Key Points
Postsplenectomy TPO-RA use was not independently linked to higher thrombosis risk.
Platelet count alone does not predict risk of bleeding or thrombosis.
Visual Abstract
Immune thrombocytopenia (ITP) carries an increased risk of thrombosis, which may be further amplified by splenectomy and thrombopoietin receptor agonists (TPO-RAs). Although each intervention has been individually studied for its thrombotic risk, data are lacking on the safety of postsplenectomy TPO-RA use. We conducted a retrospective cohort study of adult patients with ITP who underwent splenectomy between 2011 and 2024. Patients were stratified on the basis of postsplenectomy TPO-RA use. The primary outcome was incidence of thrombosis. Secondary outcomes included bleeding, mortality, and hematologic response. Time-to-event analyses and multivariate Cox regression were performed. Among 88 patients, 37 (42%) patients received TPO-RAs after splenectomy. Thrombosis occurred in 17 patients in the TPO-RA group vs 14 patients in the non–TPO-RA group (P = .07). The 10-year cumulative incidence of thrombosis was numerically higher in the TPO-RA group (57% vs 36%), with clustering of events within the 3 months of postsplenectomy TPO-RA initiation. Venous events predominated; arterial events were rare. No significant differences were observed in bleeding or mortality rates between groups. Platelet counts alone did not independently affect bleeding or thrombosis. TPO-RA use after splenectomy was not significantly associated with increased thrombosis risk. However, early initiation may coincide with a high-risk period, and the sustained numerical difference in cumulative incidence throughout follow-up highlights potential clinical relevance. These findings support the need for individualized thromboprophylaxis and prospective evaluation of TPO-RA safety in this setting.
Introduction
Traditionally viewed as a hemorrhagic disorder, immune thrombocytopenia (ITP) is increasingly recognized for its paradoxical association with thrombosis, particularly venous thromboembolism, which occurs at twice the rate of the general population.1-5 This dual risk has introduced clinical complexity, especially in relapsed or refractory disease, for which splenectomy and thrombopoietin receptor agonists (TPO-RAs) are key options.
Despite the emerging recognition, the thrombogenic potential of ITP and its therapies remains underappreciated. Splenectomy, once the cornerstone of second-line treatment, offers high response durability (60%-70%)6,7 but carries increased thrombotic risk, especially in the early postoperative period.2,6,8-10
TPO-RAs (eg, romiplostim, eltrombopag, avatrombopag) provide nonsurgical alternatives with good efficacy and safety.5,6,11-17 However, data also link TPO-RAs to thrombotic complications via enhanced platelet activation and procoagulant changes, with some studies suggesting a trend toward higher thromboembolic event rates.1,4,14,18-25
Importantly, with the baseline increased risk of thrombosis among patients with ITP, the cumulative risk may be amplified when combined with splenectomy and TPO-RA exposure given that each of these therapies have been linked to an increase in thrombotic risk independently.4,8,9,18-22 However, this concern is often overlooked when selecting therapies for relapsed or refractory disease. In clinical practice, it is common for patients who relapse after splenectomy to start on TPO-RAs without careful assessment of thrombosis risk.
Previous studies examined whether splenectomy modified thrombosis risk among TPO-RA users.26 However, whether TPO-RA exposure further increases thrombotic risk among patients already at high risk due to splenectomy has not been systematically investigated.
We aimed to determine whether the initiation or continuation of TPO-RAs after splenectomy increases thrombosis risk among patients with ITP and identify predictors of thrombotic events in this underexplored cohort. Given the long-term follow-up of patients who underwent splenectomy before the widespread use of TPO-RAs, many now receive these agents later in their disease course. Understanding how TPO-RAs affect thrombosis risk in this specific population is essential to guide safer, individualized treatment decisions.
Methods
Study design and setting
A multisite, single-institution retrospective cohort study was conducted across Mayo Clinic Enterprise. Institutional review board approval was obtained, classifying it as a minimal-risk retrospective study and granting a waiver of informed consent before study initiation.
