TO THE EDITOR:
Light-chain (AL) amyloidosis remains a devastating disorder in which misfolded free light chain immunoglobulins produced by clonal CD38+ plasma cells (PCs) aggregate into amyloid fibrils in the periphery and deposit into tissues, causing widespread organ damage. The cardiac and renal systems are the 2 most commonly involved, and overall prognosis is driven by the degree of their dysfunction.1-3 Current standard-of-care regimens target underlying PCs and have improved both hematologic and organ responses.4,5 Nevertheless, morbidity and mortality rates remain high among patients with advanced organ involvement, and organ recovery occurs months after therapy initiation.6
CAEL-101 (anselamimab) is an IgG1 monoclonal antibody (mAb) that binds to an epitope present on human kappa and lambda AL amyloid fibrils.7 Preclinical studies demonstrated 11-1F4 localization to amyloid and fibril elimination via antibody-mediated phagocytosis.7,8 We previously reported results of the open-label phase 1a/1b clinical trial of CAEL-101 (NCT02245867): the drug was well tolerated, with organ response in 63% of patients.9 We report long-term outcomes.
The study protocol was previously published.9 Briefly, patients with previously treated systemic AL amyloidosis with persistent organ dysfunction received CAEL-101 as 1 IV infusion (phase 1a) or 4 weekly infusions (phase 1b). A dose-escalation “up and down” design was used for both phase 1a and 1b. Patients were followed for 12 weeks. We retrospectively obtained hematologic, cardiac, and renal biomarker data for 6 years after enrollment.
Descriptive statistics of patient-, disease-, and treatment-related factors were generated. Graded organ response criteria were applied.10,11 For organ response, we defined baseline clinical measurements as measurements taken at time of trial enrollment. Given the tendency for N-terminus pro-brain natriuretic peptide (NT-proBNP) and urine protein estimates to fluctuate over time, we report organ responses which lasted at least 1 month. We estimated organ and hematologic progression-free survival (PFS) and overall survival (OS) with the Kaplan-Meier method. Organ progression was defined according to standard established criteria; patients were censored at time of hematologic progression.3
Between September 2014 and April 2017, 22 patients enrolled in the CAEL-101 study: 8 patients in phase 1a and 19 patients in phase 1b, with 5 patients participating in both phases. Median time from diagnosis to enrollment was 1.6 years. Median time from PC-directed therapy completion was 2.6 months in phase 1a and 7.4 months in phase 1b.
Baseline characteristics are listed in supplemental Table 1. Cardiac involvement was present in 54.6% (12/22) patients and renal involvement in 45.5% (10/22); of these, 2 had exhibited cardiac response and 4 had exhibited renal response prior to trial enrollment. Both organs were involved in 18.2% (4/22). Substantial proportions of trial patients exhibited organ involvement in ≥2 (68.2%, 15/22) and ≥3 (40.2%, 9/22) systems. All patients with cardiac disease had Mayo 2004 stage 2 (9/12, 75.0%) or 3A (3/12, 25.0%) disease. Patients with renal disease had a median baseline estimated glomerular filtration rate (eGFR) of 65.1 mL/min per 1.73 m2 (interquartile range, 44.6-83.1), with median baseline proteinuria of 2668 mg per day (1124-4415). Most patients were in hematologic complete response (CR; 45.5%, 10/22) or very good partial response (VGPR; 36.4%, 8/22); all experienced ≥ hematologic partial response (PR) at the time of enrollment.
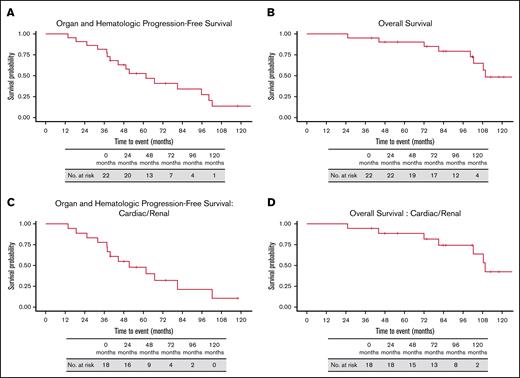
Over a median study follow-up of 112.9 months (95% confidence interval [CI], 101.6-NR, calculated from diagnosis), 45.5% (10/22) experienced hematologic progression and received further anti-PC therapy. Organ progression was observed in 45.5% (10/22). Since trial initiation, 9 patients have died (Figure 2): 5 from progressive AL amyloidosis; 1 from progressive multiple myeloma; 1 from unrelated gastric adenocarcinoma; 1 from lung transplantation complications; and 1 from venous thromboembolic sequelae. As reported previously, median time to organ response was 3 weeks after initial infusion.9 Median organ and hematologic PFS was 61.6 months (95% CI, 44.6-102.3; Figure 1A), and median OS was 109.6 months (95% CI: 102.3-NR; Figure 1B).
Survival from diagnosis. (A) Organ and hematologic PFS, (B) OS, (C) organ and hematologic PFS in patients with cardiac and/or renal involvement. (D) OS in patients with cardiac and/or renal involvement.
