TO THE EDITOR:
Advances in hematopoietic stem cell transplantation (HSCT) have led to a substantial increase in its utilization in older adults, with transplant procedures doubling in patients aged ≥65 years, including those aged ≥75 years over the past decade.1-3 Concurrently, posttransplant cyclophosphamide (PTCy)–based graft-versus-host disease (GVHD) prophylaxis, initially developed for haploidentical donor transplantation,4-6 has become widely adopted across various donor types. This approach has been successfully implemented with matched sibling donors (MSDs), matched unrelated donors (MUDs), and mismatched unrelated donors (MMUDs).7-10 Despite this increasing adoption, comparative data remain limited.11-13
Herein, we report the outcomes of HSCT with PTCy in older patients. We included all consecutive patients aged ≥65 years with any hematologic malignancy who underwent their first HSCT with 8/8 MUD (n = 371), MSD (n = 123), haploidentical donor (n = 87), or 7/8 Human Leucocyte Antigen (HLA) MMUD (n = 29) at MD Anderson Cancer Center from 2017 to 2024. The institutional review board (IRB 2024-0040) approved the study, which was conducted in accordance with the Declaration of Helsinki. All participants provided informed consents.
The primary outcome of interest was overall survival (OS). Secondary outcomes included relapse, nonrelapse mortality (NRM), acute GVHD, and any chronic GVHD. Multivariable analyses were performed using Cox proportional hazards models for cause-specific hazards. All variables listed in Table 1 were included in the univariate analyses (supplemental Table 1A-B). Variables with a P value <.10 in univariate analyses were included in the multivariable models. Stepwise selection with both forward and backward selections was used to identify a more parsimonious model retaining all significant variables (P ≤ .05). Donor type was retained in the final multivariate models regardless of its statistical significance. Variables violating the proportional hazards assumption were adjusted through stratification.
A total of 610 patients were included (Table 1). Recipient age was similarly distributed across donor groups (median, 68-69 years), and ∼35% to 40% of patients in each group were aged ≥70 years. Donor age varied across groups: MUDs and MMUDs had a median age of 29 to 30 years. By contrast, the MSD group had a median donor age of 65 years, whereas the haploidentical group had a median donor age of 40 years (supplemental Figure 1). Most patients were males except for the MMUD group. Reduced-intensity/nonmyeloablative conditioning was more frequently used in the haploidentical (47%) than in the other groups (33%-41%). Bone marrow grafts were more common in the haploidentical (60%) than the unrelated donor groups (11%-21%), whereas all patients in the MSD group received peripheral blood grafts. A substantial proportion of MMUD HSCTs (66%) were performed in 2022 or later, compared with 45% to 53% in the other groups. Consequently, the median follow-up among survivors was shorter in the MMUD group (13 months) than in the other groups (24-26 months).
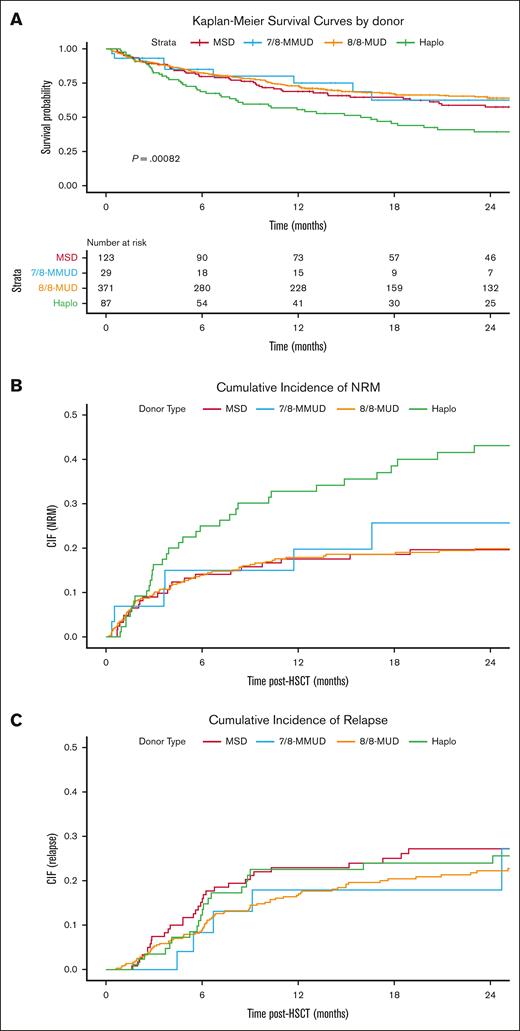
The Kaplan-Meier curves depicting OS by donor type are presented in Figure 1A; supplemental Figure 2 shows the same for patients aged ≥70 years. The cumulative incidences of NRM (Figure 1B), relapse (Figure 1C), acute GVHD (supplemental Figure 3), and chronic GVHD (supplemental Figure 4) are also shown.
Transplantation Outcomes by Donor Type. Kaplan-Meier estimate of OS (A), cumulative incidence of NRM (B), cumulative incidence of relapse (C) comparing MSD (red line), MUD (orange line), haploidentical (green line), and MMUD groups (blue line). CIF, cumulative incidence function; Haplo, haploidentical.
Transplantation Outcomes by Donor Type. Kaplan-Meier estimate of OS (A), cumulative incidence of NRM (B), cumulative incidence of relapse (C) comparing MSD (red line), MUD (orange line), haploidentical (green line), and MMUD groups (blue line). CIF, cumulative incidence function; Haplo, haploidentical.