Patient identification
Patients aged ≥18 years who underwent splenectomy for ITP between 1 January 2011 and 31 December 2024 were identified using electronic health record search tools (SlicerDicer [Epic Systems] and Advanced Text Explorer). Cases were identified on the basis of documentation of “ITP,” “immune thrombocytopenia,” or “idiopathic thrombocytopenia” in the medical record.
Eligibility criteria
Inclusion required a diagnosis of ITP (primary or secondary) and splenectomy during the study period, regardless of where the surgery was performed. Patients were excluded if (1) ≥3 postsplenectomy platelet count measurements were missing at predefined time points (at baseline, 1 week, 4 weeks, 3 months, 6 months, and 12 months after splenectomy) or (2) follow-up was shorter than 6 months from splenectomy, unless death occurred.
Data collection
Extracted variables included demographics, date of splenectomy, duration of ITP before splenectomy, surgical approach, Caprini risk score, use of pharmacologic thromboprophylaxis after splenectomy, history of venous thromboembolism before splenectomy, presence of additional thrombotic risk factors (eg, malignancy, obesity, thrombophilia), serial platelet counts at the predefined time points, use and timing of TPO-RA use, TPO-RA agent(s) used, characteristics of thrombotic and bleeding events (including timing and site), and survival status.
The Caprini risk score was retrospectively calculated for each patient on the basis of available clinical documentation before splenectomy.
Outcomes
The primary outcome was the 10-year cumulative incidence of thrombotic events (venous and/or arterial events) following splenectomy, comparing patients who received TPO-RA therapy with those who did not. All thrombotic events, except isolated splenic vein thrombosis, were included regardless of anatomical site, as documented in the medical record.
Secondary outcomes included 1-year cumulative incidence of thrombotic events, timing and type of thrombotic events, platelet count trajectories across standardized time points and at the time of thrombotic events, incidence and type of clinically significant bleeding events according to the International Society on Thrombosis and Haemostasis definition,27 hematologic responses at 12 months following splenectomy,28 and overall survival. The date of splenectomy was defined as the index date for analyses.
Statistical analysis
Baseline characteristics were summarized using descriptive statistics. Continuous variables were reported as medians with interquartile ranges (IQR) and compared using Wilcoxon rank-sum tests. Categorical variables were reported as frequencies and compared using the Fisher exact test or χ2 tests, as appropriate.
Time-to-event outcomes, including thrombosis and overall survival, were analyzed using cumulative incidence functions and Kaplan-Meier curves, with comparisons made using log-rank tests.
Cox proportional hazards regression was used to evaluate predictors of thrombosis after splenectomy. Variables assessed in univariate models included age, sex, ITP type, platelet count at baseline, surgical approach, TPO-RA use (before and after splenectomy), Caprini score risk category, postsplenectomy thromboprophylaxis, and prior thrombosis. A multivariable model was constructed using no more than 3 clinically relevant covariates to minimize the risk of overfitting.
All analyses were performed using JMP version 18.0 (SAS Institute Inc). A P value <.05 was considered statistically significant.
This study received institutional review board approval. The requirement for informed consent was waived because of the retrospective nature of the analysis.
Results
Patient characteristics
A total of 88 adult patients with ITP who underwent splenectomy between 2011 and 2024 were included. Of these, 51 patients did not receive postsplenectomy TPO-RAs, whereas 37 did. The cohort selection process is illustrated in supplemental Figure 1.
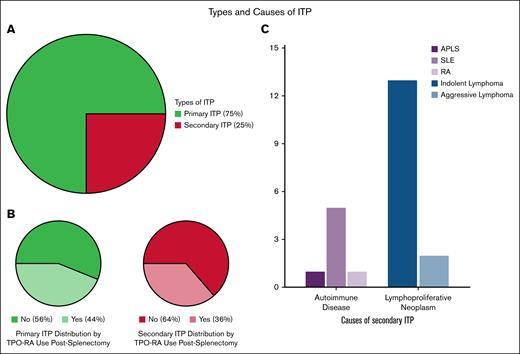
Baseline characteristics are summarized in Table 1. The 2 groups were similar in age at diagnosis and splenectomy, sex, ITP type (primary vs secondary), surgical approach (laparoscopic vs open), and Caprini risk score. Additional thrombotic risk factors were similarly distributed across groups (supplemental Figure 2), with obesity and active malignancy being the most prevalent comorbidities. The median presplenectomy platelet counts were significantly lower in the postsplenectomy TPO-RA group (14 × 109/L vs 34 × 109/L; P = .01). Primary ITP accounted for 75% of the cohort. Among the 22 patients with secondary ITP, underlying etiologies included autoimmune disorders (eg, antiphospholipid antibody syndrome, systemic lupus erythematosus, rheumatoid arthritis) and lymphoproliferative neoplasms (Figure 1).