Survival from diagnosis. (A) Organ and hematologic PFS, (B) OS, (C) organ and hematologic PFS in patients with cardiac and/or renal involvement. (D) OS in patients with cardiac and/or renal involvement.
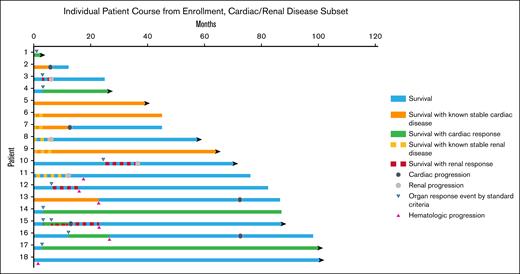
Course of CAEL-101 patients with cardiac and/or renal involvement at enrollment. Cardiac and renal responses were calculated from trial enrollment. Patients 6, 7, 9, and 15 had both cardiac and renal involvement. Patients 4 and 13-17 experienced cardiac response by NT-proBNP measurement; patients 3, 10, 12, 15, and 18 experienced renal response by proteinuria measurement. Patients 2, 7, 13, 15, and 16 developed cardiac progression; patients 3, 8, 10, and 11 developed renal progression by eGFR measurement. Patients 11-13, 15, 16, and 18 developed hematologic progression. Patients 2, 3, 7, 12, and 13 died from progressive AL amyloidosis (patient 12 from novel heart failure not present at time of CAEL-101 initiation); patient 6 died from venous thromboembolic complications; patient 14 died from gastric cancer; and patient 16 died from progressive multiple myeloma.
Course of CAEL-101 patients with cardiac and/or renal involvement at enrollment. Cardiac and renal responses were calculated from trial enrollment. Patients 6, 7, 9, and 15 had both cardiac and renal involvement. Patients 4 and 13-17 experienced cardiac response by NT-proBNP measurement; patients 3, 10, 12, 15, and 18 experienced renal response by proteinuria measurement. Patients 2, 7, 13, 15, and 16 developed cardiac progression; patients 3, 8, 10, and 11 developed renal progression by eGFR measurement. Patients 11-13, 15, 16, and 18 developed hematologic progression. Patients 2, 3, 7, 12, and 13 died from progressive AL amyloidosis (patient 12 from novel heart failure not present at time of CAEL-101 initiation); patient 6 died from venous thromboembolic complications; patient 14 died from gastric cancer; and patient 16 died from progressive multiple myeloma.
Among 18 patients with cardiac or renal involvement, 4 had both organs affected, resulting in 22 total instances of organ involvement. Any organ response lasting at least 1 month and occurring before hematologic progression was seen in 40.9% (9/22) of instances, with 36.4% (8/22) within 12 months. CR rate was 9.1% (2/22, 2 renal, at 3 and 6 months); VGPR rate was 9.1% (2/22, 1 cardiac achieved at 1 month, 1 renal at 12 months); and PR rate was 27.2% (6/22, 5 cardiac at median 3 months, 1 renal at 24 months; supplemental Table 2). At median follow-up of 112.9 months (95% CI, 83.8-NR), median organ and hematologic PFS was 51.4 months (95% CI, 37.9-NR; Figure 1C) and median OS was 109.6 months (95% CI, 102.3-NR; Figure 1D). Persistent response or organ stability was observed in 50.0% (9/18) of patients, and cardiac or renal ≥PR was maintained until death, end of follow-up, or hematologic progression in 33.3% (6/18) patients. Hematologic progression occurred in 33.3% (6/18) patients. In 16.7% (3/18) patients, it developed within 12 months after organ deterioration.
In brief, no new safety signals in patients with relapsed/refractory AL amyloidosis who received the antifibril mAb CAEL-101 in trial NCT02245867, were seen after a decade of follow-up. Additionally, patients treated on trial exhibited long-term survival. Multiple patients with cardiac and/or renal AL amyloidosis achieved prolonged survival with stable organ function or better.
The impact of novel frontline anti-CD38+ mAb–containing regimens on organ response, major organ deterioration progression free survival (MOD-PFS), and OS has been established.5 However, deep hematologic responses improve organ function and OS by eliminating toxic fibril precursors12 and preventing interstitial fibril deposition. Preexisting AL fibrils still drive morbidity and mortality, and cardiac involvement still portends early death.4,5,13,14 Currently, there are no US Food and Drug Administration–approved therapies that target amyloid fibrils. The first antifibril monoclonal antibody tested in humans, birtamimab (NEOD-001), showed promising early-phase results (NCT01707264), but later-phase studies (NCT04973137) suggested that only patients with advanced cardiac disease, derived benefit.15,16
CAEL-101 (anselamimab), a mAb which induces phagocytosis of AL fibrils,7,8,17 was well tolerated in a previously reported phase 1a/1b clinical trial.3 After limited treatment, 8 of 12 patients with cardiac disease experienced organ response within 1 month; 4 had stable disease. In patients with renal involvement, 2 of 10 experienced organ response, 6 had stable disease, and 2 had progressive disease by 2-level criteria. No patient had received prior anti-CD38+ mAb.
