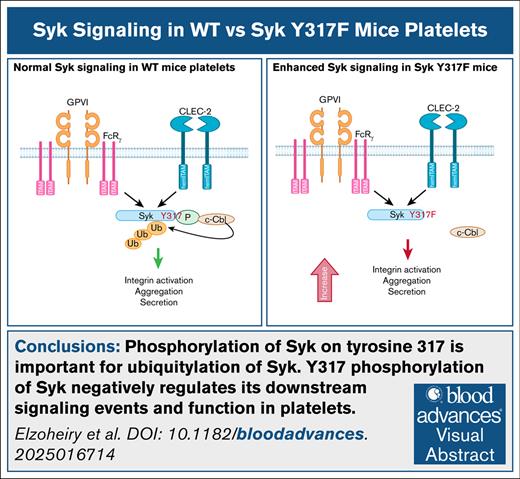
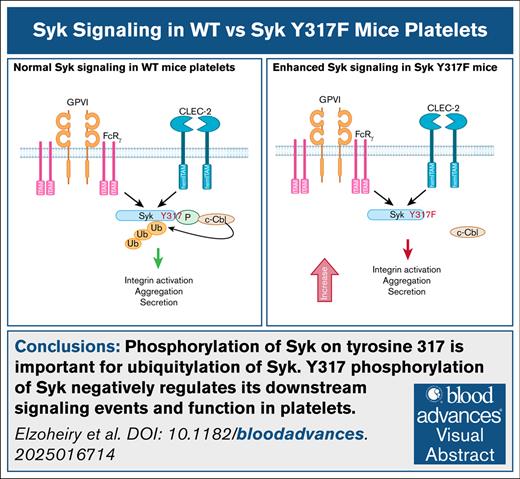
Key Points
Phosphorylation of Syk on tyrosine 317 is important for ubiquitylation of Syk.
Y317 phosphorylation of Syk negatively regulates its downstream signaling events and function in platelets.
Visual Abstract
Spleen tyrosine kinase (Syk) is expressed in a variety of hematopoietic cells. Its phosphorylation regulates downstream signaling events upon stimulation of receptors containing an immunoreceptor tyrosine-based activation motif (ITAM), like glycoprotein VI, or a hemITAM, including the C-type lectin-like receptor 2 (CLEC-2). This study focuses on the role of a specific phosphorylation site, tyrosine 317, in the regulation of Syk function. Tyrosine 317 is located in the linker region of Syk that separates the amino-terminal, tandem pair of SH2 domains from the carboxyl-terminal catalytic domain. The amino acid sequence surrounding phosphotyrosine 317 binds to the matching recognition sequence in the tyrosine kinase–binding domain of Cbl, an E3 ubiquitin-protein ligase. To evaluate the function of this phosphorylation site, we generated mice expressing Syk(Y317F) using the CRISPR/Cas9 technique. Platelets from homozygous Syk(Y317F) mice showed enhancement of platelet signaling and physiological responses after stimulation with collagen-related peptide (CRP) and CLEC-2 cross-linking. This enhancement did not occur after stimulation with AYPGKF, a protease-activated receptor 4 agonist, or 2-methylthioadenosine diphosphate, a purinergic agonist. CRP- or CLEC-2 monoclonal antibody–induced downstream signaling events, including phosphorylation of LAT and phospholipase C γ2, were enhanced in Syk(Y317F) platelets compared with platelets from wild-type (WT) littermates. Besides an increase in platelet responses in vitro, the time to occlusion in the FeCl3 injury model was decreased in Syk(Y317F) mice compared with WT littermates. However, there was no significant difference in the tail bleeding times. Taken together, these data reveal that tyrosine 317 negatively regulates Syk signaling and functions in mouse platelets.
Introduction
Spleen tyrosine kinase (Syk) is a nonreceptor protein tyrosine kinase (PTK), which is associated with cell surface receptors to amplify receptor-activated signals inside the cells regulating a variety of cellular processes.1-4 It is expressed in most hemopoietic lineage cells,1 and other tissues including fibroblasts, epithelial cells, breast tissue, hepatocytes, neuronal cells, and vascular endothelial cells.5 The Syk-family ZAP-70 PTK, expressed mainly in T and natural killer cells, performs similar functions.6
Syk mediates various signaling pathways triggered by immunoreceptor tyrosine-based activation motif (ITAM) and a hemi-immunoreceptor tyrosine-based activation motifhemITAM-containing receptors. The ITAM sequences, containing 2 YXX(L/I) motifs, are present in the signal-transducing subunits of the B-cell receptor, T-cell receptor, and glycoprotein VI (GPVI) on platelets, as well as in the cytoplasmic tail of the FcγRIIA receptor.7 Other receptors, including the C-type lectin-like receptor 2 (CLEC-2) and dectin-1, contain a hemITAM sequence corresponding to a single YXX(L/I) motif; these receptors are expressed in multiple hematopoietic cells, including platelets, and signal through a Syk-dependent pathway.8,9
Syk deficiency leads to perinatal lethality due to defective separation of lymphoid and blood vessels.10-12 Reconstitution of the hematopoietic system with Syk-null stem cells results in production of multiple cell types except mature B cells.11-13
In myeloid cells, Syk is a key protein mediating Fc receptor and integrin-mediated signaling, and also facilitates downstream signaling of C-type lectins like dectin-1 recognizing fungal antigens.14 Selective Syk inhibition abrogated inflammasome activation by fungal infection.15 Deficiency of Syk produced profound defects in neutrophil/macrophage integrin signaling and responses to immune complexes, resulting in significantly reduced stimulation of respiratory burst, degranulation, and cell spreading.16,17 Syk-deficient bone marrow chimeras proved to be completely protected from autoantibody-induced arthritis due to suppression of immune complex– or integrin ligand–activated neutrophils.17-19
Consistent with the critical role of Syk in many biological and pathological processes, pharmacological inhibitors of Syk exert clinically beneficial effects in various diseases, including rheumatoid arthritis,20 B-cell malignancies,21,22 immune thrombocytopenic purpura,23 and allergic rhinitis.24
It has been shown that Syk encoded by monoallelic syk variants associated with multiorgan inflammatory diseases such as colitis, arthritis and dermatitis, and diffuse large B-cell lymphomas exhibit the enhanced phosphorylation and downstream signaling, indicating gain-of-function mutations.25 The same study documented that the Syk(S544Y) knockin (KI) mouse model of the patient variant of Syk (p.S550Y) recapitulated aspects of the human disease that could be partially treated with a Syk inhibitor or transplantation of bone marrow from wild-type (WT) mice.