Types and causes of ITP among patients undergoing splenectomy. The large pie chart (top left) illustrates the overall distribution of ITP types in the cohort (N = 88), with primary ITP comprising 75% and secondary ITP 25% of cases. The 2 smaller pie charts (bottom left) show the proportion of postsplenectomy TPO-RA use within each ITP type: 44% of patients with primary ITP and 36% of those with secondary ITP received TPO-RA after splenectomy. The bar chart (right) outlines the underlying causes of secondary ITP, with lymphoproliferative neoplasms (predominantly indolent lymphoma) being the most common. Indolent lymphomas included CLL, LPL, MZL, and FL, whereas aggressive subtypes included HL and DLBCL. One patient with HL also had hepatitis C infection. Among autoimmune causes, SLE was most frequent, with 1 cause attributed to APLS. APLS, antiphospholipid antibody syndrome; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; LPL, lymphoplasmacytic lymphoma; MZL, marginal zone lymphoma; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Types and causes of ITP among patients undergoing splenectomy. The large pie chart (top left) illustrates the overall distribution of ITP types in the cohort (N = 88), with primary ITP comprising 75% and secondary ITP 25% of cases. The 2 smaller pie charts (bottom left) show the proportion of postsplenectomy TPO-RA use within each ITP type: 44% of patients with primary ITP and 36% of those with secondary ITP received TPO-RA after splenectomy. The bar chart (right) outlines the underlying causes of secondary ITP, with lymphoproliferative neoplasms (predominantly indolent lymphoma) being the most common. Indolent lymphomas included CLL, LPL, MZL, and FL, whereas aggressive subtypes included HL and DLBCL. One patient with HL also had hepatitis C infection. Among autoimmune causes, SLE was most frequent, with 1 cause attributed to APLS. APLS, antiphospholipid antibody syndrome; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; LPL, lymphoplasmacytic lymphoma; MZL, marginal zone lymphoma; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
All patients received at least 1 line of ITP-directed therapy before splenectomy, including corticosteroids, IV immunoglobulin, rituximab, and/or TPO-RAs (supplemental Table 1). Patients who received TPO-RAs after splenectomy were more likely to have been treated with TPO-RAs before splenectomy (57% vs 35.2%; P = .04), and more frequently continued the same agent thereafter. A detailed analysis of TPO-RA sequences is provided in supplemental Table 2. Romiplostim and eltrombopag were the most commonly used TPO-RA agents before splenectomy.
The median year of splenectomy was 2019 (IQR, 2016-2021), with a notable spike in 2021 (supplemental Figure 3). Most splenectomies were laparoscopic (84% in non–TPO-RA vs 97% in TPO-RA group; P = .13).
Among patients exposed to postsplenectomy TPO-RAs, the median cumulative duration of TPO-RA therapy following splenectomy was 213 days (IQR, 18.5-521). By agent, median postsplenectomy exposure was 228 days (IQR, 20-898) for eltrombopag, 103 days (IQR, 1-366) for romiplostim, and 409 days (IQR, 374-444) for avatrombopag, accounting for treatment interruptions.
Thrombotic occurrence and characteristics
Thrombosis occurred in 31 patients (35%) during the follow-up period: 14 (27%) in the non–TPO-RA group and 17 (46%) in the TPO-RA group (P = .07). Cumulative incidence of thrombosis at 10 years and 1 year after splenectomy is shown in Figure 2. The 10-year incidence was higher in the TPO-RA group (57% vs 36%), with visual divergence emerging in the first year after splenectomy, but the difference did not reach statistical significance (P = .06). At 1 year, the cumulative incidence was 24.7% in the TPO-RA group and 15.4% in the non–TPO-RA group (P = .35).
