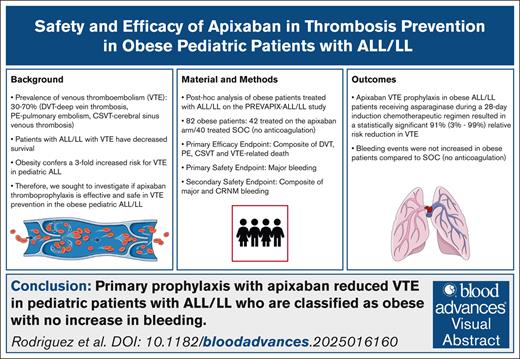
Key Points
Children with obesity with ALL/LL are at risk of VTE complications.
Apixaban prophylaxis resulted in statistically significant reduction in VTE in children with obesity, without an increase in bleeding risk.
Visual Abstract
Pediatric patients with acute lymphoblastic leukemia and lymphoma (ALL/LL) and obesity are at increased risk for venous thromboembolism (VTE). The PREVAPIX-ALL trial was an open-label, randomized, controlled trial assessing the safety and efficacy of apixaban for VTE prevention in pediatric patients with ALL/LL. An a priori subgroup analysis of patients with obesity in the PREVAPIX-ALL trial was planned because of increased VTE risk in this group. Patients with obesity, aged ≥2 to <18 years, central venous catheter, and chemotherapy containing asparaginase were randomized to apixaban (prophylactic dose) vs standard of care (SOC; no anticoagulation) during induction chemotherapy. The primary efficacy end point was a composite of nonfatal symptomatic and asymptomatic VTE and VTE-related death. The primary and secondary safety outcomes were major bleeding and a composite of major and clinically relevant nonmajor (CRNM) bleeding, respectively. A total of 82 PREVAPIX-ALL participants presented with obesity, of whom 42 were randomized to apixaban. For the primary efficacy end point, a significant decrease in VTE events was present in the apixaban arm (1/42 [2.4%]) as compared with the SOC arm (10/40 [25%]; relative risk [RR], 0.09; 95% confidence interval [CI], 0.01-0.97; P = .007). There was a statistically significant treatment obesity interaction, P = .03. No statistically significant difference was observed for the primary efficacy end point among the nonobese group (RR, 0.85; 95% CI, 0.53-1.37; P = .50). No statistically significant difference in major or CRNM bleeding was observed. Apixaban prophylaxis in patients with obesity and ALL/LL resulted in a statistically significant VTE risk reduction with no increase bleeding. This trial was registered at www.clinicaltrials.gov as #NCT02369653.
Introduction
Children and adolescents with acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LL) are at risk for venous thromboembolism (VTE), which primarily occurs during induction therapy.1-6 Multiple factors influence this risk for developing VTE including an active oncologic diagnosis, presence of a central venous catheter (CVC), and administration of chemotherapeutic drugs known to have prothrombotic properties such as asparaginase and corticosteroids.2,4
The prevalence of symptomatic VTE in this patient population is ∼5%; however, 30% to 70% prevalence is reported in studies that include asymptomatic VTE.2,4,5,7,8 Overall fatality in patients with ALL attributed to VTE is reported to occur in up to 4.8%.1,9,10 In addition, VTE affects therapy delivery and is associated with inferior long-term survival in pediatric ALL.8,11 Even in survivors, the morbidity associated with VTE (symptomatic and asymptomatic) includes a lifetime risk for recurrent thrombosis, neurologic changes, catheter removal, postthrombotic syndrome (PTS), and bleeding risk in those requiring antithrombotic therapy.1,5,12
Pharmacologic thromboprophylaxis in pediatric ALL/LL is a rational approach to prevent VTE complications and their sequalae. Therefore, we conducted the PREVAPIX-ALL trial, an open-label randomized controlled trial, which, to our knowledge, was the first of its kind to study the efficacy and safety of a direct oral anticoagulant apixaban as pharmacologic VTE prophylaxis in children and adolescents with newly diagnosed ALL/LL undergoing induction chemotherapy with asparaginase.13-15 The primary analyses showed incorporation of apixaban was safe, with no increase in major bleeding events but did not result in a statistically significant difference in VTE incidence in the overall cohort (12.1% in the apixaban arm vs 17.6% in the standard-of-care (SOC) arm (no anticoagulation, P = .08).15
Obesity is an independent risk factor for VTE in children.16 Hemostatic alterations such as increased thrombin generation and elevated fibrinogen, markers of VTE risk, have been observed in individuals with obesity.17-21 In a retrospective cohort study of children treated for ALL, obesity conferred 3.8-fold increased odds for symptomatic VTE.22 In the design of the PREVAPIX-ALL trial, we therefore included a planned subgroup analysis (post hoc) of the efficacy and safety of apixaban in children and adolescents with obesity concurrent with newly diagnosed ALL/LL, a population at particularly high risk of VTE.