The mutations in Syk causing immune dysregulation25 are located in either Syk domain or the linker between its tandem SH2 and kinase domains termed interdomain B.26,27 The interdomain B has several tyrosine residues that are phosphorylated by Src-family kinases or autophosphorylation, which regulate Syk functions and, hence, Syk-mediated signal transduction.28-37 In platelets, we have demonstrated that phosphorylation of Syk Y342, which is located inside the interdomain B, is important for the ability of Syk to transduce signals through GPVI and CLEC-2 receptors.38 We have also shown that phosphorylation of Syk Y346, a site neighboring Y342, appears to negatively regulate ITAM-mediated signaling and responses in platelets.39
Studies in several cell types indicated that mouse Syk Y317, which corresponds to tyrosine residue 323 in human Syk (Tyr323), is involved in the regulation of Syk functions; Syk mutants lacking Y317 exhibit the enhanced ability of B-cell receptors to induce signaling and activation of transcription factors.32,40 This mechanism has been shown to be mediated by Cbl-dependent ubiquitylation of activated Syk, which is phosphorylated on Y317, followed by its degradation.41
Consistent with a key role of Syk Y317 in Cbl-dependent downregulation of Syk and the critical involvement of Syk in activation of multiple cell types of the hematopoietic lineage, Syk(Y317F) mutants enhance signaling and physiological responses of basophils/mast cells.31,34 It has also been documented that expression of Syk Y317F in Syk-null osteoclasts enhanced the macrophage colony-stimulating factor receptor- and αV/β3 integrin–induced phosphorylation of the cytoskeleton-organizing molecules accelerating the resorptive capacity of these cells.42
Despite the essential function of Syk in platelet signaling and physiological responses,43-45 the active stimulation-induced ubiquitylation of Syk in platelets,46 and the ubiquitous importance of Syk pY317 in the process of Cbl-dependent Syk regulation,31,32,34,40-42 the role of Syk pY317 in the regulation of Syk in platelets has never been studied. One reason why the role of Syk pY317 has not been examined in platelets is the lack of adequate experimental systems. Because platelets are anucleate cells, their protein levels cannot be manipulated using RNA interference and/or expression of transfected/transduced complementary DNA.
To address this issue, we used Syk(Y317F) KI mice, in which platelets express Syk with Y317 mutated to a phenylalanine, thereby preventing phosphorylation at this site, to evaluate the role of Y317 phosphorylation in Syk signaling and physiological functions of platelets. In this study, we demonstrate that platelet aggregation and secretion are potentiated in Syk(Y317F) mice compared with WT mice, and this potentiation is associated with the enhanced signaling downstream of the GPVI and CLEC-2 receptors.
Materials and methods
Materials
All reagents were purchased from Thermo Fisher Scientific unless otherwise stated. Chrono-lume used for the detection of secreted adenosine triphosphate (ATP) was purchased from Chrono-log corporation (Havertown, PA). Anti–phosphorated Syk (pSky) Y525/526 (mouse Y519/520; catalog no. 2710), anti–phosphorylated phospholipase C γ2 (PLC2) Y1217 (catalog no. 3871), anti-pSyk Y323 (mouse Y317; catalog no. 2715), anti-pSyk Y352 (mouse Y346; catalog no. 2701), and anti-pLAT (linker for activation of T cells) Y191 (catalog no. 3584) were purchased from Cell Signaling Technology (Danvers, MA). Anti-Syk (catalog no. sc-51703) and anti-PLC2 (catalog no. sc-5283) were purchased from Santa Cruz Biotechnology. Anti-LAT (catalog no. TA327925) was purchased from OriGene (Rockville, MD). Blocking buffer was purchased from Genesee Scientific, and secondary antibodies (IRDye 800CW goat anti-rabbit and IRDye 680LT goat anti-mouse) were purchased from LI-COR (Lincoln, NE). Cross-linked collagen-related peptide (CRP-XL) was purchased from CambCol Ltd (Cambridge, United Kingdom). The CLEC-2 activating antibody was purchased from BioLegend (San Diego, CA), and the donkey anti-rat immunoglobulin was purchased from Novex (San Diego, CA). AYPGKF peptide was purchased from GenScript (Piscataway, NJ). ChromoTek Ubiquitin-trap magnetic agarose (anti-ubiquitin immunoprecipitating reagent) was purchased from Proteintech (Rosemont, IL).
Generation and housing of Syk(Y317F) mice
Syk(Y317F) KI mice were generated using the CRISPR/Cas9 technique on the C57BL/6 background by Taconic/Cyagen (Santa Clara, CA). The Syk Y317F mutation was confirmed by both sequencing and digesting the polymerase chain reaction (PCR) product with the restriction enzyme, AdhI. The oligonucleotides used for PCR are forward primer 5ʹ-TGAAGAATGAGATGAGCCTTTCTGA-3ʹ and reverse primer 5ʹ-TAGACAGGACTGCTTGTTTTATGGA-3ʹ. Mice were housed in a specific pathogen-free facility, and all animal procedures were approved by the Temple University Institutional Animal Care and Use Committee (protocol no. 4864).