Cumulative incidence of thrombosis following splenectomy in patients with ITP, stratified by TPO-RA use. (A) The 10-year cumulative incidence of thrombosis after splenectomy, which was numerically higher among patients who received postsplenectomy TPO-RAs (57%) than those who did not (33%; P = .06). The divergence between groups emerged early, primarily within the first year. (B) The 1-year cumulative incidence of thrombosis after splenectomy, again showing a numerically higher incidence in the TPO-RA group (25% vs 15%; P = .35). Although neither comparison reached statistical significance, likely because of sample size and censoring imbalance, the data suggest a potential trend toward increased thrombotic risk with TPO-RA use in the early postsplenectomy period.
Cumulative incidence of thrombosis following splenectomy in patients with ITP, stratified by TPO-RA use. (A) The 10-year cumulative incidence of thrombosis after splenectomy, which was numerically higher among patients who received postsplenectomy TPO-RAs (57%) than those who did not (33%; P = .06). The divergence between groups emerged early, primarily within the first year. (B) The 1-year cumulative incidence of thrombosis after splenectomy, again showing a numerically higher incidence in the TPO-RA group (25% vs 15%; P = .35). Although neither comparison reached statistical significance, likely because of sample size and censoring imbalance, the data suggest a potential trend toward increased thrombotic risk with TPO-RA use in the early postsplenectomy period.
The median time from splenectomy to thrombosis did not significantly differ between groups (10 vs 5.5 months; P = .64) (supplemental Table 3). The median time from TPO-RA initiation to thrombosis was 3 months (IQR, 0.5-23). Notably, 25% of events occurred within 2 weeks of TPO-RA initiation, and nearly 50% occurred within 3 months.
In addition to timing, we examined anatomical distribution and type of thrombotic events, as shown in Figure 3A. Venous events predominated (n = 27), particularly extremity deep vein thromboses (41%). Arterial events occurred in 3 patients, and 1 patient had both arterial and venous thromboses concurrently. Thrombosis patterns stratified by postsplenectomy TPO-RA exposure are shown in Figure 3B. Venous events were more frequent in both groups, but anatomical sites varied slightly. Thrombosis type and site did not significantly differ by TPO-RA agent or dose (P = .51 and P = .76, respectively).
Types and anatomical sites of postsplenectomy thrombotic events among patients with ITP. (A) The bar chart displays the distribution of thrombotic event types among all patients (N = 88) after splenectomy, with venous thrombosis accounting for most events, followed by arterial and combined arterial and venous events. The pie charts detail the anatomical sites involved: DVT and PE were most common among venous events, whereas coronary and cerebral arteries were the leading arterial sites. (B) Thrombotic events are stratified by postsplenectomy TPO-RA use. Venous events remained dominant in both groups; however, combined events occurred exclusively among patients who received TPO-RAs. The corresponding pie charts outline the anatomical distribution for each group. In the TPO-RA group, DVT and PE remained the most frequent, whereas the non–TPO-RA group exhibited a broader distribution of both venous and arterial sites, including cardiac and renal arteries. The multisite VTE observed in the TPO-RA group involved lower-limb DVT, PE, and SVT affecting the portal and mesenteric veins. Isolated splenic vein thrombosis events were not counted as thrombotic events because they are considered postsplenectomy findings and not clinically relevant unless extension beyond the splenic vein occurs. CVT, cerebral venous thrombosis; DVT, deep vein thrombosis; PE, pulmonary embolism; RVT, renal vein thrombosis; SVT, splanchnic vein thrombosis; VTE, venous thromboembolism.
