Visual Abstract
TO THE EDITOR:
Chronic lymphocytic leukemia (CLL) is characterized by malignant B lymphocytes and dysregulated B-cell receptor (BCR) signaling, promoting cell survival and proliferation through chronic activation by (auto)antigens or pathway mutations.1,2 Bruton tyrosine kinase (BTK), a key component of this pathway, is critical for malignant B-cell growth.3 Its therapeutic targeting led to the development of ibrutinib, a BTK inhibitor approved after demonstrating safety and efficacy in phase 1/2 trials in 2013.4-6 Ibrutinib is now widely used as monotherapy or in combination with other drugs, though combination therapies have shown limited additional benefit.7-10 Second-generation, more selective BTK inhibitors and third-generation noncovalent inhibitors are in development to minimize toxicity.4 However, ibrutinib resistance, either primary (13%-30% of patients) or acquired, remains a major clinical obstacle.7,11-17 This study investigates genes involved in ibrutinib’s downstream pathways to enhance understanding of its mechanism, and identify potential contributors to resistance. We hypothesize that ibrutinib regulates miR-181a and miR-181b, crucial genes in CLL biology and drug resistance.18-22
To investigate whether miR-181a and miR-181b are associated with the reduction of peripheral blood cell populations after ibrutinib in patients with CLL, we analyzed a retrospective case-cohort comprising 60 sequential samples from 12 patients with CLL undergoing ibrutinib therapy. Patient characteristics are detailed in supplemental Table 1, and the percentages of CD19+CD5+ and CD19+CD5– cells are provided in supplemental Table 2. The median follow-up duration was 285.5 days (interquartile range [IQR], 184-757.5).
Among these patients, 3 (CLL45, CLL157, CLL168) received ibrutinib as first-line treatment, while the remaining 9 had previously received other therapies. For this latter group, baseline samples were collected when patients were treatment free (median time from previous therapy, 346.5 days; IQR, 156.75-574.25). At each time point, miR-181a/b expression was measured in purified primary CLL cells.
Initial analysis revealed a significant increase in miR-181a/b expression from baseline to the final time point (P = .0034 and P = .0049, respectively; Wilcoxon matched-pairs test; Figure 1A). Expression levels at each time point were then correlated with concurrent white blood cell (WBC) counts. An inverse relationship emerged: miR-181a/b levels were significantly elevated in patients whose WBC count either declined by >50% from the baseline or presented physiologic values at the last time point (PmiR-181a = .0156 and PmiR-181b = .0156; Wilcoxon matched-pairs test; Figure 1B-C). These patients were also classified as responders based on clinical parameters and physician assessment.
Ibrutinib affects miR-181a and miR-181b expression in vivo. (A) Evaluation by reverse transcription quantitative polymerase chain reaction (RT-qPCR) of miR-181a and miR-181b relative expression in purified primary CLL cells collected at the first time point (FTP) and at the last time point (LTP) of ibrutinib treatment follow-up. RNU44 was used as an internal control. The expression of miRNAs was normalized to the level of the basal time point at each time point. The Wilcoxon matched-pairs test was used for statistical significance. (B) Evaluation by RT-qPCR of miR-181a and miR-181b expression (left y-axis) in purified primary CLL cells collected during the follow-up of ibrutinib treatment (x-axis; days); RNU44 was used as an internal control. The expression of miRNAs was normalized to the level of the basal time point at each time point. WBC count at each time point was reported on the right y-axis. Black arrows indicate when the WBC count of patients decreased by >50% from baseline, or reached physiologic values at the last time point (WBC with properties). Conversely, gray arrows represent time points where the WBC count of patients decreased by <50% from baseline or remained unchanged (WBC without properties). (C) Graphs illustrate the corresponding expression levels of miR-181a and miR-181b in patients with CLL, as indicated. Wilcoxon matched-pairs test was used for statistical significance; ∗P<.05.
