | 694 | 87 | 1335 | 164 | 1846 | 244 |
| Unknown | 186 | 54 | 345 | 103 | 421 | 102 |
| Median age, y | 83 | 92 | 83 | 92 | 83 | 92 |
| Site | ||||||
| Nodal | 1486 | 217 | 2839 | 407 | 3727 | 536 |
| Extranodal | 753 | 122 | 1597 | 288 | 2013 | 401 |
| . | Cohort 1 . | Cohort 2 . | Cohort 3 . | |||
|---|---|---|---|---|---|---|
| . | Group A . | Group B . | Group A . | Group B . | Group A . | Group B . |
| Number of cases | 2239 | 339 | 4436 | 695 | 5740 | 937 |
| Sex | ||||||
| Male | 875 | 114 | 1858 | 228 | 2540 | 359 |
| Female | 1364 | 225 | 2578 | 467 | 3200 | 578 |
| Race | ||||||
| White | 2058 | 314 | 3979 | 619 | 5030 | 817 |
| Black | 54 | 4 | 125 | 25 | 180 | 20 |
| Other | 126 | 20 | 326 | 46 | 493 | 95 |
| Unknown | 1 | 1 | 6 | 5 | 37 | 5 |
| Ann Arbor stage | ||||||
| Stage I | 677 | 114 | 1282 | 236 | 1404 | 303 |
| Stage II | 409 | 64 | 855 | 128 | 1075 | 190 |
| Stage III | 273 | 20 | 619 | 64 | 994 | 98 |
| Stage IV | 694 | 87 | 1335 | 164 | 1846 | 244 |
| Unknown | 186 | 54 | 345 | 103 | 421 | 102 |
| Median age, y | 83 | 92 | 83 | 92 | 83 | 92 |
| Site | ||||||
| Nodal | 1486 | 217 | 2839 | 407 | 3727 | 536 |
| Extranodal | 753 | 122 | 1597 | 288 | 2013 | 401 |
SPSS, version 24.0 (IBM Corporation, Armonk, NY), was used for statistical analyses. Three-, 6-, 12-, and 24-month overall survival (OS) and disease-specific survival (DSS) for group A and group B were calculated using the Kaplan-Meier method, and equality of the survival distributions across the cohorts was compared using the log-rank test. OS was calculated as the time from diagnosis until death from any cause or date of last follow-up, whereas DSS was calculated as the time from diagnosis until death due to DLBCL or date of last follow-up (cases with death due to other causes were censored on the date of death). We performed multivariate analysis on 24-month DSS using the Cox proportional hazards regression model with age, sex, race, site (nodal/extranodal), time of diagnosis, and Ann Arbor stage as covariates.
Institutional review board waiver was obtained from the University of Tennessee Health Science Center prior to conducting this study.
Cohort 1 comprised cases diagnosed between 1983 and 1996; cohort 2 comprised cases diagnosed between 1997 and 2005, and cohort 3 comprised cases diagnosed between 2006 and 2013. Group A contains cases aged between 80 and 89 years at diagnosis, and group B contains cases aged 90 years and above.