Types and anatomical sites of postsplenectomy thrombotic events among patients with ITP. (A) The bar chart displays the distribution of thrombotic event types among all patients (N = 88) after splenectomy, with venous thrombosis accounting for most events, followed by arterial and combined arterial and venous events. The pie charts detail the anatomical sites involved: DVT and PE were most common among venous events, whereas coronary and cerebral arteries were the leading arterial sites. (B) Thrombotic events are stratified by postsplenectomy TPO-RA use. Venous events remained dominant in both groups; however, combined events occurred exclusively among patients who received TPO-RAs. The corresponding pie charts outline the anatomical distribution for each group. In the TPO-RA group, DVT and PE remained the most frequent, whereas the non–TPO-RA group exhibited a broader distribution of both venous and arterial sites, including cardiac and renal arteries. The multisite VTE observed in the TPO-RA group involved lower-limb DVT, PE, and SVT affecting the portal and mesenteric veins. Isolated splenic vein thrombosis events were not counted as thrombotic events because they are considered postsplenectomy findings and not clinically relevant unless extension beyond the splenic vein occurs. CVT, cerebral venous thrombosis; DVT, deep vein thrombosis; PE, pulmonary embolism; RVT, renal vein thrombosis; SVT, splanchnic vein thrombosis; VTE, venous thromboembolism.
Among the 17 patients in the TPO-RA group who experienced thrombosis, 11 (65%) were receiving TPO-RAs at the time of the event: 5 patients received eltrombopag, 5 romiplostim, and 1 avatrombopag. All events in this subgroup were venous, with 1 patient also experiencing a concurrent arterial event. Therapy was continued at the time of the event in 6 patients (3 with dose reductions) and discontinued in 5 (45.5%). Three of the 5 patients subsequently resumed TPO-RA therapy (median time from discontinuation to resumption was 395 days [IQR, 12-838]); in 1 case, eltrombopag was switched to romiplostim ∼838 days after eltrombopag discontinuation.
The median platelet count at thrombosis was not significantly different between TPO-RA users (219 × 109/L) and nonusers (385 × 109/L; P = .27), as shown in supplemental Figure 4. Among patients who received TPO-RA agents, romiplostim users had higher median counts at thrombosis (295 × 109/L; IQR, 206-617.5) than eltrombopag users (100 × 109/L; IQR, 32.5-132.5; P = .02). One avatrombopag-associated event occurred at a platelet count of 880 × 109/L.
Cox regression analysis and thrombosis risk
Univariate Cox regression analysis did not identify statistically significant predictors of thrombosis (all P ≥ .05) (supplemental Table 4). A multivariate model including Caprini risk score, postsplenectomy thromboprophylaxis, and prior thrombosis was constructed on the basis of clinical relevance. Caprini score was the only variable that approached but did not reach statistical significance with adjustment (P = .05) (supplemental Figure 5).
Bleeding events
Clinically significant bleeding (per International Society on Thrombosis and Haemostasis criteria) occurred in 12 patients, 6 in each group (supplemental Figure 6). Gastrointestinal bleeding was most common (42%), followed by musculoskeletal/soft tissue (33%), central nervous system or ophthalmic (17%), and surgical site bleeding (8%).
Bleeding events occurred across a wide range of platelet counts. The median platelet count at bleeding was higher in the non–TPO-RA group (152 × 109/L; IQR, 29-189) than in the TPO-RA group (71 × 109/L; IQR, 6.75-83.5), although this difference was not statistically significant (P = .12) (supplemental Figure 6).
Platelet count trajectories
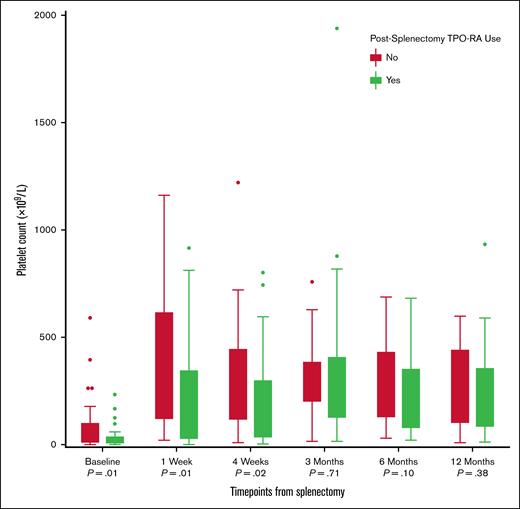
Postsplenectomy platelet recovery differed by TPO-RA exposure (Figure 4). Presplenectomy counts were lower in the TPO-RA group (14 × 109/L [IQR, 4.5-36] vs 34 × 109/L [IQR, 8-96]; P = .01). This difference persisted after splenectomy at 1 week (146 × 109/L vs 281.5 × 109/L; P = .01) and 4 weeks (108.5 × 109/L vs 246 × 109/L; P = .02). By 3 months and beyond, platelet counts were similar between the groups (3 months, 271 × 109/L vs 317 × 109/L [P = .71]; 6 months, 235 × 109/L vs 311 × 109/L [P = .10]; and 12 months, 254 × 109/L vs 275 × 109/L [P = .38]).