We confirm in this longitudinal study that CAEL-101 is safe and well tolerated over 10 years. Additionally, despite low cumulative dose, patients who received the trial therapy demonstrated long-term survival without organ deterioration: in patients enrolled at a median of ∼1.5 years from diagnosis, median organ and hematologic PFS was >5 years, and median OS was >9 years. These findings compare favorably to published survival estimates pre–anti-CD38+ mAb. Median PFS estimates were 1.0 to 1.5 years in relapsed/refractory AL amyloidosis18 and 2.5 years in newly diagnosed patients.19 The most comparable estimate for median OS is 4.6 years.20
We observed promising outcomes in patients with cardiac and renal disease. Through the first 12 months, >80% of patients with cardiac involvement experienced at least stable disease; >40% ultimately achieved any response; and 75% were alive at 5 years. In patients with renal disease, 50% experienced at least stable disease through the first 12 months; 40% achieved any renal response; and 60% were alive at 5 years. Our survival findings are comparable to the Mayo Clinic’s reported 5-year OS rates in patients alive ≥1 year from diagnosis: 44% and 69% in patients without cardiac response and with cardiac PR; and 61% and 70% in those without renal response and with renal PR.10,11 Of note, CAEL-101 patients received 1 month maximum of CAEL-101, which has an expected half-life of 30 days.21 As time progressed from infusion, recurrent fibril deposition seen in the natural course of relapsed AL amyloidosis would have resulted in recurrent organ disease and mortality, driving eventual progression and mortality.
Our sample size and statistical power are limited in this analysis. Graded organ response criteria were applied post hoc. We did not include historical comparators to avoid lead-time bias or other confounders. We anticipate the results of the ongoing phase 3 randomized controlled trials (NCT04512235 and NCT04504825), evaluating the impact of serial CAEL-101 infusions. We stress that the results we describe from this phase 1 study derive from a small sample, are hypothesis-generating, and should not be prematurely viewed as suggesting a causal link between CAEL-101 and improved survival. The strengths of our study are its survival data over a long time span and its granular data on cardiac and renal AL disease trajectories.
In conclusion, patients with relapsed/refractory AL amyloidosis who received a low cumulative amount of the novel antiamyloid CAEL-101 in a phase 1a/1b study initially exhibited a high degree of objective organ response.9 We report here lengthy OS and PFS in those patients for nearly over 10 years. The CAEL101-301 and CAEL101-302 phase 3 clinical trials are underway to confirm these findings.
Institutional review board approval was granted for retrospective follow-up of the initial institutional review board–approved clinical trial.
Contribution: M.S.H., D.B., R.C., J.R., M.S.M., M.Y.M., A.E., and S.L. conceptualized and designed the study; M.S.H., S.P., and D.B. analyzed and interpreted the data; all authors provided study materials or patients, collected and assembled the data, and wrote and approved the final manuscript.
Conflict-of-interest disclosure: M.S.H. was supported by grants from the National Institutes of Health/National Cancer Institute (grant T32CA203703) and a 2024 ASCO/Conquer Cancer Astrazeneca Young Investigator Award. R.C. reports consulting/advisory for Adaptive Biotechnologies, Janssen, and Sanofi Pasteur. M.S.M. reports consulting/advisory and research funding from Alnylam, Eidos Therapeutics, Ionis Pharmaceuticals; consulting/advisory for Intellia Therapeutics; and received research funding from Pfizer. M.Y.M. reports ownership interest and intellectual property in Caelum Biosciences; received honoraria consulting and advisory and research funding from Ossium Health; honoraria from Incyte; and reports consulting/advisory from Bluebird Bio, Crispr Therapeutics, and Vertex. S.L. reports leadership and ownership at Caelum Biosciences; consulting/advisory at Adaptive Biotechnologies, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Pfizer, Regeneron, Karyopharm Therapeutics, Alexion Pharmaceuticals; speakers’ bureaus for PeerView, Clinical Care Options, BioAscent, and RedMedEd.com; consulting/advisory and research funding from Sanofi; and is the owner of an intellectual property, 11-1F4 mAb, for use in AL amyloidosis. D.B. reports consulting/advisory and research funding from Sanofi. The remaining authors declare no competing financial interests.
Correspondence: Michael Sang Hughes, Division of Hematology Oncology, Columbia University Irving Medical Center, Herbert Irving Pavilion 9-960, 161 Ft. Washington Ave, New York, NY 10032; email: mh4266@cumc.columbia.edu.
References
Author notes
The data that support the findings of this study are available upon reasonable request from the corresponding author, Michael Sang Hughes (mh4266@cumc.columbia.edu). Access to the data may be subject to institutional, ethical, or regulatory approvals to ensure compliance with privacy and confidentiality standards.
The full-text version of this article contains a data supplement.