Methods
Study description
The PREVAPIX-ALL trial was an open-label, randomized, controlled trial performed in 74 pediatric hospitals in 9 countries.15
Trial population
In summary, children and adolescents aged ≥1 to <18 years, with newly diagnosed ALL (pre–B cell or T cell) or LL (B or T cell immunophenotype), and a new CVC placed between day −7 and day 4 of induction that was planned to remain in place until at least day 29 of induction therapy were eligible for enrollment in the PREVAPIX study. The CVC (eg, external tunneled line, implantable port, or peripherally inserted central catheter) must have been inserted before the initiation of apixaban. Eligible induction chemotherapy regimens must have included a corticosteroid (eg, prednisone or dexamethasone), vincristine, single or multiple doses of asparaginase, with or without an anthracycline following the chemotherapy backbone for induction as per Children’s Oncology Group. Other antithrombotic agents such as antiplatelet agents were prohibited except for heparin flushes to maintain CVC patency and local alteplase administration for malfunctioning CVCs.15 Previous history of VTE, organ dysfunction, severe uncontrolled hypertension, coagulopathy, extreme hyperleukocytosis (eg, >200 000 cells per μL or a history of leukapheresis) were exclusion criteria.15 Obesity was defined according to the Centers for Disease Control and Prevention body mass index (BMI) classification (BMI ≥ 95th percentile for age and sex specific).23 Children aged <2 years were excluded because BMI is not used in this age group. Patients were not stratified on obesity status as part of the randomization stratification for the PREVAPIX-ALL overall study.
Study interventions
For those receiving apixaban, the study medication had to be initiated before, or within 12 hours of, the first dose of asparaginase or by day 4 ± 3 of planned 3 to 4 drug systemic drug induction chemotherapy, whichever was first. The duration of apixaban therapy was until the end of induction (day 29). The apixaban dose regimen was age and weight based, with those with a weight of ≥35 kg receiving the adult dose of 2.5 mg twice daily. SOC was defined as no anticoagulation therapy. At each clinical visit, efficacy and safety outcomes were recorded. On day 29 (±5 days), all randomized participants who remained in the study were evaluated with a Doppler venous ultrasound of the extremity in which the CVC was placed and an echocardiogram (ECHO) to assess for right atrial thrombosis. Participants with signs and symptoms of VTE at any time during the study underwent imaging as determined by the treating physician.15
Outcome measures
The primary efficacy outcome was based on the definitions recommended by the Perinatal and Paediatric Haemostasis Subcommittee of the Scientific Standardization Committee of the International Society on Thrombosis and Haemostasis for pediatric clinical trials in VTE and was defined as the composite of nonfatal deep vein thrombosis (symptomatic or asymptomatic), pulmonary embolism, cerebral sinus venous thrombosis, and VTE-related death.24 VTE events were first confirmed by blinded adjudication solely based on imaging results and then classified as symptomatic or clinically unsuspected (asymptomatic) based on the investigator’s report. The secondary efficacy outcomes included the individual components of the composite primary efficacy outcome. Symptomatic VTE were included in the analysis only if they occurred during the intended treatment period. However, asymptomatic VTE were included if imaging studies were completed by day 40.
