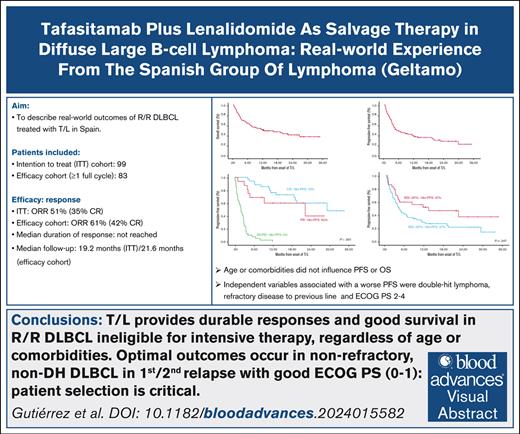
Key Points
T/L provides durable responses and good survival in R/R DLBCL ineligible for intensive therapy, regardless of age or comorbidities.
Patient selection is critical: optimal outcomes occur in nonrefractory, non-double-hit, first/second relapse with good performance status.
Visual Abstract
Relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL) remains challenging to treat, especially in patients ineligible for intensive therapy or chimeric antigen receptor T cells. Tafasitamab plus lenalidomide (T/L) is an effective option based on the phase 2 L-MIND trial findings, although real-world evidence studies have not consistently confirmed these results. We aimed to describe real-world outcomes of R/R DLBCL treated with T/L in Spain. A total of 99 patients received at least 1 dose of tafasitamab (intent-to-treat [ITT] cohort), with 83 completing at least 1 full cycle of T/L (efficacy cohort). Respectively for ITT and efficacy cohorts, at a median follow-up of 19.2 and 21.6 months, the overall response rate was 51% and 61% (complete response [CR], 35% and 42%). Median duration of response was not reached, and patients achieving a CR had excellent outcomes. The median progression-free survival (PFS) was 4.9 and 10.9 months, and overall survival (OS) was 12.2 and 21.8 months, respectively for both ITT and efficacy cohorts. Neither age nor cumulative illness rating score influenced survival. Better PFS was obtained in first/second relapse but only poor Eastern Cooperative Oncology Group performance status 2 to 4, double-hit lymphoma, and those with refractory/progressing disease after the previous therapy, were independently associated with worse PFS. Treatment was generally well tolerated, with manageable toxicity. Relative dose intensity of lenalidomide significantly affected response, PFS, and OS. In summary, T/L is both well tolerated and effective, irrespective of age or comorbidities. Our findings provide valuable insights into the real-world application of T/L and reinforce its role as a key treatment option for patients with R/R DLBCL.
Introduction
Approximately 20% to 25% of patients with diffuse large B-cell lymphoma (DLBCL) experience relapse, and ∼10% to 15% are refractory to frontline R-CHOP (rituximab, cyclophosphamide, doxorubicin, Oncovin [vincristine], and prednisone) therapy.1 For these patients, for whom first-line treatment fails, outcomes are often suboptimal, particularly for those with refractory disease or early relapse, who have a median survival of ∼6 months.2 In cases of high-risk relapsed/refractory (R/R) disease, novel therapies such as chimeric antigen receptor T cells (CAR-Ts) have demonstrated significant improvements in outcomes.3,4 Patients experiencing later relapses may undergo salvage immunochemotherapy followed by high-dose chemotherapy with autologous hematopoietic stem cell transplantation (ASCT).5 In general, more patients are eligible for CAR-T therapy than for ASCT. However, despite the curative potential of these approaches, which are recommended by major guidelines for early and late treatment failures, a considerable proportion of patients remain ineligible due to age, comorbidities or a combination of these factors, along with other barriers such as long distance to CAR-T–approved centers or limited accessibility. This poses a significant challenge, as treatment options for these patients remain limited.