Ibrutinib affects miR-181a and miR-181b expression in vivo. (A) Evaluation by reverse transcription quantitative polymerase chain reaction (RT-qPCR) of miR-181a and miR-181b relative expression in purified primary CLL cells collected at the first time point (FTP) and at the last time point (LTP) of ibrutinib treatment follow-up. RNU44 was used as an internal control. The expression of miRNAs was normalized to the level of the basal time point at each time point. The Wilcoxon matched-pairs test was used for statistical significance. (B) Evaluation by RT-qPCR of miR-181a and miR-181b expression (left y-axis) in purified primary CLL cells collected during the follow-up of ibrutinib treatment (x-axis; days); RNU44 was used as an internal control. The expression of miRNAs was normalized to the level of the basal time point at each time point. WBC count at each time point was reported on the right y-axis. Black arrows indicate when the WBC count of patients decreased by >50% from baseline, or reached physiologic values at the last time point (WBC with properties). Conversely, gray arrows represent time points where the WBC count of patients decreased by <50% from baseline or remained unchanged (WBC without properties). (C) Graphs illustrate the corresponding expression levels of miR-181a and miR-181b in patients with CLL, as indicated. Wilcoxon matched-pairs test was used for statistical significance; ∗P<.05.
Among the 5 patients who did not meet the WBC count response criteria, 3 (CLL135, CLL136, CLL145) were nonresponders. In these cases, microRNA (miRNA) trends largely mirrored WBC dynamics, with some exceptions: for example, in CLL166, only miR-181a slightly increased when WBC count dropped, while CLL168 was in the ibrutinib-associated lymphocytosis phase. Absolute lymphocyte count (ALC) trends paralleled those of WBC count, further suggesting a link between the expressions of these miRNAs, and lymphocyte burden (supplemental Figure 1).
We next examined whether miR-181a/b expression dynamics were associated with prognostic markers, such as IGHV mutational status, ZAP70 expression, and cytogenetic abnormalities.23 Fold change analyses comparing either the final vs the first time point or a clinically relevant time point vs baseline showed no statistically significant differences between favorable and unfavorable prognostic groups (supplemental Figure 2).
Given prior findings that miR-181b enhances CD8+ T-cell cytotoxicity,24 we assessed granzyme B expression in CD8+ T cells from samples showing significant WBC count reduction or normalization. However, no changes were detected at the analyzed time points (supplemental Figure 3).
To assess whether miR-181a/b upregulation is specific to ibrutinib, we evaluated 22 longitudinal samples from 5 patients with CLL treated with other BCR signaling inhibitors: idelalisib plus rituximab, or acalabrutinib. Idelalisib targets phosphoinositide 3-kinase, rituximab is an anti-CD20 antibody, and acalabrutinib is a second-generation BTK inhibitor. The median study duration was 112 days (IQR, 60.5-242.5). One patient (CLL152) received acalabrutinib as a first-line treatment, while others had different prior therapies. Baseline samples were collected after a median treatment-free interval of 491.5 days (IQR, 25.75-1438).
Although statistical significance was not calculated due to small sample size, miR-181a/b expression increased in 4 out of 5 patients at both final vs initial time points (supplemental Figure 4A), and at time points with normalized or significantly reduced WBC counts (relative increase: miR-181a = 16.16×, miR-181b = 9.04×; Figure 2B). miRNAs trends in WBC counts also paralleled absolute lymphocyte count dynamics (supplemental Figure 4B-C).
