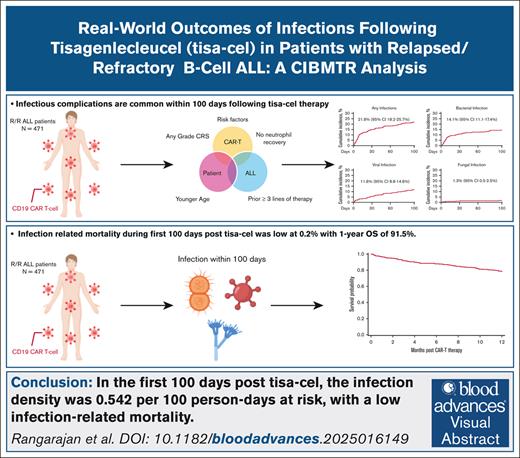
Key Points
In 471 patients with B-ALL, by day 100 after tisa-cel, the infection density was 0.542 per 100 person-days at risk.
Risk factors for infections were ≥3 prior lines of therapy, any-grade cytokine release syndrome, and lack of neutrophil recovery.
Visual Abstract
Tisagenlecleucel (tisa-cel) is a CD19-directed chimeric antigen receptor T-cell therapy for relapsed/refractory precursor B-cell acute lymphoblastic leukemia (R/R B-ALL). We report infectious complications for 100 days (D100) following tisa-cel therapy in 471 pediatric and young adults (median age 13.8 years) with R/R B-ALL reported from September 2017 to June 2022. By D100, 137 (29%) patients had an infectious event, with an infection density of 0.542 per 100 person-days at risk. D100 cumulative incidences of bacterial, viral, and fungal infections were 14.1%, 11.6%, and 1.3%, corresponding to infection density scores of 0.296, 0.213, and 0.033 per 100 person-days at risk, respectively. In a multivariable analysis, receipt of ≥3 lines of therapy before tisa-cel (hazard ratio [HR], 1.86; 95% confidence interval [CI], 1.13-3.08; P = .015), any-grade cytokine release syndrome (HR, 1.78; 95% CI, 1.17-2.71; P = .007), and lack of neutrophil recovery (HR, 2.63; 95% CI, 1.47-4.69; P = .001) were associated with an increased risk for any infection. Similar associations were observed for bacterial infections, with the addition of younger age as an adverse risk (<6 vs 6-15 years; HR, 2.38; 95% CI, 1.23-4.61; P = .01). Risk factors for viral infections included increasing age (1-year increase; HR, 1.05; 95% CI, 1.01-1.09; P = .016), prior history of any infection (HR, 2.76, 95% CI, 1.40-5.46; P = .004), and prior hematopoietic cell transplant (HR, 2.10; 95% CI, 1.18-3.71; P = .011). D100 infection-related mortality (IRM) rate was low at 0.2% (95% CI, 0.0-0.8). In this multicenter real-world study, we observed a high incidence of infectious complications but a low IRM following tisa-cel for R/R B-ALL.
Introduction
Adoptive chimeric antigen receptor (CAR) T-cell immunotherapy is a viable treatment option for patients with refractory/relapsed (R/R) B-cell malignancies, and its use is expected to increase with expanding indications in autoimmune diseases and other malignancies.1-4 Tisagenlecleucel (tisa-cel), the first United States Food and Drug Administration-approved CAR T-cell therapy product, targets CD19,5-7 a surface antigen universally expressed on most B-cell malignancies. Following tisa-cel therapy, an overall response rate ranging between 73% and 87% has been observed in pediatric and adolescent and young adult patients with R/R precursor B-cell acute lymphoblastic leukemia (B-ALL).5-8 However, CAR T-cell therapies have associated complications. While toxicities like cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are well studied,9 infectious complications have been primarily described through retrospective, single-center studies, or reported as part of clinical trials.5,10-14 Among B-cell malignancies, individuals with B-ALL are reported to have the highest risk of infections after CD19 CAR T-cell therapies.10,11 Postulated risk factors for infections after CD19 CAR T-cell therapy include pretreatment factors, such as impaired immune function due to primary malignancy and cytotoxic therapies, and post-treatment factors, like CRS and associated use of steroids and tocilizumab, need for intensive care, and lastly prolonged cytopenia, including B-cell aplasia and associated hypogammaglobulinemia.15-19
In a pooled safety analysis of 137 patients enrolled in clinical trials, infections occurred in 43% of patients, with grade 3 to 4 infections occurring in 19% of patients through 8 weeks after CD19 CAR T-cell therapy.5,14 Single-center retrospective studies have reported infectious complications in 20% to 40% of patients within the first month after CAR T-cell therapy, despite antimicrobial prophylaxis.10-13 However, multicenter, real-world studies detailing infectious complications following CD19 CAR T-cell therapy are lacking. Given the rapid advancement and successful application of CD19 CAR T-cell immunotherapy for B-cell malignancies, a critical need exists to determine the scope of infectious complications, and to define strategies to mitigate these potentially life-threatening events. Therefore, we studied the epidemiology, risk factors, and impact of infectious complications during the first 100 days (D100) after tisa-cel in a large cohort of pediatric and young adult patients with R/R B-ALL reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). To our knowledge, this is the largest study to date reporting on infectious complications after CD19 CAR T-cell therapy in these patients with R/R B-ALL.