For patients ineligible for CAR-T or ASCT, regardless of clinical trial availability, treatment recommendations include regimens with a more favorable toxicity profile, such as R-GemOx (rituximab combined with gemcitabine and oxaliplatin)6 or bendamustine with rituximab (BR).7 Recently, new therapeutic strategies have emerged for patients with DLBCL with R/R disease, including polatuzumab vedotin8 and tafasitamab. Tafasitamab is a humanized monoclonal antibody that targets CD19, a protein expressed on B lymphocytes throughout their development until they differentiate into plasma cells and is also present on B-cell–derived neoplasms, including DLBCL. The constant region of the antibody has been modified to enhance its binding to Fcγ receptors, thereby increasing antibody-dependent cell cytotoxicity, antibody-dependent cell phagocytosis, and direct cytotoxic effects (apoptosis) on tumor cells.9
Preclinical data support the combination of tafasitamab and lenalidomide (T/L), as lenalidomide promotes the activation of natural killer cells, potentially enhancing the antibody-dependent cell cytotoxicity of tafasitamab.10 The primary evidence for the efficacy of this combination comes from the pivotal phase 2 single-arm L-MIND study involving 81 patients, which yielded encouraging results, including a high overall response rate (ORR), prolonged duration of response (DOR), progression-free survival (PFS), and overall survival (OS) in patients with R/R DLBCL who are not eligible for ASCT. The efficacy observed in the L-MIND study indicates an improvement over the results obtained with T/L as individual agents, suggesting a synergistic effect when used together. Specifically, the L-MIND study reported an ORR of 57.5% and a complete response (CR) rate (CRR) of 41.3%, with a median DOR that was not reached after 45.6 months of median follow-up, which is clinically significant for patients with R/R DLBCL who are not eligible for ASCT. The safety profile of T/L is manageable, with adverse events primarily being reversible and clinically manageable.11,12
Beyond this pivotal trial, several real-world (RW) retrospective studies have reported variable results. To enhance the international RW data, we conducted a real-life study assessing the efficacy and safety of this combination in Spain, after the initiation of the expanded access program in 2021.
Methods
Patients and study design
We conducted a retrospective, observational, multicenter, RW evidence (RWE) study evaluating patients with R/R DLBCL treated with T/L in Spain. This study included 39 institutions from the Spanish Group of Lymphoma (GELTAMO [Grupo Español de Linfomas/Trasplante Autólogo de Médula Ósea]) and covered patients consecutively enrolled in the expanded access program in Spain from June 2021 to September 2022, as well as an additional period extending to December 2023 after the drug’s approval and commercial availability in Spain. The study was approved by the ethics committee of the Balearic Islands (Comité de Ética de la Investigación con medicamentos de las Islas Baleares, [CEIm-IB]; reference number IB 5210/23).
Inclusion criteria encompassed adult patients with histologically confirmed DLBCL, including those with DLBCL transformed from a previous indolent lymphoma. Patients had to have disease R/R to at least 1 line of therapy that included an anti-CD20 agent and must have received at least 1 dose of salvage therapy with T/L. Patients were also required to sign the informed consent form at the time of inclusion in this retrospective study. Exclusion criteria included any histologies other than those mentioned earlier (eg, indolent BCLs, primary mediastinal BCL, or Burkitt lymphoma). To accurately describe the RW experience, all patients who received at least 1 dose of T/L were included in the study’s intent-to-treat (ITT) population. For the purposes of efficacy analysis, we evaluated both the entire ITT cohort and the subset of patients who received at least 1 full cycle of T/L (efficacy population), to account for the high likelihood that this therapy is often reserved for patients who are extremely unfit, which may otherwise hinder a proper assessment of efficacy.
Treatment
Treatment was prescribed according to recommendations: coadministration of tafasitamab (12 mg/kg) and lenalidomide (25 mg/d; 3 of every 4 weeks) for up to 12 cycles (28 days each), followed by tafasitamab monotherapy (in patients with at least stable disease) until disease progression or unacceptable toxicity, as originally reported in L-MIND trial and approved.12 In the design of the study, we focused our analysis on the first year of combination therapy because this period corresponds to the time when most of the toxicity was reported in the L-MIND trial, primarily associated with lenalidomide administration. Consequently, this is also the phase during which potential reductions in dose intensity are most likely to affect treatment outcomes.