The primary safety outcome was major bleeding based on criteria recommended by the International Society on Thrombosis and Haemostasis for pediatric clinical trials in VTE and was defined as (1) fatal bleeding; (2) clinically overt bleeding associated with a decrease in hemoglobin of at least 20 g/L (ie, 2 g/dL) in 24 hours; (3) bleeding that was retroperitoneal, pulmonary, intracranial, or otherwise involves the central nervous system; and/or (4) bleeding that required surgical intervention in an operating suite, including interventional radiology. The secondary safety outcome was the composite of major and clinically relevant nonmajor (CRNM) bleeding. CRNM bleeding was defined as (1) overt bleeding for which blood product was administered and not directly attributable to the patient’s underlying medical condition and/or (2) bleeding that required medical or surgical intervention to restore hemostasis, other than in an operating room. Other safety outcomes included (1) minor bleeding, defined as any overt or macroscopic evidence of bleeding that does not fulfill the criteria for major or CRNM bleeding, and (2) the number of platelet transfusions administered during the study.24
Biomarker substudy
A biomarker substudy was conducted for participants enrolled in the trial who consented for participation in this substudy. The main aim was to assess the ex vivo effect of apixaban on biomarkers of endogenous thrombin generation and fibrin generation capacity (supplemental Material Section 1). Blood samples were collected at baseline and days 8, 15, 22, and 29 of induction. Samples were frozen and sent to a central laboratory at the University of Alberta, Edmonton, Canada, for analysis.
Statistical analysis
The analyses of all efficacy end points were based on the randomized/intent-to-treat population. The primary analysis of safety events was based on the safety (all randomized) population. Obesity was a prespecified subgroup of interest in the PREVAPIX-ALL statistical plan. A post hoc analyses comparing those with obesity in the apixaban and SOC arms for the primary efficacy, safety, and secondary safety outcomes end points included event rate, 95% confidence interval (CI) for event rate, and P value (nominal). Participants with no symptomatic VTE and no evaluable ultrasound or ECHO assessment were counted as not having an event. Ninety-five percent (95%) CIs for the ratio of event rates were computed based on the Mantel-Haenszel method stratified by age (<10 years, ≥10 to <18 years), sex (male, female), and subtype (T-cell, B-cell precursor, or other). 95% CIs for single event rates were constructed based on the Agresti-Coull method. P values and 95% CI for relative risk were calculated using the Cochran-Mantel-Haenszel test stratified by age at baseline. Relative risk (RR) reduction is calculated as 1-RR. Adjusted RR of event rates takes into consideration age at baseline as a stratification factor (<10 years; ≥10 to <18 years). The analysis of the biomarker data is included in the supplemental Material. The protocol for this trial was reviewed and approved by regulatory authorities, the Scientific Council of the Children’s Oncology Group, and ethics committees at each site. Written informed consent was obtained from legal guardians of all participants along with assent when applicable. An independent data safety monitoring committee was used.15
Results
Patient population characteristics
A total of 82 participants were classified as obese, of whom 42 were randomized to apixaban and 40 were randomized to SOC (no anticoagulation; Table 1; Figure 1). Demographics characteristics among the obese-apixaban and obese-SOC subgroups were comparable.
Among patients with obesity assigned to the apixaban arm (n = 42), 6 of 42 did not have both ultrasound venous Doppler (US) and ECHO; the remaining participants had both imaging studies completed (n = 36; 85.7%). For patients assigned to the SOC arm (n = 40), 3 of 40 did not have both US and ECHO, 1 of 40 had US but not ECHO, and all others had both (n = 37; 92.5%).
End point analysis
For the primary efficacy outcome, 1 event ([2.38%]; 95% CI, 0.00-13.44) was observed in the apixaban arm compared with 10 (25%) events in the SOC arm (95% CI, 14.02-40.6); (RR, 0.09; 95% CI, 0.01-0.97; nominal P = .007; Table 2). The primary safety end point (major bleeding) was reported in 1 of 42 participants (2.38%) (retroperitoneal hematoma) (95% CI, 0.00-13.44) in the apixaban arm and in 2 of 40 (5%) participants (n = 1 intracranial hemorrhage and n = 1 menorrhagia) in the SOC arm (95% CI, 0.50-17.39). For the secondary safety end point, 3 events in 42 (7.14%) participants (CRNM bleeding: n = 1 epistaxis and n = 1 hematochezia; major bleeding: n = 1 hemorrhage; 95% CI,1.77-19.70) were reported in the apixaban arm and 2 events in 40 (5%) participants (major bleeding: n = 1 intracranial hemorrhage and n = 1 menorrhagia; 95% CI, 0.50-17.39; P = .69) in the SOC arm.