Methods
Study design and data source
CIBMTR is a research collaboration between the Medical College of Wisconsin and NMDP (formerly known as the National Marrow Donor Program), comprised of a working group of >360 participating centers worldwide that contribute detailed data on hematopoietic cell transplant (HCT) and other cellular therapies. Participating centers in the United States are required to report data on allogeneic HCTs to the Stem Cell Therapy Outcomes Database, while reporting for other cellular therapies is voluntary.20 Studies conducted by CIBMTR are performed in compliance with all applicable United States federal regulations pertaining to the protection of human research participants. Patients participating in CIBMTR-related research have been consented with such research approved by the NMDP Institutional Review Board.
Data collection
Patients who received commercial tisa-cel at participating CIBMTR centers from 1 September 2017 to 30 June 2022 were identified. About 95% (n = 195) of United States transplant centers voluntarily report CAR T-cell therapy data to the CIBMTR. Only patients who provided consent and with complete follow-up data were included. Patients with no prior therapy reported (n = 5), with inadequate follow-up (n = 9), and who received tisa-cel under post-authorization safety study (n = 61) during the study period were excluded (supplemental Figure 1). For patients who received >1 CAR T-cell therapy infusion, only infection events occurring after the first CAR T-cell therapy infusion and before the second CAR T-cell therapy infusion were captured. Data on patient, disease characteristics and CAR T-cell therapy-related factors and clinical outcomes, including CRS, ICANS, infectious events, survival, and cause of death, were extracted from the database. Centers were required to enter data on these variables including lines of therapy21 as per the instructions provided in CIBMTR forms manual.
Baseline comorbidities, as defined by the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI), including baseline infections at any time prior to day (D) –10 from tisa-cel infusion, were documented.22 The baseline infection history included a composite record of documented infection, fever of unknown origin, and infection requiring continued antimicrobial therapy beyond D0, as outlined by the HCT-CI, and recorded in the CIBMTR case report form.23 CRS was graded according to the American Society for Transplant and Cellular Therapy (ASTCT) Consensus Grading.9 CAR T-cell therapy-associated toxicity scores (through December 2019)24 and immune effector cell-associated encephalopathy scores (from January 2020) were used to derive ICANS scoring as per ASTCT consensus grading.9,25 Neutrophil recovery, defined as the time at which the absolute neutrophil count reached ≥500/mm3 (or ≥0.5 × 109/L) for 3 consecutive laboratory measurements obtained on separate days, was recorded. Hypogammaglobulinemia was based on the lowest level of immunoglobulin G (IgG) levels recorded following tisa-cel and prior to IV IgG replacement; with cut-offs being <600 mg/dL in patients >10 years of age, and <500 mg/dL in patients aged 4 to 10 years. In patients aged <4 years, hypogammaglobulinemia was recorded by the site if the levels were below the age-normative values CIBMTR.26
Infection data were collected on the CIBMTR comprehensive report forms, with only clinically significant infections (microbiologically documented or clinically documented, or both) requiring treatment being reported.27,28 Infection data included organism, site of infection, and date of onset. No data on prophylaxis, diagnostic methodology, or infection treatment were collected. Viral load and specific information on preemptive protocols for pathogen surveillance were not collected. Analysis of infectious complications was restricted to the first 100 days after CAR T-cell therapy (D100), given the lack of uniform follow-up beyond this time. Data completeness was 100% up to D100.
Statistical analysis
The primary study objective was to describe the D100 incidence, pattern, and outcomes of clinically significant infections in patients with R/R B-ALL. Primary outcomes included infection density and cumulative incidence of infections following tisa-cel infusion. Unique infections with the same organism were required to have positive tests outside prespecified time frames.28 The cumulative incidences of infections at D30, D100, and D100 infection-related mortality (IRM), along with 95% confidence intervals (CIs), were described using the Aalen-Johansen estimator. Infection density for each patient was defined as the total number of infections per patient-days at risk among all patients in the cohort29 (supplemental Methods), and was calculated for bacterial, viral, fungal, and all-cause infections for the time intervals D0 to D30, D31 to D100, and D0 to D100. Relapse/progression, HCT after CAR T-cell therapy, second CAR T-cell therapy infusion, and death were treated as competing risks for the calculations of the cumulative incidence of infection, but not infection densities. Median times of all-cause and type-specific infections were reported among infected patients. Descriptive statistics were provided as mean and standard deviation, or median and interquartile range (IQR) for continuous variables, and percentages for categorical variables. Patient-related, disease-related, and CAR T-cell infusion–related factors were compared among groups using Pearson’s χ2 test for categorical variables, and Kruskal-Wallis/Wilcoxon tests for continuous variables.