Study outcomes
The primary objective was to assess the efficacy of the combination of T/L in terms of ORR (CR plus partial response [PR]) in patients with R/R DLBCL included in the efficacy cohort. Secondary objectives included the disease control rate (defined as CR plus PR plus stable disease [SD]), DOR, PFS, OS, toxicity of the combination, and the impact of relative dose intensity (RDI). To evaluate the impact of age and comorbidities on study outcomes, the cumulative illness rating score (CIRS), which quantifies the presence and severity of comorbidities across 14 organ systems, was recorded in each case.13 Primary refractory cases were considered those cases not achieving a CR or relapsing within 6 months after completing frontline therapy. Response assessments were performed by positron emission tomography/computed tomography after 3, 6, and/or 12 cycles, according to clinical practice, using the Lugano criteria.14
Statistical analysis
In this RWE setting, no hypothesis was tested, and the analysis was descriptive. Variables following binomial distributions are expressed as frequencies and percentages. Comparisons between qualitative variables were performed using the Fisher exact test or the χ2 test. Comparisons between quantitative and qualitative variables were conducted using nonparametric tests (Mann-Whitney U test or Kruskal-Wallis test). To evaluate the impact of RDI of lenalidomide on DOR and PFS we used receiver operating characteristic curves. The role of prognostic factors on treatment response was assessed using univariate and multivariate binary logistic regression analyses.
For survival analysis, time-to-event variables (OS and PFS) were measured from the date of therapy initiation and estimated using the Kaplan-Meier method. Comparisons between the variables of interest were performed using the log-rank test. Multivariate analyses were carried out for the variables that were significant in the univariate analysis, according to the Cox proportional hazards regression model. All P values reported are 2-sided, and statistical significance was defined as P value of <.05.
Results
Characteristics of patients
A total of 99 patients who had received at least 1 dose of tafasitamab were identified, constituting the global/safety ITT cohort. Of these patients, 83 received at least 1 full cycle of the T/L regimen, forming the efficacy cohort. Among the 16 patients who were unable to complete at least 1 full cycle of T/L, the most common reasons were poor clinical condition: 7 patients (44%) had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 3 to 4 and/or were aged >85 years (aged 87 or 89 years). In addition, 2 patients (12%) had significant comorbidities, such as advanced HIV with grade 4 infections, or myelodisplastic syndrome with grade 4 cytopenias, which precluded tolerance to T/L. The remaining cases were due to early disease progression (37%), often in the context of reduced lenalidomide RDI (63%) or severe toxicity (1 case of grade 4 pulmonary thromboembolism, 6%).
Table 1 presents the main characteristics of patients at diagnosis and at the initiation of T/L treatment. Briefly, at diagnosis, 83% of patients had advanced Ann Arbor stage III to IV, 71% had a high-risk Revised International Prognostic Index (R-IPI) score of 3 to 5, and 63% had primary refractory disease. At the initiation of T/L, to highlight, the median age was 78 years, with 41% of patients aged ≥80 years, the median CIRS score was 6 (range, 0-21), and the median number of previous therapy lines was 2 (range, 1-13).
Of patients treated in this RWE study, 77% would not have been eligible for the L-MIND trial. The most common reasons for noneligibility in the ITT and efficacy cohorts were primary refractory disease (63% and 58%, respectively), having received >3 previous lines of therapy (14% and 13%, respectively), or renal failure (12%). In addition, 10% of patients in the ITT cohort would have been excluded because of an ECOG PS score of 3 to 4, which was twice the rate observed in the efficacy cohort (5%).