Platelets preparation
Mouse blood was collected, and platelets were isolated as described previously.47 The isolated platelets were counted using a Hemavet 950FS blood cell analyzer, and adjusted to a final concentration of 1.5 × 108 cells per mL in a modified Tyrode’s solution (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 10 mM HEPES [N-2-hydroxyethylpiperazine-Nʹ-2-ethanesulfonic acid], 0.1% glucose, and 0.2 U/mL apyrase, pH 7.4). All platelet aggregation and secretion experiments were carried out using a lumi-aggregometer (Chrono-log) at 37°C under stirring conditions. Platelet aggregation was measured using light transmission, and ATP secretion was measured using Chrono-lume (a luciferin/luciferase assay).
Western blotting
Western blotting procedures were performed as described previously.47 Briefly, platelets were stimulated for 3 minutes with CRP or for 5 minutes with the CLEC-2 antibody with stirring at 37°C. The reaction was stopped by precipitating the platelet proteins with HClO4 at a final concentration of 0.6 N. The pellet was washed once with deionized water before the addition of sample loading buffer. Platelet protein samples were then boiled at 95°C for 5 minutes before resolution by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes using an iBlot (ThermoFisher) for 10 minutes at 20 V. Membranes were blocked with Prometheus OneBlock Western-FL blocking buffer (Genesee Scientific) and incubated overnight at 4°C with primary antibodies against the indicated protein. The membranes were then washed 3 × 10 minutes with Tris-buffered saline containing 0.1% Tween-20 before incubation with appropriate secondary antibodies for 1 hour at room temperature. The membranes were washed again as previously described and scanned using a LI-COR Odyssey infrared imaging system.
Ubiquitin-trap assay
Platelets were isolated from Syk(Y317F) mice or WT littermates as previously described. The platelet count was adjusted to 0.6 × 109/mL. Samples (200 μL) were incubated with 1 μg/mL CRP or vehicle for 60 seconds at 37°C with stirring. The reaction was stopped by addition of 50 μL of 5 × TNE lysis buffer (1 × 10 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1% NP-40) containing protease/phosphatase inhibitors. Samples were rotated at +4°C for 30 minutes, and centrifuged at 12 000g/+4°C for 10 minutes. Supernatants were transferred to clean tubes, and 25 μL of gently resuspended ubiquitin-trap magnetic beads, containing nanobodies to ubiquitin, were added, and samples were rocked for 1 hour at 4°C. The beads were isolated by magnet, and 30 μL of the supernatant was mixed with 10 μL of 4 × sample buffer (unbound) before the beads being washed 3×. Ubiquitylated proteins were solubilized with 50 μL of 2 × sample buffer (bound). Samples were boiled for 5 minutes before protein resolution on 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Western blotting was performed as previously described.
The proportion of ubiquitylated Syk to nonubiquitylated Syk was quantified in the following way: the intensity of all Syk p346-reactive bands over background was determined using LI-COR scanning. The intensity of the lowest band, which was the major pY346-reactive band and comigrated with the total Syk band, was considered a measure of the nonubiquitylated form’s amount. The total intensity of all pY346-reactive bands running above the major band of nonubiquitylated Syk was taken as a measure of the total amount of ubiquitylated forms. The ratio was determined by division. This operation was not performed for the unstimulated/control sample because the Syk pY346 signal was very low both for the lowest, nonubiquitylated band and for the higher-molecular-weight ubiquitylated bands, as expected for unstimulated platelets.
Flow cytometry
Isolated platelets (106/100 μL Tyrode’s buffer per test) were stained for GPVI using 5 μL anti-GPVI (catalog no. M011-1; Emfret analytics) or isotype control immunoglobulin G–fluorescein isothiocyanate (catalog no. P190-1; Emfret Analytics), kept at room temperature in the dark for 15 minutes, and then fixed with 1% paraformaldehyde in phosphate-buffered saline.
To determine platelet P-selectin exposure or αIIbβ3 activation after stimulation with different concentrations of CRP and CLEC-2 antibody, isolated platelets were diluted in Tyrode’s buffer containing 1 mM CaCl2 (106 per test), then stimulated with the agonist for 15 minutes at room temperature. The platelets were then stained with anti-CD41 (catalog no. 133914; BioLegend), anti–CD62P P-selectin fluorescein isothiocyanate (catalog no. D200; Emfret Analytics), and anti–JON/A-PE (catalog no. D200; Emfret Analytics) at room temperature for 15 minutes in the dark. The reactions were stopped by addition of 500 μL 1% paraformaldehyde in phosphate-buffered saline to each tube.
For measuring annexin V binding, isolated platelet samples were diluted in 1 × annexin V binding buffer (catalog no. 51-66121E; BD Pharmingen) with 2 mM MgCl2 and 0.2 U/mL apyrase, then stimulated for 20 minutes at room temperature with different concentrations of CRP. The isolated platelets (106/100 μL buffer) were stained with anti–CD41-BUV395 (catalog no. 752966; BD Bioscience) and Alexa Fluor 647 Annexin V (catalog no. 567356; BD Pharmingen). The reactions were stopped by addition of 1 mL of 1× annexin V binding buffer, and samples were acquired immediately with 50 000 genuine platelets events recorded based on CD41 expression.
FACSymphony A5 flow analyzer was used for data acquisition, and FlowJo was used for flow analysis.