Platelet count trajectories following splenectomy in patients with ITP, stratified by postsplenectomy TPO-RA use. Box plots illustrate platelet counts (×109/L) at predefined time points: baseline (before splenectomy) and 1 week, 4 weeks, 3 months, 6 months, and 12 months after splenectomy. Although both groups experienced a rapid rise in platelet counts during the first week, a broader range and higher peak counts were observed in the non–TPO-RA group. Over time, platelet counts declined and stabilized in both groups, with overlapping medians from 3 months onward. The use of TPO-RAs after splenectomy was more common among patients with lower or less sustained platelet responses, which is reflected in the slightly lower median counts in this group beyond the initial postoperative period.
Platelet count trajectories following splenectomy in patients with ITP, stratified by postsplenectomy TPO-RA use. Box plots illustrate platelet counts (×109/L) at predefined time points: baseline (before splenectomy) and 1 week, 4 weeks, 3 months, 6 months, and 12 months after splenectomy. Although both groups experienced a rapid rise in platelet counts during the first week, a broader range and higher peak counts were observed in the non–TPO-RA group. Over time, platelet counts declined and stabilized in both groups, with overlapping medians from 3 months onward. The use of TPO-RAs after splenectomy was more common among patients with lower or less sustained platelet responses, which is reflected in the slightly lower median counts in this group beyond the initial postoperative period.
Hematologic response 12 months after splenectomy
At 12 months after splenectomy, complete response was achieved in most patients, more frequently among those not receiving postsplenectomy TPO-RAs (32 vs 23 patients) (Figure 5). Among those who achieved complete response, two-thirds required additional ITP therapy after splenectomy. In the non–TPO-RA group, 38% required further treatment (excluding TPO-RAs).
Hematologic response at 12 months after splenectomy among patients with ITP, stratified by TPO-RA use. The left panel shows hematologic response rates, CR, PR, and NR, at 12 months after splenectomy, stratified by postsplenectomy TPO-RA use. CR was the most common outcome in both groups but occurred more frequently among patients who did not receive TPO-RAs. PR and NR were more evenly distributed across groups. The right panel shows the proportion of patients who required additional ITP-directed treatment following splenectomy; 38% achieved CR with additional ITP therapies other than TPO-RAs. CR, complete response; NR, no response; PR, partial response.
Hematologic response at 12 months after splenectomy among patients with ITP, stratified by TPO-RA use. The left panel shows hematologic response rates, CR, PR, and NR, at 12 months after splenectomy, stratified by postsplenectomy TPO-RA use. CR was the most common outcome in both groups but occurred more frequently among patients who did not receive TPO-RAs. PR and NR were more evenly distributed across groups. The right panel shows the proportion of patients who required additional ITP-directed treatment following splenectomy; 38% achieved CR with additional ITP therapies other than TPO-RAs. CR, complete response; NR, no response; PR, partial response.