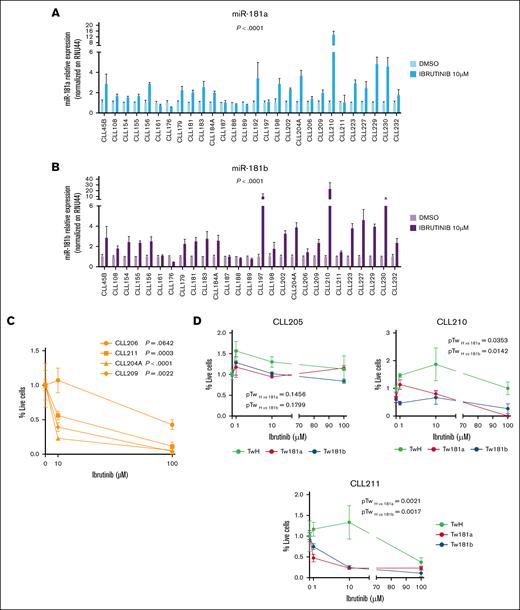
miR-181a and miR-181b expression increases after ibrutinib treatment in patients with CLL in vitro. The relative expression of miR-181a (A) and miR-181b (B) was evaluated by RT-qPCR in purified primary CLL cells treated for 72 hours with ibrutinib 10 μM, or DMSO as a control. RNU44 was used as an internal control. The relative expression of miRNAs for each patient was normalized to the DMSO-treated sample. miR-181a expression was assessed in 28 patients with CLL, with 21 analyzed in experimental duplicates and 7 (CLL45B, CLL176, CLL192, CLL197, CLL198, CLL210, and CLL211) analyzed in a single experiment. Similarly, miR-181b expression was evaluated in 27 patients with CLL, with 24 analyzed in duplicates and 3 (CLL161, CLL176, and CLL198) in a single experiment. The Wilcoxon matched-pairs test was used for statistical significance. (C) Cell viability of purified primary CLL cells was assessed after treatment with increasing doses of ibrutinib or DMSO (0, 10, and 100 μM) for 72 hours using the MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium)) assay. Statistical significance was determined by 1-way analysis of variance (ANOVA). (D) Cell viability of primary CLL cells transfected with either pTween_181b, pTween_181a, or pTween_CTRL vectors was evaluated after a 72-hour treatment with increasing concentrations of ibrutinib or DMSO (0, 10, and 100 μM). Cell viability was determined using the MTS assay. Statistical significance was assessed by 1-way ANOVA. DMSO, dimethyl sulfoxide.
miR-181a and miR-181b expression increases after ibrutinib treatment in patients with CLL in vitro. The relative expression of miR-181a (A) and miR-181b (B) was evaluated by RT-qPCR in purified primary CLL cells treated for 72 hours with ibrutinib 10 μM, or DMSO as a control. RNU44 was used as an internal control. The relative expression of miRNAs for each patient was normalized to the DMSO-treated sample. miR-181a expression was assessed in 28 patients with CLL, with 21 analyzed in experimental duplicates and 7 (CLL45B, CLL176, CLL192, CLL197, CLL198, CLL210, and CLL211) analyzed in a single experiment. Similarly, miR-181b expression was evaluated in 27 patients with CLL, with 24 analyzed in duplicates and 3 (CLL161, CLL176, and CLL198) in a single experiment. The Wilcoxon matched-pairs test was used for statistical significance. (C) Cell viability of purified primary CLL cells was assessed after treatment with increasing doses of ibrutinib or DMSO (0, 10, and 100 μM) for 72 hours using the MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium)) assay. Statistical significance was determined by 1-way analysis of variance (ANOVA). (D) Cell viability of primary CLL cells transfected with either pTween_181b, pTween_181a, or pTween_CTRL vectors was evaluated after a 72-hour treatment with increasing concentrations of ibrutinib or DMSO (0, 10, and 100 μM). Cell viability was determined using the MTS assay. Statistical significance was assessed by 1-way ANOVA. DMSO, dimethyl sulfoxide.
These data suggest that ibrutinib, as well as alternative BCR inhibitors, may promote miR-181a/b expression in CLL, particularly in patients who experience effective WBC count reduction during treatment.