Secondary objectives included evaluating risk factors of infection in patients treated with tisa-cel, and determining associations between infectious complications and clinical outcomes following tisa-cel. Secondary outcomes included infection-free survival (IFS), D100 IRM, overall survival (OS), and cause of death. D100 IRM was defined as the cumulative incidence of death directly attributable to infection as either the primary cause or a contributing cause, with relapse and death from noninfectious causes as competing events. IFS was defined as survival after tisa-cel without infection. Surviving patients who were infection-free were censored at the time of the last follow-up. OS was defined as survival from tisa-cel infusion to death from any cause. Surviving patients were censored at the time of the last follow-up. Probabilities of 1-year OS and D100 IFS were described using the Kaplan-Meier estimator. To assess the potential impact of infections on subsequent mortality risk, a landmark analysis was performed for OS at D100 (ie, only patients still at risk at D100 were included in the analysis), with patients classified into 4 groups based on their infection history after CAR T-cell therapy: no infection during D1 to D100, infection during D1 to D30 only, infection during D31 to D100 only, and infections during both D1 to D30 and D31 to D100.
Cox proportional hazards models were used to evaluate potential risk factors impacting OS and the cause-specific hazards of infections and IRM. A stepwise selection procedure was used to identify variables for inclusion in the final models, with a significance level of 0.05 used as the inclusion criterion. Risk factors considered for these multivariable models were age, performance score, history of prior infection (described as part of the HCT-CI), prior lines of treatment for underlying disease (1-2 vs >2), HCT before tisa-cel infusion, disease status (clinical remission [CR] vs no CR), the occurrences of any-grade CRS and ICANS, and neutrophil recovery. CRS, ICANS, and neutrophil recovery were handled as time-dependent covariates. For the landmark model of OS, HCT after CAR T-cell therapy was also considered for inclusion as a time-dependent variable. For candidate variables with missing values, a separate missing category was created for its consideration in variable selection. Two-way interactions between significant variables were tested. The proportional hazard assumptions for each variable were tested, and if the assumption was violated, the variable was added as a time-dependent covariate. The existence of center effects was tested using the score test of Commenges and Andersen.30 When center effects were detected, a marginal Cox model was used to account for the heterogeneity of outcomes between centers.31 Analyses were conducted using SAS 9.4 (Cary, NC) and R version 4.3.0.
Results
Baseline clinical characteristics
A total of 471 patients from 72 participating centers met the study inclusion criteria (Table 1; supplemental Figure 1). The median age of the study cohort was 13.8 years (IQR, 7.8-19.5), with patients equally distributed across 3 age groups (ie, ≤10 years, 11-17 years, and 18-39 years). The majority of patients were males (60.5%), of white race (72.2%), and non-Hispanic and non-Latino ethnicity (51.8%). Most patients (63.5%) had a good performance score (≥90%), and HCT-CI of 0 (41.2%) or 1 to 2 (31.6%). Baseline infection was documented before tisa-cel infusion as part of the HCT-CI in 11.3% of patients. Most patients (59%) had poor risk cytogenetics, and 63% were not in CR at the time of tisa-cel infusion, with only 19% of patients having negative measurable residual disease status before infusion. In this heavily pretreated population, 49% received ≥4 lines of therapy; and 25% received prior HCT, 21% blinatumomab, and 11% inotuzumab before CD19 CAR T-cell therapy. In the HCT group (n = 119), 7 patients had 2 prior allogeneic HCTs before CAR T-cell therapy. HCT details, including donor source and conditioning regimens, are described in supplemental Table 1. Most patients received HCT using myeloablative conditioning (90%), without serotherapy (75%), and grafts from matched siblings (30%) or unrelated donors (22%).
CAR T-cell therapy–associated characteristics
Detailed information for tisa-cel therapy and related events is shown in Table 2. The median time from initial diagnosis of ALL and initiation of last non-HCT therapy to tisa-cel infusion was 31.8 months (IQR, 12.2- 59.9) and 17.9 months (IQR, 5.1-44.5), respectively. The median time from HCT to tisa-cel infusion was 14.5 months (IQR, 9.2-21.3), with many patients (59%) having had HCT ≥12 months before tisa-cel infusion. All but 6 patients received fludarabine and cyclophosphamide-based lymphodepletion prior to CAR T-cell therapy infusion. The median time from start of lymphodepletion therapy to tisa-cel infusion was 6 days (IQR, 6.0-7.0). The median CAR T-cell therapy dose was 1.4 × 108 (IQR, 4.22 × 107 to 5.4 × 108). Neutrophil recovery occurred at a median of 14 days (IQR, 9.0-21.0) following tisa-cel infusion. Any-grade CRS and ICANS were seen in 56% and 9.5% of the study cohort at medians of 5 (IQR, 2.0-7.0) and 7 (IQR, 5.0-9.0) days after infusion, respectively. Severe CRS and ICANS (grade ≥3) affected 18.7% and 7% of patients, respectively. Use of steroids, tocilizumab, anakinra, and siltuximab were documented in 13.2%, 31.4%, 0.6%, and 3.2% of patients, respectively. Through D100, 68% of the patients had hypogammaglobulinemia, and 64% of them received IV IgG therapy. Among patients who received tisa-cel after HCT, a history of acute and chronic graft-versus-host disease was documented in 22.1% and 27.4% of patients, respectively.