Treatment and toxicity
A total 551 cycles of T/L were evaluated. The median number of cycles during the first year in the ITT and efficacy cohorts was 4 and 5. The median RDI for lenalidomide in the ITT and efficacy cohorts during the first year of treatment was 72% and 74% respectively, with 75% and 76% of patients requiring dose reductions, and 56% and 57% experiencing treatment interruptions, respectively. Treatment was generally well tolerated, as shown in Table 2. The most common grade 3 to 4 adverse events were neutropenia (42%), infections (28%), and anemia (21%). The primary cause of death was disease progression, accounting for 41 of 54 (76%) cases. Eight patients died because of infections (including 3 cases of severe COVID-19 pneumonia), 1 case of progressive multifocal leukoencephalopathy, 2 secondary malignancies, and 2 deaths from other causes. At last follow-up, 45 patients were alive (44 in the efficacy cohort) and 27 (32%) were still receiving T/L therapy, representing 61% of surviving patients.
Efficacy
As shown in Table 3, the ORR and CRR were 51% and 35% in the ITT cohort, and 61% and 42% in the efficacy cohort, respectively.
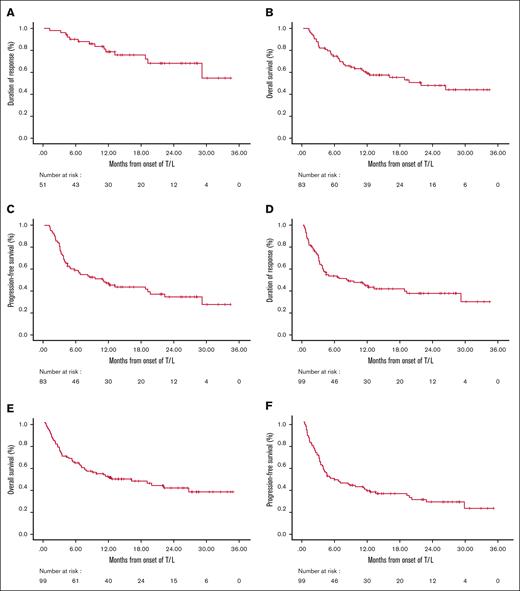
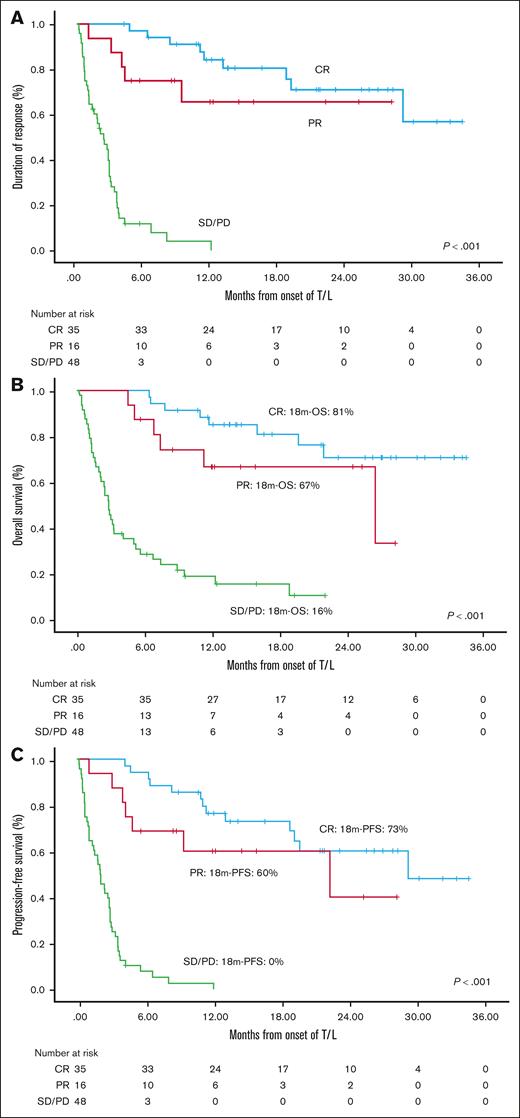
After a median follow-up of 21.6 months (95% confidence interval [CI], 15.3-27.9) in the efficacy cohort and 19.2 months in the ITT cohort, the median DOR has not yet been reached for patients achieving CR, PR, or even SD. The median PFS was 10.9 months (95% CI, 4.7-17) in the efficacy cohort and 4.9 months (95% CI, 1.2-8.6) in the ITT cohort. The median OS was 21.8 months (95% CI, 10.3-33.3) and 12.2 months (95% CI, 3.1-21.4), for the ITT and efficacy cohorts, respectively (Figure 1). Results of DOR, PFS, and OS according to response are shown in Figure 2 and were highly consistent across both cohorts, illustrating that, once achieved, responses tend to be durable. Median PFS for patients achieving a CR, PR, or SD/progressive disease was 29.1, 22.2, and 2 to 2.9 months, respectively (P < .001). Median OS was not reached for patients with a CR, whereas it was 26.4 months, and 2.8 to 4.1 months for those with a PR and SD/progressive disease, respectively (P < .001).