In multivariable analyses, the haploidentical group was associated with lower OS (hazard ratio [HR] 1.59; 95% confidence interval [CI], 1.08-2.34; P = .02) than the MSD group (reference). For the MUD and MMUD groups, the HRs were 0.87 (95% CI, 0.63-1.21) and 1.04 (95% CI, 0.54 - 2.02), respectively (supplemental Table 2).
Lower OS in the haploidentical group was predominantly driven by a high NRM (HR, 2.10; 95% CI, 1.25-3.53; P = .005). The increased NRM in the haploidentical group did not appear to be driven by either grade 2 to 4 acute GVHD (HR, 0.86; 95% CI, 0.46-1.62; P = .65 vs MSD) or chronic GVHD (HR, 0.88; 95% CI, 0.41-1.86; P = .73). This suggests the high NRM might be related to other factors such as infection or organ toxicity. For relapse, the HR for haploidentical group compared to MSD was 0.88 (95% CI, 0.52-1.52; P = .66).
The most common cause of death across groups was relapse, but the proportion was higher in recipients of matched (MSD 52%; MUD 44%) compared with mismatched donors (haploidentical 35%; MMUD 36%). Infections accounted for 27% of NRM deaths in the MMUD group, 30% in the MUD group, and 33% in the haploidentical group. In the MSD group, infections and organ failure contributed equally to NRM deaths, each accounting for 18%. MUD recipients had higher proportions of bacterial infections (38%) than the MSD (22%) and haploidentical groups (18%). The haploidentical group had a slightly higher proportions of viral infections (24%) than the MSD (22%) and MUD groups (18%). In contrast, the MSD group had a lower fungal infection rate (11%) than the MUD (18%), haploidentical (18%), and MMUD groups (33%) (supplemental Table 3).
Although PTCy has been shown to effectively mitigate GVHD across various donor sources and has expanded access to HSCT, our data suggest that in older recipients, haploidentical HSCT may carry a higher risk of NRM than other donor types. Both the MUD and MMUD groups were noted to have comparable OS to the MSD group in this cohort, suggesting a unique vulnerability associated with haploidentical donors in older individuals.
Although infections emerged as the leading cause of NRM across all groups, it is plausible that older recipients of haploidentical donors are more susceptible to severe infections or experience delayed immune reconstitution, contributing to higher infection-related mortality. Furthermore, donor age, a known significant predictor of HSCT survival especially in the non-PTCy setting14 and more recently with cohorts including PTCy,15 was notably higher in the haploidentical group than the unrelated donor groups. Due to these substantial differences, reliable adjustment for donor age in the analysis was not possible. Older haploidentical donors have been associated with significantly worse NRM, especially in older patients.16 It is possible that older recipients may exhibit diminished tolerance to the combined effects of an older graft that is also HLA mismatched.17 Further analysis of HSCT outcomes using younger haploidentical donors into older recipients is needed. Donor pool can be expanded to younger donors by using non–first-degree relatives.18-20
Our findings should be interpreted with caution, in the context of the study's strengths and limitations. We acknowledge the limitations associated with a retrospective analysis, absence of detailed HLA-match data, and the notable correlation between donor type and donor age. The shorter follow-up in the MMUD group, while reflecting more recent practice, necessitates a cautious interpretation of long-term outcomes in this donor category. Furthermore, a limitation is the observed patient sex imbalance across donor types, the reasons for which are not fully elucidated by our data. We acknowledge that although we have controlled for all available key prognostic variables, the potential for residual confounding remains. However, these limitations are balanced by several notable strengths. The relatively large sample size, representing a consecutive cohort of patients from a major transplant center, reflects contemporary HSCT practices for older adults in the PTCy era. Additionally, the inclusion of multiple donor types and the specific focus on an older patient population, often underrepresented in the studies, are key assets. In conclusion, optimizing donor choice for older HSCT recipients using PTCy requires ongoing research and careful risk assessment.
Acknowledgment: The authors gratefully acknowledge Kai Cao (Laboratory Medicine) for expert assistance with HLA data acquisition and curation, which was essential to the completion of this manuscript.
Contribution: Y.M.A. abstracted the study design, interpreted data, and wrote the manuscript; R.S.M. conceptualized and designed the study, performed statistical analysis, interpreted data, helped with the manuscript writing, and had the final responsibility to submit for publication; J.R., P.S., G.C., P.K., U.P., B.O., K.R., R.E.C., and E.J.S. reviewed and interpreted data, reviewed the manuscript, and provided critical feedback; Y.M.A. and R.S.M. had full access to raw data; and all authors approved the manuscript.
Conflict-of-interest disclosure: E.J.S. serves as a consultant for Cimeio Therapeutics AG, New York Blood Center, and Celaid Therapeutics Inc; serves as a scientific advisor for Adaptimmune Limited, Navan Technologies, Axio Research, and FibroBiologics; is on the board of directors/management for National Marrow Donor Program; has license agreement with Affimed, Takeda Pharmaceuticals, and Rege Nexus; and is a speaker for Dava Oncology. K.R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceuticals and Affimed GmbH. K.R. participates on the scientific advisory board for Avenge Bio, Virogin Biotech, Navan Technologies, Caribou Biosciences, Bit Bio Limited, Replay Holdings, oNKo Innate, The Alliance for Cancer Gene Therapy, Innate Pharma, and Shinobi Therapeutics; and is the scientific founder of Syena. R.S.M. served as a consultant reviewer for Orca Bio; and received honorarium from Lumanity for providing expert opinion. The remaining authors declare no competing financial interests.
Correspondence: Rohtesh S. Mehta, Department of Stem Cell Transplantation, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: rmehta1@mdanderson.org.
References
Author notes
Original data are available on request from the corresponding author, Rohtesh S. Mehta (rmehta1@mdanderson.org).
The full-text version of this article contains a data supplement.