Infection data
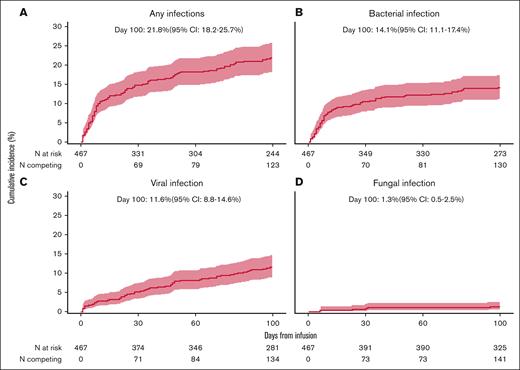
Within the initial 100 days, 137 patients (29.1%) experienced ≥1 episode of infection; resulting in an infection density of 0.542 per 100 person-days at risk (Figure 1; supplemental Figure 2A). This translated into a D100 cumulative incidence of infection of 21.8% (95% CI, 18.2-25.7) (Figure 2A). Multiple infections (>1) were observed in 11.4% of patients. Of the 137 patients who reported infections, two-thirds (n = 92) had an infection by D30 after tisa-cel. By D100, the proportion of patients with bacterial, viral, and fungal infections comprised 19.7% (n = 93), 15.3% (n = 72), and 3.0% (n = 14) of the entire cohort, respectively. In the first 30 days following tisa-cel therapy, the proportion of patients with bacterial infections (13.8%) was higher than that for viral infections (7.5%). However, between D31 and D100, the proportions of patients developing bacterial and viral infections were comparable (8% vs 9.2%). The D100 cumulative incidences of bacterial, viral, and fungal infections were 14.1% (95% CI, 11.1-17.4), 11.6% (95% CI, 8.8-14.6), and 1.3% (95% CI,0.5-2.5), respectively (Figure 2B-D), with corresponding infection densities of 0.296, 0.213, and 0.033 per 100 person-days at risk (Figure 1; supplemental Figure 2A). The infection density for bacterial infection was higher in the first 30 days vs 31 to 100 days (0.179 vs 0.113), whereas the reverse was true for viral infections (0.095 vs 0.117). Infection densities were nearly similar for fungal infections (0.015 vs 0.018) for the 2 periods (Figure 1; supplemental Table 2B-C).
Normalized infection density by organism and time of tisa-cel therapy for R/R B-ALL. Each figure is normalized for the specified nonoverlapping time period.
Normalized infection density by organism and time of tisa-cel therapy for R/R B-ALL. Each figure is normalized for the specified nonoverlapping time period.
Cumulative incidence of infection with competing events following CD19 CAR T-cell therapy for R/R B-ALL. (A) Any infection; (B) bacterial infection; (C) viral infection; (D) fungal infection.
Cumulative incidence of infection with competing events following CD19 CAR T-cell therapy for R/R B-ALL. (A) Any infection; (B) bacterial infection; (C) viral infection; (D) fungal infection.
Details of the D100 infectious complications according to organism subtypes and site of infections are shown in supplemental Tables 3-5. The most frequent pathogens causing bacterial infection were Staphylococcus spp., Clostridioides difficile, Enterococcus sp, Enterobacteriaceae sp, and non-Enterobacteriaceae sp (supplemental Figure 3A). The most frequent sites of bacterial infections were bacteremia (57%) and lower gastrointestinal infection (33.3%; supplemental Figure 3B). Bacterial bloodstream infections (BSIs) occurred in 11.3% of the entire cohort, and Clostridium difficile was the most common organism of lower gastrointestinal infections (supplemental Figure 3C). For viral infections, community-acquired respiratory viruses were the most frequent (63%), followed by non-cytomegalovirus (non-CMV) herpes viruses (25%), and CMV (13.9%; supplemental Figure 4A). The most common sites of viral infections were sinus and/or upper respiratory tract and viremia (supplemental Figure 4B). Fungal infections were most frequently seen in lungs and blood, with mold (50%) accounting for half of the fungal infections (supplemental Table 5).