Overall survival
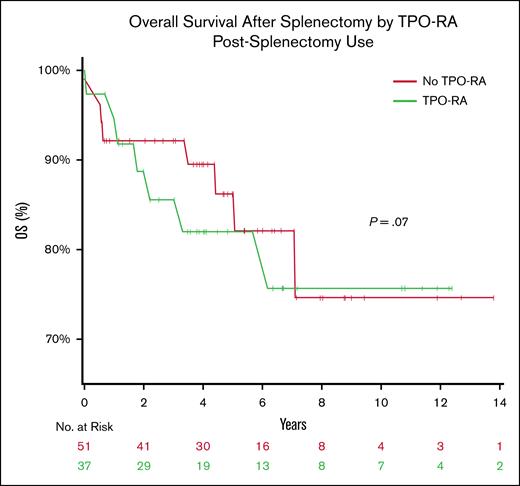
The 10-year overall survival was similar between groups (76% in the TPO-RA group vs 78% in the non–TPO-RA group) (Figure 6). No statistically significant difference was observed (P = .7, log-rank test).
OS following splenectomy among patients with ITP, stratified by TPO-RA use. Kaplan-Meier curves illustrate OS after splenectomy among patients with ITP who did (green) or did not (red) receive postsplenectomy TPO-RA therapy. No significant difference in OS was observed between groups (P = .7). A total of 12 deaths occurred during the study period (6 deaths in each group). Patients who remained alive were censored at the date of the last follow-up. The number at risk at each time point is shown below the x-axis. OS, overall survival.
OS following splenectomy among patients with ITP, stratified by TPO-RA use. Kaplan-Meier curves illustrate OS after splenectomy among patients with ITP who did (green) or did not (red) receive postsplenectomy TPO-RA therapy. No significant difference in OS was observed between groups (P = .7). A total of 12 deaths occurred during the study period (6 deaths in each group). Patients who remained alive were censored at the date of the last follow-up. The number at risk at each time point is shown below the x-axis. OS, overall survival.
Most deaths occurred within the first 5 years after splenectomy, after which the survival curves plateaued. The limited numbers of patients at risk beyond 8 years (≤8 patients per group) reduced the precision of long-term survival estimates.
Fifteen patients died during the study period. Three deaths were attributed to thrombotic events: 2 in the non–TPO-RA group (1 from acute cerebrovascular and myocardial infarction and 1 from pulmonary embolism) and 1 in the TPO-RA group. Causes of death by TPO-RA exposure group are summarized in supplemental Table 5.
Discussion
This study explores the intersection of 2 independently thrombogenic interventions in ITP, splenectomy and TPO-RA therapy, a setting where clinical decisions are often made with limited evidence. Although previous reports have examined thrombosis risk with TPO-RAs or splenectomy individually, this analysis provides, to our knowledge, novel insights into the cumulative thrombotic implications when TPO-RAs are used in patients who have already undergone splenectomy.
Although the 10-year cumulative incidence of thrombosis was numerically higher in the TPO-RA group, the difference did not reach statistical significance. Nonetheless, the temporal distribution of events is noteworthy: 25% of thrombotic events occurred within 2 weeks of TPO-RA initiation, and nearly half within the first 3 months. The median time from splenectomy to TPO-RA initiation was 24.5 days (IQR, 3-248), often overlapping with the known postoperative prothrombotic window. This clustering raises concerns for a vulnerable early phase in which the additive thrombotic effects of recent surgery and TPO-RA exposure may converge. Although clinical significance has not been established, possibly because of the modest sample size, these numerical and temporal patterns, including divergence that persisted through long-term follow-up, underscore the need for careful patient selection, cautious timing of TPO-RA initiation, and clinical vigilance, especially during the early period following splenectomy or in patients with additional thrombotic risk factors.