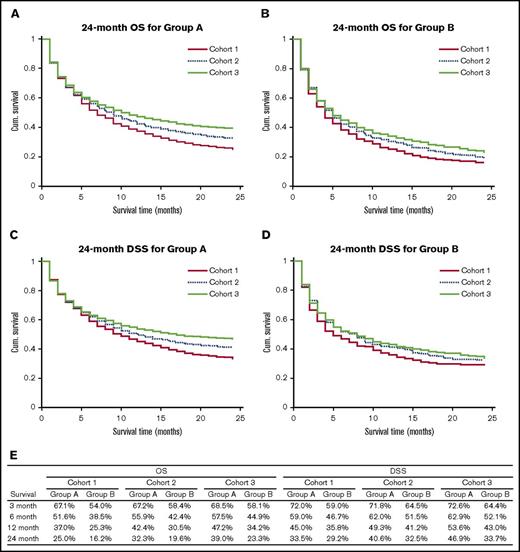
Cohort 1 had 2578 cases (group A: 2239 and group B: 339); cohort 2 had 5131 cases (group A: 4436 and group B: 695), and cohort 3 had 6677 cases (group A: 5740 and group B: 937). Figure 1 shows the OS and DSS characteristics of the study population and the 24-month OS and DSS curves. OS and DSS for both groups and for all survival periods increased from cohort 1 to cohort 2 to cohort 3, and the increase in survival for group A while moving from cohort 1 to cohort 3 was more marked than for group B. The log-rank test showed that for group A cases, 6-, 12-, and 24-month OS (P ≪ .005) and DSS (P = .04 for 6 months and P ≪ .005 for 12 and 24 months) showed a statistically significant increase while moving from cohort 1 to cohort 3. For group B cases, there was a statistically significant increase in the 12- and 24-month OS from cohort 1 to cohort 3, but there was no significant difference between DSS for the 3 cohorts. Multivariate analysis using Cox regression indicated that in addition to year of diagnosis (hazard ratio [HR]: 0.867 for cohort 2 compared with cohort 1, 95% confidence interval [CI]: 0.811-0.926, P ≪ .005; HR: 0.757 for cohort 3 compared with cohort 1, 95% CI: 0.710-0.808, P ≪ .005), increasing age at diagnosis (HR: 1.428 for group B compared with group A, 95% CI: 1.334-1.529, P ≪ .005), and Ann Arbor stage (HR: 1.266, 95% CI: 1.241-1.292, P ≪ .005) had a statistically significant impact on survival.
Survival outcomes of the study population. (A) 24 month OS for group A. (B) 24-month OS for group B. (C) 24-month DSS for group A. (D) 24 month DSS for group B. (E) OS and DSS rates for the study population for different survival periods. Cum., cumulative.
Survival outcomes of the study population. (A) 24 month OS for group A. (B) 24-month OS for group B. (C) 24-month DSS for group A. (D) 24 month DSS for group B. (E) OS and DSS rates for the study population for different survival periods. Cum., cumulative.
In conclusion, our analysis showed significantly improved OS and DSS at 6, 12, and 24 months for patients aged between 80 and 89 years and significantly improved OS at 12 and 24 months for patients aged >90 years. Our study has several limitations due to the nature of SEER data because information regarding comorbidities and modalities of treatment is not available. We divided the study population into 3 cohorts based on milestones in the introduction of R and observed significant improvement in survival from cohort 1 to cohort 2 to cohort 3. Several factors, for example, (1) increased number of cases managed with R-based therapy, (2) advanced medical care with better control of comorbidities, and (3) improved performance status in the elderly, which could have contributed to more physicians and patients willing to proceed with treatment, could have contributed to this improvement in survival.
With the advent of genomic profiling, the detailed molecular mechanism and heterogenicity of DLBCL have been well established,18,19 and various novel agents and targeted therapies (lenalidomide, protein kinase C β inhibitor, enzastaurin, Bruton tyrosine kinase inhibitor, ibrutinib, etc.) are available.20-23 Because molecular abnormalities in DLBCL increase with age,20 some of these novel agents with relatively low toxicity can potentially modify the therapeutic options for the elderly.
A search of clinicaltrials.gov on September 21, 2016 using the key word diffuse large B-cell lymphoma yielded 187 open studies actively recruiting patients with DLBCL, only 1 of which focused on the 80+ age group. More prospective studies are needed in the very elderly, separately for patients aged between 80 and 89 years and >90 years, taking into consideration the comorbidities associated with these age groups in order to clearly define guidelines for management of DLBCL in this population.
Contribution: M.G.M. developed the concept for the study and reviewed and critically revised the manuscript; U.G. reviewed literature, designed the study, collected and analyzed data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Upama Giri, University of Tennessee Health Science Center, 956 Court Ave, Suite H314, Memphis, TN 38163; e-mail: ugiri@uthsc.edu.