Risk factors for infection after CD19 CAR T-cell therapy
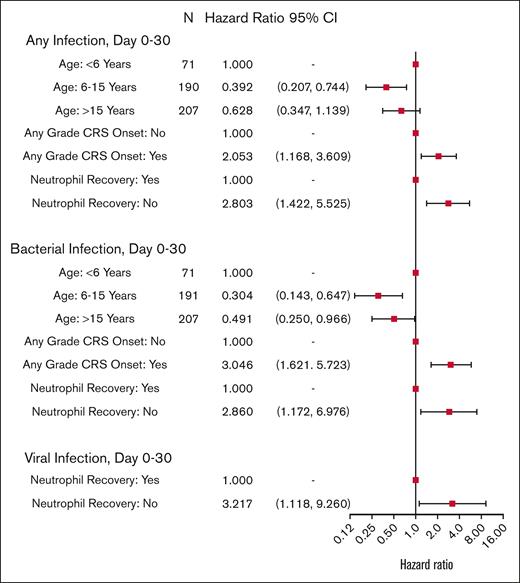
Risk factors for bacterial, viral, and all-cause infection within 30 days after tisa-cel infusion are shown in Figure 3 and supplemental Table 6. Older age was protective against all-cause infections (patient age 6-15 years vs <6 years; [hazard ratio [HR], 0.392; 95% CI, 0.207-0.744]; and patient age >15 years vs <6 years; [HR, 0.628; 95% CI, 0.347-1.139; overall P = .016]), whereas any-grade CRS was associated with increased risk of all-cause infections (HR, 2.053; 95% CI, 1.168-3.609; P = .012). Similar risk factors were observed when the analysis was restricted to bacterial infections alone. Neutrophil recovery was associated with decreased risk for all-cause, bacterial, and viral infections. Risk factors for fungal infection were not assessed due to low incidence within 30 days.
Cox model multivariable analysis of cause-specific hazard rate of infections during D0 to D30 after CAR T-cell therapy.
Cox model multivariable analysis of cause-specific hazard rate of infections during D0 to D30 after CAR T-cell therapy.
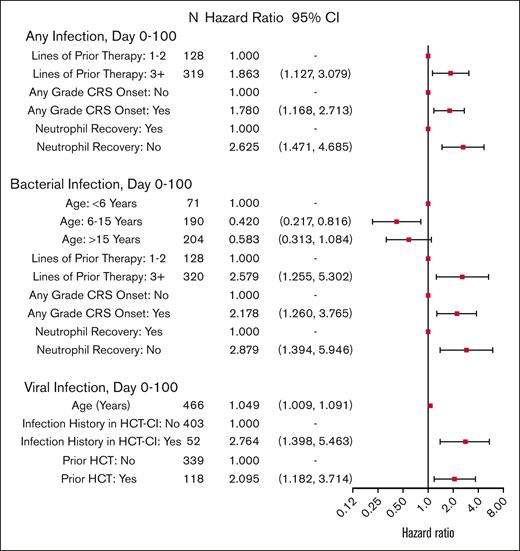
Infection risks through D100 after tisa-cel are shown in Figure 4 and supplemental Table 7. Risk of any infection through D100 was higher in patients who received ≥3 lines of prior therapy than those who received 1 to 2 lines of prior therapy (HR, 1.863; 95% CI, 1.127-3.079; P = .015). Additionally, patients with CRS were more likely to develop an infection than those without CRS (HR, 1.780; 95% CI, 1.168-2.713; P = .007). Risk factors for a bacterial infection were similar, with the addition of older age being protective of bacterial infections (age 6-15 years vs <6 years; [HR, 0.420; 95% CI, 0.217-0.816]; and age >15 years vs <6 years; [HR, 0.583; 95% CI, 0.313-1.084; overall P = .037]). Risk factors for viral infections included older age (1-year increase; [HR, 1.049; 95% CI, 1.009-1.091; P = .016]), an infection history before tisa-cel therapy (HR, 2.764; 95% CI, 1.398-5.463), and prior HCT (HR, 2.095; 95% CI, 1.182-3.714). Center effects were detected for all-cause infections within 30 days, and bacterial infections within 100 days (P = .004); these were accounted for in the multivariable analysis using marginal Cox models.
Cox model multivariable analysis of cause-specific hazard rate of infections during D0 to D100 after CAR T-cell therapy.
Cox model multivariable analysis of cause-specific hazard rate of infections during D0 to D100 after CAR T-cell therapy.