Tail-bleeding assay
Tail bleeding times were determined according to the procedure described by Patel et al.48 Mice aged 4 to 6 weeks were anesthetized before amputation of the distal 3 mm of the tail. The tail was then immersed in 37°C saline, and bleeding was monitored. The bleeding was halted manually by applying pressure if it continued longer than 600 seconds.
Carotid artery injury
FeCl3 was used to injure the carotid artery as previously described.48 Mice aged 10 to 12 weeks were anesthetized, and the carotid artery was exposed. A baseline blood flow reading was obtained using a Transonic T402 flow meter. The carotid artery was injured using a 1 × 1 mm piece of filter paper saturated with 7.5% FeCl3 for 90 seconds. The filter paper was removed, and blood flow was recorded.
Statistics
Data are presented as mean ± standard error of the mean of at least 3 independent experiments. Means were compared by Mann-Whitney U test for comparison of 2 groups, 1-way analysis of variance for comparison of >2 groups, and 2-way analysis of variance for comparison of different groups with different factors, followed by Tukey post hoc multiple comparison test using GraphPad Prism, version 10 (GraphPad Software, Inc, San Diego, CA). A P value <.05 was considered statistically significant.
The study was approved by the Institutional Animal Care and Use Committee at Temple University.
Results
Generation and characterization of Syk Y317 KI mice
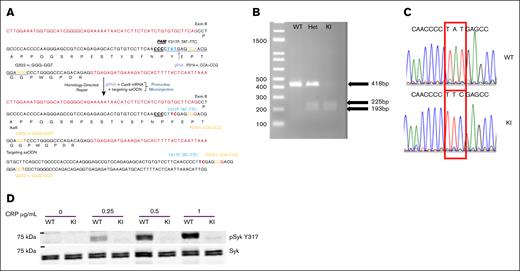
To determine the role of Syk Y317 phosphorylation in ITAM receptor–mediated platelet signaling and responses, we generated a Syk(Y317F) KI mouse using the CRISPR/Cas9 approach. The Syk Y317F mutation was confirmed by both sequencing and digesting the PCR product with the restriction enzyme AdhI. After digestion, the following products were detected: WT (+/+) 418 base pairs (bp); heterozygous (+/−) 225, 193, and 418 bp; and homozygous KI (−/−) 225 and 193 bp (Figure 1A). In addition, Sanger sequencing of PCR products of WT and Y317F homozygous mouse DNA shows that the relevant codon has been mutated from TAT to TTC, changing a tyrosine to a phenylalanine, as shown in Figure 1B. To confirm the Y317F mutation at the protein level, we isolated platelets from Syk(Y317F) and WT littermate mice, stimulated them with different concentrations of CRP, and then evaluated the phosphorylation of Y317 using western blot analysis. As shown in Figure 1C, there was no phosphorylation of Syk Y317 in samples obtained from Syk(Y317F) platelets treated with any concentration of CRP, whereas WT samples exhibit a robust response. Together, these data demonstrated that we successfully generated Syk(Y317F) KI mice. Both heterozygous and homozygous Syk(Y317F) mice bred normally and produced pups at expected Mendelian ratios. Blood cell counts were not altered in Syk(Y317F) KI mice (Table 1). This represents the first in vivo model harboring a mutation at the Y317 site of Syk.
Generation of Syk Y317 KI mice. (A) The process of creating the mutant mouse via CRISPR is schematically presented. The targeted DNA codon is changed from TAT to TTC, changing the amino acid from tyrosine to phenylalanine. (B) Restriction enzyme AdhI digestion of PCR products from Syk(Y317F) homozygous KI(SykY317F/Y317F), Syk(Y317F) Het(SykY317F/WT), and WT littermate control(SykWT/WT) DNA. The oligonucleotides used for PCR are forward primer 5ʹ-TGAAGAATGAGATGAGCCTTTCTGA-3′ and reverse primer 5ʹ-TAGACAGGACTGCTTGTTTTATGGA-3′. WT PCR product is 418bp, whereas KI products are 225 and 193 bp. (C) Sanger sequencing of PCR products of WT and Syk(Y317F) homozygous mouse DNA shows that a DNA codon changed from TAT to TTC, changing the tyrosine amino acid to phenylalanine. (D) A representative western blot reveals the absence of phosphorylated Syk Y317 in Syk(Y317F) mouse platelets compared with WT platelets after stimulation with CRP at the concentrations indicated for 3 minutes. Het, heterozygous.
Generation of Syk Y317 KI mice. (A) The process of creating the mutant mouse via CRISPR is schematically presented. The targeted DNA codon is changed from TAT to TTC, changing the amino acid from tyrosine to phenylalanine. (B) Restriction enzyme AdhI digestion of PCR products from Syk(Y317F) homozygous KI(SykY317F/Y317F), Syk(Y317F) Het(SykY317F/WT), and WT littermate control(SykWT/WT) DNA. The oligonucleotides used for PCR are forward primer 5ʹ-TGAAGAATGAGATGAGCCTTTCTGA-3′ and reverse primer 5ʹ-TAGACAGGACTGCTTGTTTTATGGA-3′. WT PCR product is 418bp, whereas KI products are 225 and 193 bp. (C) Sanger sequencing of PCR products of WT and Syk(Y317F) homozygous mouse DNA shows that a DNA codon changed from TAT to TTC, changing the tyrosine amino acid to phenylalanine. (D) A representative western blot reveals the absence of phosphorylated Syk Y317 in Syk(Y317F) mouse platelets compared with WT platelets after stimulation with CRP at the concentrations indicated for 3 minutes. Het, heterozygous.