General efficay plots. DOR in the efficacy cohort (A) and ITT cohort (D); OS in the efficacy cohort (B) and ITT cohort (E); and PFS in the efficacy cohort (C) and ITT cohort (F).
General efficay plots. DOR in the efficacy cohort (A) and ITT cohort (D); OS in the efficacy cohort (B) and ITT cohort (E); and PFS in the efficacy cohort (C) and ITT cohort (F).
Impact of RDI for lenalidomide. (A) DOR, and (B) PFS according to RDI for lenalidomide in the efficacy and (C) ITT cohorts. CR, complete response; PR, partial response; SD, stable disease; PD, progression of disease.
Impact of RDI for lenalidomide. (A) DOR, and (B) PFS according to RDI for lenalidomide in the efficacy and (C) ITT cohorts. CR, complete response; PR, partial response; SD, stable disease; PD, progression of disease.
Prognostic factors
Table 4 also presents prognostic factors related to response. In the efficacy cohort, the CRR was significantly higher in nonprimary refractory cases (P = .025) and those who experienced a relapse than those with refractory disease or with progressive disease at T/L (P = .007). In the ITT cohort, similar findings were observed, with higher CRR also seen in patients with ECOG PS score of 0 to 1 (P = .009), normal lactate dehydrogenase (LDH; P = .029). or R-IPI of 0 to 2 (P = .018). The CIRS score did not influence ORR or CRR in either of the cohorts studied. Multivariate binary logistic regression analysis identified a good ECOG PS score (0-1; relative risk, 0.25; 95% CI, 0.09-0.69; P = .008) and having relapsing/nonrefractory disease (RR, 0.22; 95% CI, 0.09-0.55; P = .001) as the main independent predictors of achieving a CR.
The results of the prognostic factors for survival are summarized in Tables 5 and 6. Univariate analysis revealed that PFS was significantly better in both the efficacy and ITT cohorts for patients who relapsed (nonrefractory or progressing) after the previous line of therapy, those with an ECOG PS score of 0 to 1 at the initiation of T/L, normal LDH levels, and non–double-hit histology. In addition, in the ITT cohort, better PFS was observed in patients treated during their first or second relapse or in those with disease that was not primary refractory. Age and comorbidities did not influence PFS.
Regarding OS, worse outcomes were observed in both cohorts among patients with double-hit lymphomas, ECOG PS scores of 2 to 4 at T/L initiation, elevated LDH, high-risk R-IPI scores, and those relapsing/progressing after the previous line of therapy. In addtiion, in the ITT cohort, OS was worse in patients who had primary refractory disease or who had advanced stage disease (Ann Arbor stage III-IV) at T/L initiation. In multivariate analysis (Table 6), poor ECOG PS (score of 2-4), double-hit lymphoma, and refractory disease or progression after the previous line of therapy were identified as independent predictors of poor PFS. These last 2 variables, along with a high-risk R-IPI, were also independently associated with poor OS.