Survival outcomes
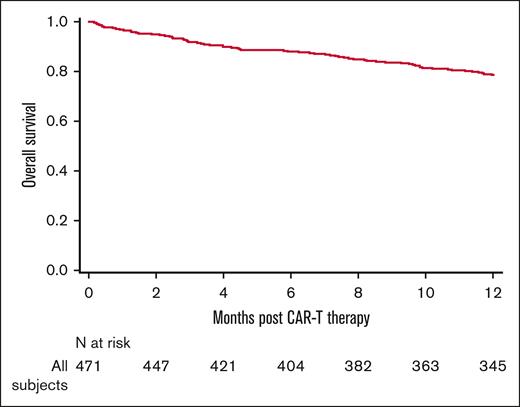
The D100 cumulative incidence of IRM was 0.2% (95% CI, 0-0.8), and the D100 IFS was 70.9% (95% CI, 66.7-75). At a median follow-up of 29.7 months (range 3-59.5 months), D100 OS and 1-year OS were 91.5% (95% CI, 88.8-93.9) and 78.7% (95% CI, 74.9-82.3), respectively (Figure 5). At last follow-up, relapse was the primary cause of death in 50% and infections in 11.7% of patients (supplemental Table 8). In a landmark analysis at D100, infections did not significantly affect overall mortality during the first 100 days after tisa-cel therapy (P = .72; Table 3). Beyond D100 CAR T-cell therapy infusion, age <6 years, performance status ≤80, active disease (non-CR) at tisa-cel infusion, and any-grade CRS onset were factors associated with increased risk of overall mortality in our cohort.
Discussion
Although a promising therapy for patients with hematologic malignancies, CAR T-cell therapy is associated with potentially life-threatening complications, including CRS, ICANS, and infection. Prior studies on infectious complications after CAR T-cell therapies have often included clinical trial participants with heterogeneous diagnoses who have received different CAR T-cell therapy constructs.10,11,13,32 This study is, to the best of our knowledge, the first that includes a comprehensive real-world analysis of infection complications in the largest cohort of pediatric and young adult patients with B-ALL receiving tisa-cel. Infections following tisa-cel were reported in 29% of the patients with a low D100 IRM of 0.2%. Infection density through D100 was 0.542 per 100 person-days at risk. Like many studies on CD19 CAR T-cell therapy for B-cell malignancies, two-thirds of the infections occurred in the first month after CAR T-cell therapy infusion,33,34 and bacterial and viral infections were more frequent, and fungal infections were less common. During the first month following tisa-cel therapy, any-grade CRS and younger age were risk factors for any infection. Beyond the first month, receipt of ≥3 prior lines of therapy and CRS were associated with increased infection risk.
The overall infection density per 100 person-days at risk was 0.289 through D30, and 0.248 from D31 to D100 after CD19 CAR T-cell therapy in our cohort.
Prior single-center studies have reported infection densities of 0.9 to 2.9 through D30, and 0.55 to 0.98 for D31 to D100 after tisa-cel therapy.10-13 The observed lower rates of infection densities in the current study are likely to reflect inclusion of only clinically significant infections necessitating treatment in the CIBMTR database.28 In contrast, single-center studies include all infections regardless of whether treatment was warranted. Our study cohort reflects real-world data, where lower-risk patients (eg, measurable residual disease–negative, clinical remission 1 status) received tisa-cel therapy. In contrast, in the ELIANA/ENSIGN trials, higher-risk patients were enrolled who were likely at greater risk for infection. Finally, the retrospective nature of our study may have led to underreporting infectious events, resulting in a lower observed infection rate. In line with our observation of low IRM, infection-related deaths were similarly minimal in several reports on infectious outcomes following CD19 CAR T-cell therapy.10-13,35 In the ELIANA trial, only 3 patients had fatal infections, including 2 patients <100 days following CAR T-cell therapy.14
In the first month after tisa-cel infusion, bacterial infections accounted for 71% of total infections, with many (57%) presenting as BSIs. The incidence of BSIs (11.3%) in our entire cohort was similar to prior reports, wherein the incidence ranged from 7% to 17% in the first 30 days, to 5% to 15% beyond 30 days.10,12,13 Across studies on infections after CD19 CAR T-cell therapy, viral infections constituted 19% to 47% and 60% of all infections through D30 and D31 to D100, respectively.34 We observed that viral infections constituted 25% and 31% of all infections by D30 and D31 to D100, respectively. Like many reports, community respiratory viruses were the most common viral pathogens in our study population.10,12,13 Few studies have reported viral reactivations being more frequent after CAR T-cell therapy.13,36,37 Non-CMV herpes virus and CMV virus infections contributed to 25% and 13.9% of total viral infections in the current study. Pre-emptive monitoring for viral reactivations was not uniformly used across different centers, potentially leading to an underestimation in this extensively treated population. Furthermore, patients with prior HCT were more likely to undergo pre-emptive viral monitoring after CAR T-cell therapy. Additionally, nearly a quarter of these recipients had documented acute or chronic graft-versus-host disease, which could have further increased their risk of infection. All of these may have contributed to our finding that prior HCT is a risk factor for viral infections.