Enhanced GPVI-mediated aggregation and secretion in Syk(Y317F) platelets
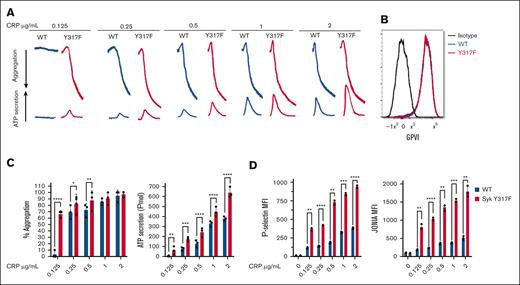
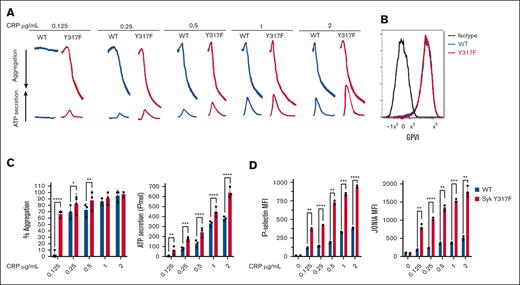
To evaluate the effect of Syk(Y317F) on GPVI-mediated aggregation and secretion, we stimulated isolated platelets from Syk(Y317F) and WT littermate mice with different concentrations of CRP. Our results indicated that there was no aggregation or ATP secretion in WT platelets at very low concentrations of CRP, whereas Syk(Y317F) platelets aggregated and secreted under this condition (Figure 2A,C). Furthermore, Syk(Y317F) platelets showed a significant increase in ATP secretion in a concentration-dependent manner compared with platelets from WT littermates (Figure 2A,C). As these differences could occur because of altered expression of GPVI on platelets in Syk(Y317F) platelets, we evaluated the GPVI expression on WT and Syk(Y317F) isolated platelets using flow cytometry. The result showed no significant difference between the 2 groups (Figure 2B). We also evaluated CRP-induced P-selectin exposure, which indicates α-granule release, and JON/A antibody binding to αIIbβ3, which indicates activation of this integrin. The results shown in Figure 2D indicate that both P-selectin and JON/A levels are increased by CRP stimulation in a concentration-dependent manner in Syk(Y317F) platelets as compared with WT control platelets. We evaluated the platelet function in Syk(Y317F) mice after stimulation of the G-protein–coupled receptor protease-activated receptor 4 with AYPGKF or the purinergic receptors P2Y1 and P2Y12 with 2-methylthioadenosine diphosphate. Syk(Y317F) platelets respond normally to these agonists (supplemental Figure 1).
Enhanced GPVI-mediated aggregation and secretion in Syk(Y317F) platelets. (A) Representative aggregation and secretion tracings of Syk(Y317F) and WT control platelets after stimulation with the indicated concentrations of CRP. (B) Expression of GPVI on CD41+ cells is shown as a flow histogram. Staining of Syk(Y317F) and WT platelets with fluorescein isothiocyanate (FITC)–conjugated anti-GPVI is compared with isotype control FITC-labeled immunoglobulin G1. (C) Quantification of aggregation (left) and ATP secretion (right). (D) Quantification of P-selectin (left) and JON/A (right) expression. Results are depicted as mean ± standard error of the mean (SEM), and differences are analyzed using 2-way analysis of variance (ANOVA) with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Enhanced GPVI-mediated aggregation and secretion in Syk(Y317F) platelets. (A) Representative aggregation and secretion tracings of Syk(Y317F) and WT control platelets after stimulation with the indicated concentrations of CRP. (B) Expression of GPVI on CD41+ cells is shown as a flow histogram. Staining of Syk(Y317F) and WT platelets with fluorescein isothiocyanate (FITC)–conjugated anti-GPVI is compared with isotype control FITC-labeled immunoglobulin G1. (C) Quantification of aggregation (left) and ATP secretion (right). (D) Quantification of P-selectin (left) and JON/A (right) expression. Results are depicted as mean ± standard error of the mean (SEM), and differences are analyzed using 2-way analysis of variance (ANOVA) with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CRP-mediated signaling is enhanced in Syk(Y317F) platelets
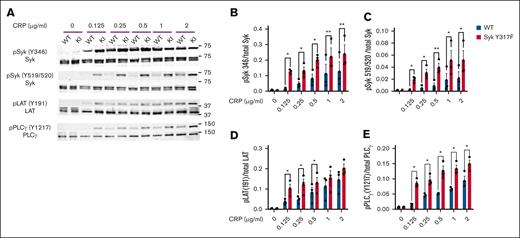
To evaluate GPVI signaling through the Syk-dependent pathway, we analyzed phosphorylation of the main proteins involved in the signaling cascade. Our results show higher phosphorylation of Syk Y346, Syk Y519/520, LAT Y191, and PLCγ2 Y1217 in Syk(Y317F) platelets compared with WT platelets (Figure 3). These data indicate that signaling downstream of GPVI is enhanced in Syk(Y317F) platelets, correlating well with an increase in the GPVI-induced aggregation and secretion.
CRP-mediated signaling is enhanced in Syk(Y317F) platelets. (A) Representative western blots showing the phosphorylated and total proteins, as indicated, in platelets from Syk(Y317F) and WT littermate mice stimulated with the indicated concentrations of CRP for 3 minutes. (B-E) Quantification of the indicated phosphorylated residues, depicted as the ratio of pY to total protein. pPLCγ, phosphorylated PLCγ.
CRP-mediated signaling is enhanced in Syk(Y317F) platelets. (A) Representative western blots showing the phosphorylated and total proteins, as indicated, in platelets from Syk(Y317F) and WT littermate mice stimulated with the indicated concentrations of CRP for 3 minutes. (B-E) Quantification of the indicated phosphorylated residues, depicted as the ratio of pY to total protein. pPLCγ, phosphorylated PLCγ.