RDI
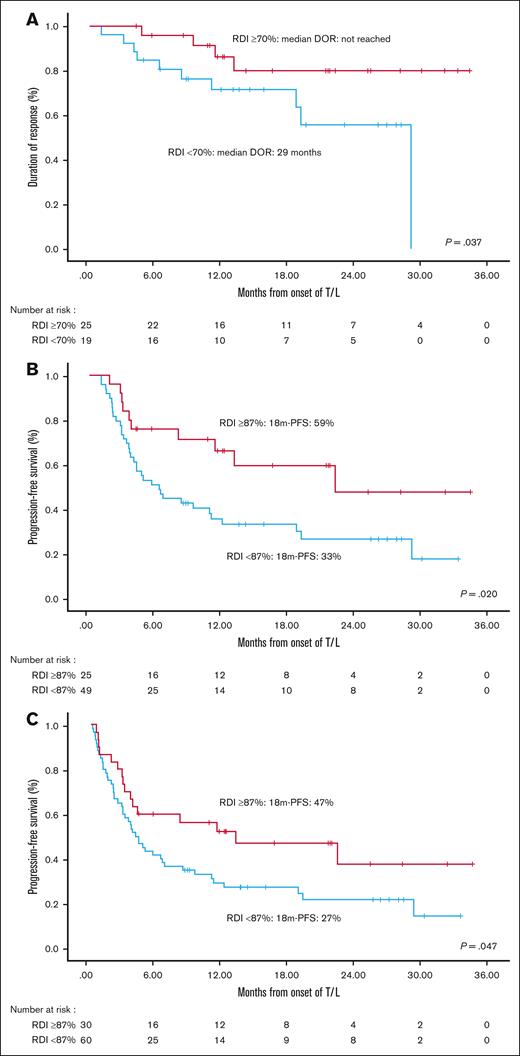
The RDI of lenalidomide had a significant impact on the median DOR: patients with an RDI of ≥70% for lenalidomide had a better DOR than those with an RDI of <70% (median not reached vs 29 months; P = .037). The best PFS outcomes were observed in patients receiving >87% RDI: both in the ITT cohort (median, 13.1 vs 4 months; P = .047) and the efficacy cohort (median, 22.2 vs 6.3 months; P = .020; Figure 3).
Impact of RDI for lenalidomide. DOR (A), and PFS according to RDI for lenalidomide in the efficacy (B) and ITT (C) cohorts.
Impact of RDI for lenalidomide. DOR (A), and PFS according to RDI for lenalidomide in the efficacy (B) and ITT (C) cohorts.
Discussion
Our work represents the Spanish RWE with the T/L combination in patients with R/R DLBCL over a period of 30 months. To our knowledge, these are the closest data to the original L-MIND trial reported in a real-life setting. These data may help illustrate the optimal role for this therapy and highlight how the characteristics of the patients can affect outcomes in RWE studies, when evaluating treatments often reserved for patients who are not candidates for intensive therapies.
The observed ORR of 51% to 61% (ITT vs efficacy cohorts), with CRR of 35% to 42%, and a median DOR not reached after a median follow-up of 19 to 21.6 months, align closely with the results of the L-MIND trial (ORR, 60%; CR, 43%).11,12,15 PFS and OS were lower than those reported in L-MIND trial, which can be attributed to differences in patient populations, because 73% to 77% of the patients in our cohorts would not have been eligible for the L-MIND trial. However, our efficacy cohort had a smaller percentage of patients with adverse prognostic factors, such as double-hit high-grade lymphoma (HGL; 6%), poor ECOG PS score of 3 to 4 (5%), or >3 previous lines of therapy (13%). Furthermore, nearly half of our patients relapsed, and were not progressing or refractory to their previous treatment line. It is remarkable that neither age nor CIRS influenced PFS or OS, illustrating the favorable safety profile of this therapy. Consistent with the L-MIND trial, our results showed that achieving a CR with T/L was associated with particularly favorable outcomes for DOR, PFS, and OS. This finding was especially relevant, because it was observed not only in the efficacy cohort but also in the ITT cohort, highlighting the durability of responses, once achieved.