CRS and ICANS lead to immune dysregulation which, in combination with the use of immunomodulatory therapies to treat these complications and accompanying prolonged cytopenia, places patients at risk of infectious complications.38-40 While the occurrence of CRS was associated with the risk of bacterial/any infections within the first 30 days and 100 days after tisa-cel therapy, we could not detect the impact of the severity of CRS on the risk of infections due to a limited number of patients in each category and a lack of information on the timing of immunosuppressive therapy in relation to onset of CRS and infections. This is in contrast to the single-center studies wherein severe CRS was independently associated with an increased risk of infection.10,41 Our inability to assess the impact of immunosuppressants used for management of CRS/ICANS, likely a main driver of increased risk of infection in this cohort, is a major limitation of our study. An increase in the number of lines of therapy before CAR T-cell therapy was a risk factor for infections in many studies, including ours.15,41 Lastly, we observed no association between ICANS and disease status (active vs remission) on the risk of infectious complications after CAR T-cell therapy.
The current study has several limitations. Although 195 centers submit CAR T-cell therapy data on patients, our study includes data only from <50% of these centers. This, along with underreporting of events, may underestimate the true incidence of infections in this cohort. As the CIBMTR manual does not provide guidance on what constitutes treatment for clinically significant infections,27,28 this could have contributed to heterogeneity in reporting between centers. Infections >100 days following tisa-cel therapy were not captured in the database. Furthermore, information was lacking on the duration of neutropenia and lymphopenia before tisa-cel, degree of neutropenia as defined by the immune effector cell-associated hematotoxicity scoring,42 the use of prophylactic strategies and growth factors (granulocyte colony-stimulating factor or granulocyte macrophage colony-stimulating factor), all of which can impact infectious outcomes following tisa-cel infusion.13 We also could not determine the impact of tisa-cel dose on infections. Immune effector cell-associated hemophagocytic lymphohistiocytosis (IEC-HLH) is a more recently described immune complication after CAR T-cell therapy, and has been shown to impact infectious outcomes in a single study.13 However, ICE-HLH is not captured in the CIBMTR database.
Despite these limitations, our study includes the largest cohort of pediatric and young adult patients with ALL treated with tisa-cel in the real-world setting. The findings from our study could help centers/organizations with strategies to formulate guidelines for anti-infective prophylaxis following CAR T-cell therapy infusion. Further studies are needed to evaluate whether the role of viral surveillance and anti-infective prophylaxis (eg, antibiotics and letermovir) may be beneficial in patients with high-risk factors, such as HCT before CAR T-cell therapy, prolonged neutropenia, or CRS after CAR T-cell therapy, as currently recommended by the ASTCT.18,19
In conclusion, while tisa-cel therapy offers significant therapeutic benefits for patients with R/R B-cell ALL, significant infections associated with tisa-cel therapy occur. Our analysis demonstrates notable infection dynamics within the first 100 days after treatment, emphasizing the need for vigilant monitoring and tailored antimicrobial strategies. The findings also underscore the importance of understanding patient-specific risk factors, such as previous therapies and delayed neutrophil recovery after CAR T-cell therapy to optimize preventive measures and therapeutic interventions.
Acknowledgments
Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Scotland grant U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; grant U24HL138660 from NHLBI and NCI; grants 75R60222C00008, 75R60222C00009, and 75R60222C00011 from the Health Resources and Services Administration (HRSA); and grants N00014-23-1-2057 and N00014-24-1-2057 from the Office of Naval Research. Additional federal support is provided by CIBMTR grants OT3HL147741, P01CA111412, R01CA100019, R01CA218285, R01CA231838, R01CA262899, R01AI128775, R01AI150999, R01AI158861, R01HL155741, R01HL171117, R21AG077024, U01AI069197, U01AI184132, U24HL157560, and UG1HL174426. Support is also provided by Boston Children’s Hospital; Fred Hutchinson Cancer Center; Gateway for Cancer Research, Inc; Jeff Gordon Children’s Foundation; Medical College of Wisconsin; National Marrow Donor Program; Patient Center Outcomes Research Institute; Pediatric Bone Marrow Transplant Foundation; St. Baldrick’s Foundation; Stanford University; Stichting European Myeloma Network; and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc; Adaptimmune LLC; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Alexion; AlloVir, Inc; Amgen, Inc; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; Autolus Limited; BeiGene; BioLineRX; Blue Spark Technologies; bluebird bio, Inc; Blueprint Medicines; Bristol Myers Squibb Co; CareDx Inc; Caribou Biosciences, Inc; CytoSen Therapeutics, Inc; German Bone Marrow Donor Center; Editas Medicine; Elevance Health; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; Gift of Life Biologics; Gift of Life Marrow Registry; HistoGenetics; In8bio, Inc; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc; Karius; Kashi Clinical Laboratories; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Labcorp; Legend Biotech; Mallinckrodt Pharmaceuticals; Med Learning Group; Medac GmbH; Merck & Co; Millennium, the Takeda Oncology Co; Miller Pharmacal Group, Inc; Miltenyi Biomedicine; Miltenyi Biotec, Inc; MorphoSys; Management Science Associates-EDITLife; Neovii Pharmaceuticals AG; Novartis Pharmaceuticals Corporation; Omeros Corporation; Orca Biosystems, Inc; OriGen BioMedical; Ossium Health, Inc; Pfizer, Inc; Pharmacyclics, LLC, An AbbVie Company; PPD Development, LP; Registry Partners; Rigel Pharmaceuticals; Sanofi; Sarah Cannon; Seagen Inc; Sobi, Inc; Sociedade Brasileira de Terapia Celular e Transplante de Medula Óssea; Stemcell Technologies; Stemline Technologies; STEMSOFT; Takeda Pharmaceuticals; Talaris Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; and Xenikos BV.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, HRSA, or any other agency of the United States Government.