Enhanced CLEC-2–mediated aggregation and secretion in Syk(Y317F) platelets
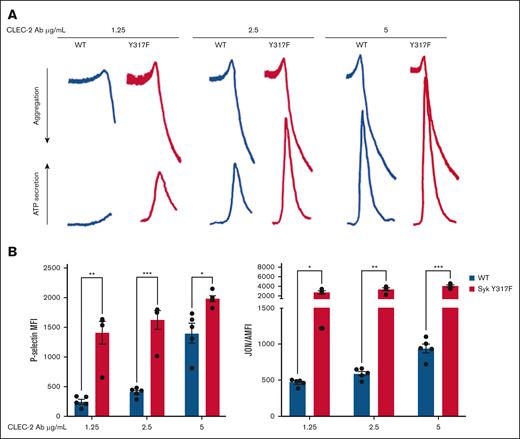
We also investigated a role of Syk Y317 phosphorylation in hemITAM-dependent signaling that is initiated through the CLEC-2 receptor by its cross-linking with different concentrations of an anti–CLEC-2 monoclonal antibody. Potentiation of responses, including aggregation, α-granule secretion, dense granule secretion, and integrin activation, were seen in the Syk(Y317F) platelets as compared with the WT platelets (Figure 4); this enhancement was similar to what was observed in GPVI-stimulated platelets receptor (Figure 2).
Enhanced CLEC-2–mediated aggregation and secretion in Syk(Y317F) platelets. (A) Representative aggregation and secretion tracings of Syk(Y317F) and WT control platelets stimulated with the indicated concentration of CLEC-2 antibody. (B) P-selectin (left), and JON/A (right) expression after CLEC-2 antibody stimulation. Results are analyzed and presented as described in the legend to Figure 2. MFI, median fluorescence intensities.
Enhanced CLEC-2–mediated aggregation and secretion in Syk(Y317F) platelets. (A) Representative aggregation and secretion tracings of Syk(Y317F) and WT control platelets stimulated with the indicated concentration of CLEC-2 antibody. (B) P-selectin (left), and JON/A (right) expression after CLEC-2 antibody stimulation. Results are analyzed and presented as described in the legend to Figure 2. MFI, median fluorescence intensities.
CLEC-2–mediated signaling is enhanced in Syk(Y317F) platelets
Similar to evaluating Syk signaling cascade downstream of GPVI, an ITAM-bearing receptor, we analyzed phosphorylation of the main signaling proteins involved in the pathway downstream of CLEC-2. After activation of the CLEC-2 receptor, there was a significant increase in phosphorylation of Syk Y346, Syk Y519/520, LAT Y191, and PLCγ2 Y1217 in Syk(Y317F) platelets compared with WT platelets (Figure 5).
CLEC-2–mediated signaling is enhanced in Syk(Y317F) platelets. (A) Representative western blots showing the phosphorylated and total proteins in Syk(Y317F) and WT littermate control platelets stimulated with the indicated concentrations of CLEC-2 antibody. (B-E) Quantification of the indicated phosphorylated residues, depicted as the ratio of pY to total protein.
CLEC-2–mediated signaling is enhanced in Syk(Y317F) platelets. (A) Representative western blots showing the phosphorylated and total proteins in Syk(Y317F) and WT littermate control platelets stimulated with the indicated concentrations of CLEC-2 antibody. (B-E) Quantification of the indicated phosphorylated residues, depicted as the ratio of pY to total protein.
In vivo thrombus formation is enhanced in Syk(Y317F) KI mice, whereas hemostasis is not affected
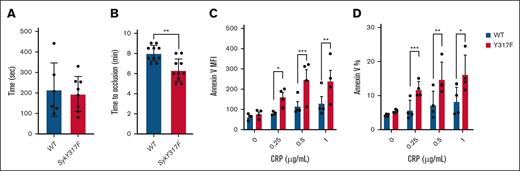
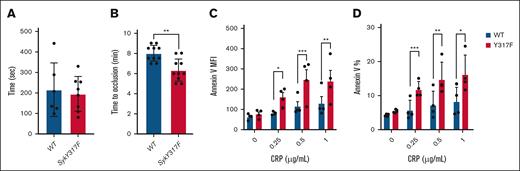
To address the impact of Syk(Y317F) on hemostasis, we performed tail-bleeding assay on litter-matched WT and Syk(Y317F) mice. Our data showed no significant difference in the cessation of bleeding between Syk(Y317F) and WT mice, indicating that Syk Y317 phosphorylation exhibits no impact on hemostasis (Figure 6A). To determine the impact of impaired phosphorylation of Syk Y317 on the thrombus formation, we used a FeCl3-injury thrombosis model, monitoring the time that is needed for occlusion of the injured carotid artery in Syk(Y317F) and WT littermate mice. Syk(Y317F) mice formed a stable occlusion in a statistically significant shorter time than WT mice did (Figure 6B). These data suggest that the potentiation of GPVI- and CLEC-2–mediated signaling and biological responses in Syk(Y317F) platelets enhance the in vivo thrombus formation, a platelet-driven process.
In vivo thrombus formation is enhanced in Syk(Y317F) KI mice with increased annexin V binding compared with WT. (A) Scatter dot plot shows the time it took for bleeding to stop during tail bleeding experiments conducted on Syk(Y317F) and WT littermate control mice 4 to 6 weeks of age in a blind fashion. (B) Scatter dot plot of the time to occlusion in WT and Syk(Y317F) mice following 7.5% FeCl3 injury on the carotid artery. Normality of the data was assessed using Shapiro-Wilk test, and data were found to be normally distributed. Accordingly, P values were determined using unpaired Student t test. (C-D) Bar graphs represent MFI and percentage of annexin V–positive platelets with different doses of CRP stimulation, respectively. Results are depicted as mean ± SEM, and differences are analyzed using 2-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, median fluorescence intensities.