To investigate whether the increase in miR-181a/b expression observed in patients is directly attributable to ibrutinib treatment or influenced by other in vivo factors, leukemia cells from 28 patients with CLL were treated in vitro with either ibrutinib or dimethyl sulfoxide (control) for 72 hours. The efficacy of ibrutinib doses was validated via western blot analysis in CLL cell lines MEC1 and HG3 (supplemental Figure 5). The results revealed a significant upregulation of miR-181a and miR-181b in most patient samples (PmiR-181a < .0001 and PmiR-181b < .0001; Wilcoxon matched-pairs test), and in MEC1 cells (Figure 2A-B; supplemental Figure 6). To assess a potential link between miR-181a/b expression and CLL cell death, primary CLL cells were exposed to increasing ibrutinib concentrations (0, 10, and 100 μM), and cell viability was assessed via MTS (3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium)) assay after 72 hours. We confirmed that ibrutinib significantly reduced leukemic cell survival in 3 of 4 patient samples at 10 μM, correlating with increased miR-181a/b expression (PCLL211 = .0003, PCLL204A ≤ .0001, PCLL209 = .0022; 1-way analysis of variance). In the remaining sample, no significant change in viability and miRNAs expression was observed (PCLL206 = .0642; Figure 2C). Expression of miRNAs was not assessed at 100 μM due to excessive cell death, compromising RNA quality. Further, live cell quantification after ibrutinib treatment showed a reduction in miR-181a/b–overexpressing live cells in 2 of 3 CLL samples, with a similar but nonsignificant trend in the third, where ibrutinib had a weaker effect (Figure 2D). To explore whether other BCR pathway inhibitors affect miR-181a/b, primary CLL cells were also treated with idelalisib. A slight increase in miR-181b was seen in only a few samples, indicating that idelalisib has less impact than ibrutinib on miR-181a/b regulation in vitro (supplemental Figure 7).
Ibrutinib, a first-generation BTK inhibitor, is widely used as a first-line treatment for CLL, demonstrating significant clinical efficacy. However, resistance, either primary or acquired, remains a common challenge. Understanding the molecular mechanisms underlying ibrutinib response is crucial to improving therapeutic outcomes. In this study, we investigated the role of miR-181a and miR-181b in patients with CLL undergoing ibrutinib therapy. Increased expression of these miRNAs was associated with a significant reduction in WBC counts, while their expression remained low in most nonresponders. Previous studies, such as that by Saleh et al,25 reported a downregulation of miR-181b during early treatment phases, which aligns with our findings of dynamic miRNA expression over time. Notably, we observed upregulation of miR-181a/b in patient samples collected a median of 285.5 days after treatment, correlating with decreased anti-apoptotic gene expression. In vitro, ibrutinib alone induced expression of both miR-181a/b and increased CLL cell death. While similar trends were seen with other BCR inhibitors in patients, in vitro results suggested additional factors may mediate this effect.
In conclusion, we identified miR-181a/b as a gene involved in the ibrutinib pathway that could be linked to the CLL cell death observed in patients and, consequently, to drug response. Indeed, a low expression of the miR-181a/b had already been associated with drug resistance in hematologic malignancies. This discovery opens new avenues to investigate mechanisms of ibrutinib resistance and potentially avoid them.
Acknowledgments: This study was supported by the Italian Ministry of Health (GR-2016-02363070) and “Associazione Lory a Colori” (R.V.).
Contribution: R.V. conceived of the study, analyzed the data, and wrote the manuscript; A.R. designed, performed, and analyzed the experiments, and cowrote the manuscript; S.P. executed the experiments, collected the data, generated the figures, and contributed to manuscript editing; M.D.M., M.M.R., C.D.C., and F.P. helped to perform reverse transcription quantitative polymerase chain reaction and western blot; L.L., I.I., F.A., and A.T. provided samples and clinical data from patients with chronic lymphocytic leukemia; and all the authors critically read, edited, and approved the final manuscript.
Conflict-of-interest disclosure: L.L. reports advisory roles for AstraZeneca, Johnson & Johnson, BeiGene, AbbVie, and Lilly during the preparation of the manuscript. The remaining authors declare no competing financial interests.
The current affiliation for M.M.R. is Albert Einstein College of Medicine, Bronx, NY.
The current affiliation for F.P. is Max von Pettenkofer-Institut, Ludwig-Maximilians-Universität, München, Germany.
Correspondence: Rosa Visone, G. d’Annunzio University, Via Luigi Polacchi 11, 66100 Chieti, Italy; email: r.visone@unich.it.
References
Author notes
A.R. and S.P. contributed equally to this work.
All the data have been deposited on the code-hosting platform GitHub (https://github.com/LabVisone/Ibrutinib-modulates-miR-181a-b-in-CLL.git).
The full-text version of this article contains a data supplement.