Authorship
Contribution: H.G.R. proposed the study concept, reviewed the literature, designed the study, drafted the study protocol, interpreted the analysis, wrote the first draft, and edited, reviewed, and approved the final version of the manuscript; P.S., M.M.H., and J.A.H. coproposed the study, provided feedback, reviewed the manuscript, and approved the final draft of the manuscript; M.C. and M.J.M. cleaned and analyzed the data, and provided the study reports; K.W. proposed the study, provided the feedback, and reviewed the manuscript; S.J., V.A.F., E.M.H., A.H.K., E.D., D.I.M., O.R., B.F., M.S.K., N.F., T.P., N.M.H., H.L., S.H., D.M., L.E.W., Z.E.B., H.S.M., M.-A.P., R.F.C., C.E.D., and A.H. provided feedback and reviewed the manuscript; M.R. and J.J.A. supervised the study, reviewed the proposal, and reviewed and edited the manuscript; and all the coauthors approved the final draft of the manuscript.
Conflict-of-interest disclosure: H.G.R. reports consultancy fees from Medexus and Vertex Therapeutics; and an honorary CD33CAR T medical monitor position with National Marrow Donor Program (NMDP). P.S. reports consultancy fees from Sobi. V.A.F. reports consultancy fees from Adaptimmune. E.M.H. reports consultancy fees from Novartis. M.S.K. reports consultancy fees and institutional research support from Merck. N.F. reports compensation from Incyte; advisory and speaker fees from Sanofi; consultancy for Omeros; medical monitor position with Bone Marrow Transplant Clinical Trials Network (BMT CTN); and data safety and monitoring board (DSMB) membership for Chronic Graft Versus Host Disease Consortium. H.L. served at the advisory board meeting for Rigel and Incyte; and received consultation fees from AbbVie in the past 24 months. D.M. reports consulting/advisory role for ADC therapeutics, Genmab, Genentech (spouse), Daiichi Sankyo (spouse), AstraZeneca (spouse); research funding for investigator initiated trials from Genentech, Genmab, Karyopharm, AstraZeneca (spouse); and expert testimony from AstraZeneca. H.S.M. reports compensation for advisory board/consultancy from CRISPR Therapeutics, Bristol Myers Squibb, Jazz, Incyte, Sobi, Autolus, Senti Bioscience; and medical monitor position for BMT CTN/NMDP. M.-A.P. reports honoraria from Adicet, Allogene, Caribou Biosciences, Celgene, Bristol Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, Takeda, VectivBio AG, and Vor Biopharma; serves on DSMBs for Cidara Therapeutics and Sellas Life Sciences; has ownership interests in Omeros and OrcaBio; and has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. R.F.C. reports compensation for advisory role for ADMA Biologics, Merck/MSD, Takeda, Shinogi, AiCuris, Astellas, Tether, Karius, Moderna, Pfizer, Invivyd, Gilead, AssemblyBio, Eurofins-Viracor, and Ansun Pharmaceuticals; and research support from Merck, Karius, AiCuris, Ansun Pharmaceuticals, Takeda, OM1, Genentech, and Eurofins-Viracor. C.E.D. reports compensation and honorarium from Omeros and Alexion Pharmaceuticals; and honorarium from Alexion for a presentation at European Society for Blood and Marrow Transplantation. J.A.H. reports consultancy for Moderna, Allovir, Gilead, Takeda, CSL Behring, Karius, Symbio, Geovax, and Sanofi; research funding from Gilead, Takeda, Merck, Geovax, and Sanofi; and significant payments from Takeda for advisory board meetings. A.H. reports honorarium from Elsevier for Clinical Overview Chapter. J.J.A. reports being part of the advisory committee for AscellaHealth. The remaining authors declare no competing financial interests.
Correspondence: Hemalatha G. Rangarajan, Pediatric Hematology, Oncology, and Blood and Marrow Transplantation, Nationwide Children’s Hospital, 700 Children’s Dr, Columbus, OH 43205; email: hemalatha.rangarajan@nationwidechildrens.org.
References
Author notes
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accord with the National Institutes of Health data sharing policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
The full-text version of this article contains a data supplement.