Tables 3 and 4 provide a summary of the primary efficacy, safety, and secondary safety outcomes for the nonobese subgroup analysis and the overall trial, respectively. No statistically significant difference was observed for the primary efficacy end point among the those in the nonobese group (apixaban, 30/210 [14.3%] and SOC, 34/206 [16.5%]; RR, 0.85 [95% CI, 0.53-1.37]; P = .50; treatment-obesity interaction, P = .03). No statistically significant difference was observed for the secondary efficacy end point (apixaban arm, 10/210 [4.76%] and SOC, 3/206 [1.46%]; RR, 3.24 [95% CI, 0.91-11.51; P = .05; treatment-obesity interaction, P = .50).
Pharmacokinetic analysis
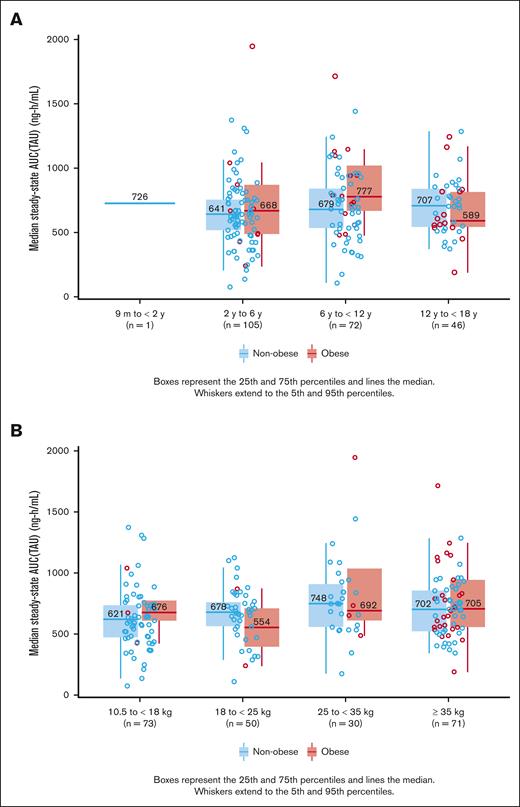
The apixaban exposure duration was similar among the subgroups: the obese group: mean exposure (standard deviation [SD]) of 23.4 days (SD, 5.55) and the nonobese group, 23.5 days (SD, 6.44). Apixaban median steady state area under the curve was completed in 224 participants of the study of whom 16.9% (n = 38/224) were categorized having obesity. No statistically significant difference was observed in the median steady state area under the curve drug exposure between obese and nonobese groups stratified by age on a linear scale: obese (n = 38) 686.94 ng h/mL (95% CI, 397.8-1314.6) and nonobese (n = 186) 663.50 ng h/mL (95% CI, 319.1-1090.9; Figure 2A-B).
Comparison of Apixaban median steady state AUC (TAU). Comparison of the median steady-state AUC (TAU) in pediatric patients without vs those with obesity, stratified by age (A) and weight on a linear scale (B). AUC, area under the curve; TAU, TAU(dosing interval)
Comparison of Apixaban median steady state AUC (TAU). Comparison of the median steady-state AUC (TAU) in pediatric patients without vs those with obesity, stratified by age (A) and weight on a linear scale (B). AUC, area under the curve; TAU, TAU(dosing interval)
Biomarker substudy
A total of 187 patients participated in the biomarker study (supplemental Tables 1-12). Overall, 153 were classified as nonobese (SOC [n = 92], apixaban [n = 61]), and 34 participants were obese (SOC [n = 15], apixaban [n = 19]). Biomarkers of in vivo hypercoagulability were assessed (supplemental Tables 1 and 2). Both D-dimer and prothrombin F1.2 showed a statistically significant decrease on days 15 and 22 when comparing apixaban with SOC in both patients with and those without obesity. In addition, D-dimer showed a statistically significant decrease on day 29 when comparing apixaban to SOC in both patients with and those without obesity. No difference was seen when comparing obese with nonobese groups in the apixaban or SOC arms.