A regimen such as T/L, primarily reserved for patients with DLBCL who are not candidates to ASCT, is often chosen for patients in a poor condition and with a high rate of comorbidities, which is not the optimal setting to analyze the efficacy of a therapeutic approach. For this reason, the design of the study included 2 cohorts, to assess efficacy while avoiding selection bias: 1 cohort comprised all patients, designated as the ITT cohort; and other cohort evaluated efficacy, which included only those patients who had received at least 1 full cycle of the combination. This approach is supported by principles outlined in the ICH E8 guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and European Medicines Agency’s clinical efficacy and safety guidelines, which emphasize the importance of aligning study populations with specific research objectives. Furthermore, the “Trial within Cohort” methodology provides a precedent for structuring RWE studies to address distinct outcomes.16 From the analysis of 16 patients of the ITT cohort excluded from the efficacy cohort, we could conclude that most of the cases (56%) had poor ECOG PS, very advanced age, or severe comorbidities. The remaining cases had severe toxicity or early disease progression in the context of reduced RDI. All of these cases should be considered in a RW setting; however, they are not optimal candidates for evaluating any therapeutic approach, and it is difficult to imagine that they would have achieved better outcomes with alternative available therapies.
It is also essential to contextualize our results with the broader landscape of published RWE studies. Some reports have used methods such as propensity score matching (eg, RE-MIND and RE-MIND2 studies)17-19 or matching-adjusted indirect comparisons,20 which have demonstrated superior survival outcomes with T/L than standard approaches such as BR, R-GemOx, polatuzumab-rituximab-BR, and rituximab-lenalidomide. In the RE-MIND2 study, even CAR-T therapy yielded similar outcomes to T/L in patients similar to those included in L-MIND trial.18
However, other RWE studies emphasize the risk of using this combination in patients who are extremely unfit or heavily pretreated, which may result in poorer outcomes. For example, the multicenter US study by Qualls et al, which included 178 patients receiving at least 1 dose of tafasitamab (as in our ITT cohort),15 reported less favorable outcomes, with an ORR of 31%; CRR of 19%; and median PFS and OS of 1.9 and 6.5 months, respectively. This cohort had higher rates of adverse prognostic factors compared with both the L-MIND trial and our efficacy cohort, including nearly triple the rate of double-hit HGL, poor ECOG PS of 3 to 4, and a higher proportion of patients in their fourth or later relapse, including 30% of patients failing a previous CAR-T. In contrast, a more favorable RWE study presented by Saverno et al in 2024 at the annual meeting of the American Society of Hemotology,21 which included a cohort with earlier lines of therapy (96% in first or second relapse), fewer primary refractory cases (26%), and half the percentage of patients progressing or refractory to the previous line of therapy, reported better outcomes: ORR of 73%, with median PFS and OS of 11.3 and 24.8 months, respectively. This study highlights that a key factor in suboptimal outcomes is the population of patients who are heavily pretreated and relapse after multiple lines of therapy, including CAR-Ts.
Our study is a retrospective RW analysis and the decision to initiate T/L was made by the physicians at each participant center, primarily based on age and comorbidities, in patients who were not candidates to ASCT or CAR-T therapy. Although it is possible that some patients who received T/L in certain centers might have been eligible for CAR-T therapy, it is important to consider other factors. For most of our study period, only a limited number of hospitals in our country (9 centers in just 6/17 autonomous communities in Spain) were authorized to administer CAR-T therapy. Furthermore, clinical experience with CAR-Ts was still limited even in these approved centers. For these reasons, Spain offered a unique opportunity to evaluate T/L in a slightly more favorable population than that reported in other studies, such as those conducted in the United States.