In vivo thrombus formation is enhanced in Syk(Y317F) KI mice with increased annexin V binding compared with WT. (A) Scatter dot plot shows the time it took for bleeding to stop during tail bleeding experiments conducted on Syk(Y317F) and WT littermate control mice 4 to 6 weeks of age in a blind fashion. (B) Scatter dot plot of the time to occlusion in WT and Syk(Y317F) mice following 7.5% FeCl3 injury on the carotid artery. Normality of the data was assessed using Shapiro-Wilk test, and data were found to be normally distributed. Accordingly, P values were determined using unpaired Student t test. (C-D) Bar graphs represent MFI and percentage of annexin V–positive platelets with different doses of CRP stimulation, respectively. Results are depicted as mean ± SEM, and differences are analyzed using 2-way ANOVA with Tukey post hoc test. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, median fluorescence intensities.
Increased annexin V binding in Y317F mice compared with WT
Measuring annexin V binding as a marker of phosphatidylserine exposure, which is representative of platelet procoagulant activity, we showed that there were no differences between resting platelets in both groups. However, after stimulation with different concentrations of CRP, both florescence of annexin V and percentages of annexin V–positive platelets (Figure 6C-D, respectively) were higher in Syk(Y317F) platelets compared with WT platelets.
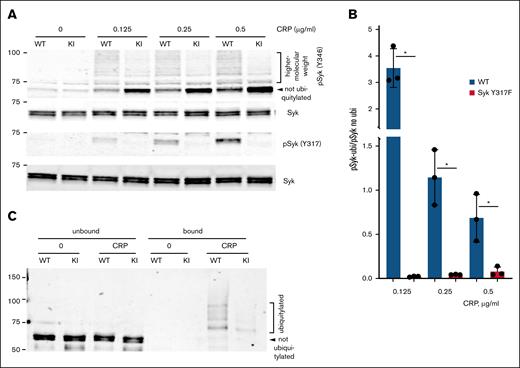
Syk ubiquitylation is reduced in Syk(Y317F) KI mice
Because several studies suggested that phosphorylated Syk Y317 binds to Cbl tyrosine kinase–binding domain, and this binding is critical for Cbl-dependent Syk ubiquitylation,41,42 we analyzed the Syk ubiquitylation in Syk(Y317F) and WT control platelets after stimulation with different concentrations of CRP. First, we examined the ladder-like pattern of higher-molecular-weight species of activated Syk indicative of its ubiquitylation using western blotting of Syk pY346 as a marker. This experiment showed that the amount of higher-molecular-weight species induced by CRP stimulation, and especially the ratio between these species and nonubiquitylated Syk, is significantly lower for Syk(Y317F) (Figure 7A-B), suggesting that the Y317F mutation reduces Syk ubiquitylation. Note that a decrease seen in Figure 7B relates to the ratio, not to the total amount of Syk pY346, which is gradually increased in both WT and Syk(Y317F) in a CRP concentration-dependent manner (Figure 3A-B). Despite this decrease, the ubiquitylated-to-nonubiquitylated ratio in WT always exceeds that in Syk(Y317F) by at least an order of magnitude. We further analyzed the difference between WT and mutated Syk using ubiquitin-trap magnetic agarose and western blotting for Syk; this experiment also showed a dramatic decrease in ubiquitylation of Syk(Y317F) compared with WT Syk (Figure 7C). Together, these results indicate that phosphorylation of Syk Y317 plays a major role in the stimulation-induced ubiquitylation of Syk in platelets.
Syk ubiquitylation is reduced in Syk(Y317F) KI mice. (A) Representative western blots showing Syk pY346 in Syk(Y317F) (KI) and WT littermate control platelets stimulated various concentrations of CRP for 3 minutes at 37°C with stirring. (B) The ratios of the total intensity of high-molecular-weight species (pSyk-ubi) to the intensity of nonubiquitylated pSyk (pSyk no ubi) were quantified, analyzed, and presented as described in the “Materials and methods.” The ratio in nonstimulated platelets was not analyzed, as all intensities were close to 0. (C) Syk(Y317F) and WT littermate control platelets were stimulated with 1 μg/mL CRP for 60 seconds, the ubiquitylated proteins from their lysates were immunoprecipitated with the ubiquitin-trap reagent and immunoblotted for Syk, as described in the “Materials and methods.” A representative western blot of 2 experiments is presented.
Syk ubiquitylation is reduced in Syk(Y317F) KI mice. (A) Representative western blots showing Syk pY346 in Syk(Y317F) (KI) and WT littermate control platelets stimulated various concentrations of CRP for 3 minutes at 37°C with stirring. (B) The ratios of the total intensity of high-molecular-weight species (pSyk-ubi) to the intensity of nonubiquitylated pSyk (pSyk no ubi) were quantified, analyzed, and presented as described in the “Materials and methods.” The ratio in nonstimulated platelets was not analyzed, as all intensities were close to 0. (C) Syk(Y317F) and WT littermate control platelets were stimulated with 1 μg/mL CRP for 60 seconds, the ubiquitylated proteins from their lysates were immunoprecipitated with the ubiquitin-trap reagent and immunoblotted for Syk, as described in the “Materials and methods.” A representative western blot of 2 experiments is presented.