The thrombotic profile in our cohort was predominantly venous, consistent with previous literature.9,29-31 Arterial events were rare and not clearly linked to TPO-RA exposure or splenectomy. When accounting for total exposure time, the pooled on-treatment (TPO-RA) thrombosis incidence was 21.3 events per 100 patient-years (95% confidence interval, 11.6-38.1). Only 1 line-associated deep vein thrombosis occurred, and isolated splenic vein thrombosis was excluded because of its known association with splenectomy.32 A French study of patients with ITP who relapsed after splenectomy found that postsplenectomy TPO-RA use was common (94% received TPO-RA), with only 4 thrombotic events reported and no excess risk observed. However, interpretation is limited by the modest sample size and absence of a control group.33
Thromboses occurred at relatively lower platelet counts in the TPO-RA group, whereas bleeding events occurred at higher counts in the non–TPO-RA group. Although not statistically significant, this inverse trend may reflect qualitative differences in platelet function with TPO-RA. Prior studies did not show alterations in platelet activity with TPO-RA, but some studies suggested that TPO-RA may enhance prothrombotic potential through mechanisms such as increased platelet microparticles and accelerated clot formation.22,34,35 In support of this, Garabet et al reported that patients with ITP receiving long-term TPO-RA therapy had increased serum P-selectin levels and transient PAI-1 (plasminogen activator inhibitor-1) elevation, both prothrombotic biomarkers, while also demonstrating that ITP is associated with increased endothelial activation and neutrophil extracellular trap formation, changes that were not further augmented by TPO-RA treatment.10,36 These findings underscore that thrombotic and hemorrhagic risks in ITP are not solely dictated by platelet count and highlight the importance of assessing the broader hemostatic context.
Neither the Caprini score nor use of thromboprophylaxis independently predicted thrombosis in our cohort. This may reflect limitations in current risk tools when applied to patients with ITP, whose pathophysiology and treatment exposures differ from those in the surgical populations for which such scores were developed. The need for an ITP-specific thrombotic risk model, particularly for postsplenectomy patients, remains pressing.
Patients who received TPO-RAs after splenectomy had features suggestive of more refractory disease, including lower presplenectomy platelet counts and more extensive preoperative treatment. Despite this, platelet trajectories between groups equalized by 3 months after splenectomy, suggesting that TPO-RAs may have supported hematologic recovery in otherwise poor responders. This observation highlights the therapeutic value of TPO-RAs in this setting but also invites scrutiny regarding timing and safety.
Bleeding events were less frequent than thromboses, although underreporting is possible given the retrospective design. Importantly, bleeding was observed across a wide platelet range, including values above conventional treatment thresholds. This again challenges reliance on platelet counts alone in therapeutic decision-making.
Regarding mortality, infection was the most common cause of death in the TPO-RA groups, consistent with immunosuppressive effects of ITP treatments and splenectomy. Only 3 deaths were attributed to thrombosis, of which 2 were arterial. Antiphospholipid antibody syndrome was documented in 3 patients with thrombosis (9.6%), suggesting that it did not account for the observed thrombotic burden. However, antiphospholipid antibody syndrome testing was not routinely performed, and occult risk factors cannot be excluded.
Study strengths include long-term follow-up and real-world applicability. Limitations include its retrospective design, small sample size, and potential for confounding by indication. The rarity of splenectomy in contemporary ITP practice constrained the cohort size, as its use has declined to <5% of treatment decisions with the availability of newer agents.6,37,38 Hematologic response was assessed at a single 12-month time point, which does not reflect the dynamic course of ITP; however, we addressed this by examining platelet trajectories over the first year and capturing subsequent therapy needs.
In conclusion, although TPO-RA use after splenectomy was not independently associated with a significantly increased risk of thrombosis, the timing and context of use, especially early initiation, may warrant heightened clinical vigilance given the observed numerical difference in thrombotic events. These findings support the continued use of TPO-RAs in appropriate postsplenectomy patients but emphasize the need for thoughtful timing, individualized thromboprophylaxis, and future prospective studies to guide clinical practice in this population.
Authorship
Contribution: R.A. conceptualized and designed the study, performed data collection, analysis, and interpretation, and wrote the manuscript; D.E.H., M.S., R.K.P., S.S., R.C.G., A.W.-.S., and R.S.G. critically reviewed and edited the manuscript; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.S. reports consultancy (includes providing expert testimony) for Sanofi; received honoraria from Argynx; and serves as a research consultant for Johnson & Johnson. The remaining authors declare no competing financial interests.
Correspondence: Ruah Alyamany, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: r.alyamany@hotmail.com; and Ronald S. Go, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; email: go.ronald@mayo.edu.
References
Author notes
Deidentified clinical and laboratory data are available on request from the corresponding author, Ronald S. Go (go.ronald@mayo.edu).
The full-text version of this article contains a data supplement.