RDI is a crucial factor in cancer treatment, as it is in DLBCL.22 Our findings further emphasize the importance of RDI in achieving optimal outcomes. In our study, RDI was significantly associated with DOR and PFS, emphasizing the necessity for close patient monitoring and the use of supportive measures, such as hematopoietic growth factors, to maintain adequate RDI.
As with any retrospective study, our work has limitations, including potential selection bias and confounding factors. However, we mitigated these by including all eligible patients from participating centers during the study period. Our primary goal was to emphasize the critical role of patient selection in achieving optimal outcomes. T/L represents an effective alternative for those patients ineligible for ASCT or CAR-T therapy, and should not be considered a metronomic palliative option. Careful selection of candidates is essential to optimize its benefits.
There have been significant advances in the treatment of patients with DLBCL in second or later lines who are not candidates for intensive therapy. T/L can currently be considered one of the most effective options for this population. However, other promising alternatives are emerging. These include the combination of the bispecific anti-CD20/anti-CD3 antibody glofitamab with GemOx, recently approved by the European Medicines Agency based on the STARGLO study23; and the less toxic CAR-T therapy, lisocabtagene maraleucel, based on the PILOT study,24 although it is still not available in several European countries. Additional chemotherapy-free or investigational therapies are also under development.
In conclusion, our Spanish RWE study demonstrates that T/L is both well tolerated and effective, irrespective of age or comorbidities. The optimal use of this combination appears to be in relapsing, nonrefractory cases, particularly in patients with a good ECOG PS (0-1) and in first or second relapse. Results were worse in double-hit HGL. RDI is a key factor in improving outcomes. These findings provide valuable insights into the RW application of T/L and reinforce its role as a key treatment option for patients with R/R DLBCL.
Acknowledgment
This study was supported by Incyte.
Authorship
Contribution: A.G., E.G.-B., and I.Z. designed and directed the study, analyzed, revised, and managed the final database, participated in the statistical analysis, and wrote the manuscript; A.G. and S.P. directed the data acquisition, revised the data, and managed the final database; F.J.P., P.M.-B., D.M., X.M., C.N., A. Ferrero, A.J.-U., M.B.-O., J.D.-V., M.V.C., M.P., G.R., A.A., A.G.-N., D.S.A., T.K., Á. Fernández, J.L., J.P.D.O., S.G.d.V., E.P., A.M., M.B.N., R.F., P.G., J.A.H., M.J.P.P., P.B., D.G.-B., H.D.L.N., S.N., P.A., F.I., L.P., E.D., A.P., and M.S.I. supplied patient data; and all authors revised and approved the final form of the manuscript.
Conflict-of-interest disclosure: A.G. has received research funding and honoraria from Janssen, AbbVie, Roche, AstraZeneca, Incyte, Takeda, Lilly, and BeiGene; M.B.-O. has received research funding and honoraria from Incyte, Roche, AbbVie, and Kite; P.A. has received honoraria from BeiGene, Janssen, Roche, Bristol Myers Squibb, AbbVie, Incyte, Gilead, and AstraZeneca. I.Z. and E.G.-B. disclose honoraria from Incyte. The remaining authors declare no competing financial interests.
Correspondence: Eva Gonzalez-Barca, Department of Hematology, Instituto Catalán d’Oncologia, Hospital Duran i Reynals, IDIBELL, AV Gran via 199-203 08908 Barcelona, Spain; email: e.gonzalez@iconcologia.net; and Antonio Gutiérrez, Department of Hematology, University Hospital Son Espases, IdISBa, Ctra de Valldemossa, 79, 07120 Palma de Mallorca, Spain; email: antoniom.gutierrez@ssib.es.
References
Author notes
The data sets generated and analyzed during this study are available upon reasonable request from the corresponding authors, Eva Gonzalez-Barca (e.gonzalez@iconcologia.net) and Antonio Gutiérrez (antoniom.gutierrez@ssib.es); no unreasonable restrictions will be applied.