Discussion
It has been shown that the regulation of Syk function is dependent on phosphorylation of multiple tyrosines, which modify Syk activity through conformational changes and/or serve as docking sites for proteins that contain SH2 or other phosphotyrosine-recognition domains.2,3 Syk Y317 is one of the important regulatory tyrosine residues that significantly regulate Syk signaling in various immune cells, including B cells and mast cells.31,32,34,40 This role of Syk pY317 is based on its binding to Cbl tyrosine kinase–binding domain, which is required for Cbl-dependent ubiquitylation of Syk causing downregulation of this PTK.30,40,41 In platelets, it has been shown that Cbl downregulates tyrosine phosphorylation of Syk, and that Syk is ubiquitylated.46,49 However, the role of Syk Y317 in the regulation of Syk functions in platelets remained unknown mostly because the key approach used to examine the role of Syk pY317 in other cells, namely expression of a mutated version of Syk using transfection or transduction, cannot be accomplished in platelets. We have overcome this limitation by generating KI mice expressing Syk(Y317F), which are overall normal and fertile. Platelets from these mice exhibit responses to multiple biological relevant agonists acting through various receptors. We observed a substantial difference in the intensity of these responses and receptor signaling between WT and Syk(Y317F) platelets when ITAM and hemITAM receptors were activated.
Our study provides evidence that the Syk Y317F mutation enhances most platelet responses examined in the entire concentration range for both agonists stimulating Syk-dependent platelet activation, CRP and anti–CLEC-2 antibody. A high concentration of agonist does not compensate for the reported differences between WT and Syk(Y317F). The only exception is aggregation, which is significantly enhanced only at low-to-moderate agonist concentrations.
The effect of Syk(Y317F) on physiological responses of platelets in vitro is consistent with its effect on receptor signaling; phosphorylation of the Syk Y519/520, a well-characterized marker of Syk activity,50-53 and PLCγ2, an enzyme critical for Syk-mediated signaling in several types of hematopoietic cells,54,55 is increased in stimulated Syk(Y317F) platelets in the wide range of agonist concentrations. Interestingly, this effect is also observed for Syk Y346, a site considered mostly a target for Src family kinases (SFKs)56,57; because Syk Y346 is also a target of Syk autophosphorylation.58 Taken together, these results unequivocally indicate that Syk Y317, like in other hematopoietic cells, acts as a negative regulatory site in platelets.
Notably, Syk(Y317F) enhances signaling and platelet activation by both CRP and CLEC-2 stimulating through the receptors bearing ITAMs and hemITAM, respectively, but not by thrombin and purinergic receptor agonists, which belong to the G-protein–coupled receptors group. This finding argues that the Y317F mutant universally affects all Syk-dependent signaling pathways, but not those largely independent of Syk.
The effects of Syk(Y317F) on platelet signaling and physiological responses in vitro are consistent with its effect in vivo. The FeCl3-injury model demonstrates a significant enhancement of thrombosis manifested by a decrease in time needed for occlusion of the carotid artery, a platelet-driven process. Although tail bleeding shows no significant change in the bleeding time, it should be noted that hemostasis is a well-balanced process with many compensatory mechanisms that may be independent of ITAM or hemITAM signaling and may even be unrelated to platelets, being mediated by the coagulation system. It appears that severe platelet dysfunction can be detected in the tail bleeding times, but enhanced platelet function might be difficult to detect in this assay as it is expected to reduce the bleeding time from 1.6 minutes in a statistically significant way. Thus, we are not surprised that we did not see a defect in hemostasis. Consistent with enhanced signaling downstream of GPVI receptors, we have observed increased annexin V binding in Y317F mice.
The difference we observed between WT and Syk(Y317F) regarding platelet signaling and responses is consistent with the role of Syk Y317 in ubiquitylation of this PTK shown previously in other hematopoietic systems41,42,59; ubiquitylation of Syk is significantly decreased in Syk(Y317F) platelets. Some residual ubiquitylation of Syk(Y317F) might be explained in several ways: (1) Cbl continues interacting with Syk through an alternative mechanism,60 (2) through a protein binding to both Cbl and Syk,61-64 (3) Syk is ubiquitylated by Cbl-b,65 or (4) by an entirely different non–Cbl E3 ubiquitin-protein ligase. However, a significant decrease in the ubiquitylation of Syk(Y317F) compared with WT Syk definitively indicates a key role of Y317F in the process of Syk ubiquitylation in platelets.
To summarize, our study shows that Syk Y317 is phosphorylated downstream of ITAM- and hemITAM-bearing platelet receptors, and that the resulting Syk pY317 is a negative regulatory site for Syk and the entire Syk-dependent signaling pathway. The inhibitory effect of Syk pY317 on signaling is relevant for platelet physiological responses as indicated experiments both in vitro and in vivo. This finding is key for asserting the general nature of this regulatory mechanism; it has already been shown in several cells of hematopoietic origin, but platelets are anucleate and differ substantially from these cells by the type of functional responses and the applicability of standard cell biology experimental approaches. Because our results have been obtained in primary cells expressing mutant Syk at a natural level and are not affected by any transfection/transduction procedures, they clearly reflect the physiological function of Syk Y317 in platelets, and strongly argue in favor of the ubiquitous role of this site in the regulation of Syk in hematopoietic cells.
Acknowledgment
This work was supported by grant R35 HL155694 from the National Institutes of Health (S.P.K.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: All authors significantly contributed to the manuscript and read and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Sol Sherry Thrombosis Research Center, Lewis Katz School of Medicine, Temple University, 3420 N. Broad St, Room 418 MRB, Philadelphia, PA 19140; email: spk@temple.edu.
References
Author notes
Original data are available on request from the corresponding author, Satya P. Kunapuli (spk@temple.edu).
The full-text version of this article contains a data supplement.