Biomarkers of ex vivo anticoagulant effect of apixaban treatment in obese and nonobese groups were assessed by both thrombin and fibrin generation capacity assays (supplemental Tables 5-9). Anticoagulant effect was indicated by statistically significantly increased lag time in the apixaban arm for both the thrombin generation assay in participants with and those without obesity (days 8, 15, and 22; and days 8, 15, 22, and 29; respectively) and the fibrin generation assay in participants with and those without obesity (day 15, and days 8 and 22, respectively). No difference was found when comparing participants with and those without obesity in the apixaban-treated or SOC arms. Furthermore, thrombin peak height was statistically significantly decreased in the apixaban arm (days 15) in those with obesity and (days 15 and 22) in those without. No difference was seen in peak height for fibrin generation, likely related to all patients having very low fibrinogen levels. Thrombin endogenous potential was statistically significantly decreased on days 15 and 22 in both the obese and nonobese groups. No difference in thrombin peak height, fibrin peak height, or time to 50% clot lysis was identified when comparing participants with obesity with those without obesity in the apixaban-treated or SOC arms.
Biomarkers of fibrinolytic capacity at baseline (pretreatment) in all patients were compared in obese and nonobese groups (supplemental Table 12). Fibrin peak height was statistically significantly increased (P = .01) in patients with obesity.
Discussion
VTE adversely affects pediatric ALL survival, and for survivors of the disease, results in increased acute morbidity (eg, organ dysfunction related to the anatomic location of the thrombosis) and long-term sequelae (eg, PTS).5,9,12 VTE prevention strategies have long been studied in pediatric ALL/LL.5,25-28 Several small studies pioneered the concept of thromboprophylaxis in this population; however, sample size limited the ability to determine the efficacy and safety of primary prophylaxis.25-29 Despite the limited sample size, primary thromboprophylaxis demonstrated a trend toward efficacy in preventing VTE while maintaining a relative low bleeding risk. The THROMBOTECT trial demonstrated the efficacy of pharmacologic VTE prophylaxis in a large adequately powered randomized clinical trial.30 However, major issues with adherence to low molecular weight heparin were encountered because of the need for subcutaneous administration. Apixaban provides an alternative for VTE prophylaxis that can be more acceptable to patients and caregivers because of its oral administration and lack of requirement for monitoring.
Patients with ALL and obesity have an increased risk for treatment-related toxicities compared with peers without obesity. In the Nordic Society of Pediatric Haematology and Oncology ALL 2008 non–high-risk protocol, children with obesity had an increased incidence rate ratio (IRR) for severe toxic events (IRR, 1.55; 95% CI, 1.07-2.50) including organ toxicity, bleeding, and unexpected severe adverse reactions, compared with children with a healthy weight. Also, children with obesity aged ≥10 years had increased IRR for asparaginase-related toxicities, such as thrombosis (IRR, 2.87; 95% CI, 1.00-8.21), compared with children with a healthy weight of the same age group.31 The increased prevalence of toxicity and a higher risk of truncation of asparaginase may play a role in the poor prognosis of children with obesity aged ≥10 years with ALL.32-34 Similarly, other studies have demonstrated the negative impact of obesity on ALL survival.3,6,8,11,22,35,36 In a retrospective cohort of children with ALL, obesity was an independent risk factor for VTE and significantly predictive of VTE irrespective of ethnicity, age, leukemia phenotype, and treatment intensity.22 These findings suggest that children with obesity with ALL are at higher risk for treatment toxicity and may benefit from pharmacologic prophylaxis. Our subgroup analysis results show the benefit of primary VTE prophylaxis in the obese group with a relative risk reduction of 91% compared with patients with obesity receiving SOC (95% CI, 3-99; P = .007) with no significant increased risk for bleeding. Further analysis in children without obesity showed no statistically significant difference for the primary efficacy and secondary safety outcome for those treated with apixaban vs SOC. The statistically significant efficacy treatment obesity interaction P value of .03 indicates that the treatment effect differs significantly between patients with obesity and those without.
Hemostatic alterations have been described in individuals with obesity, suggesting a hypercoagulable state.17-21 Elevation of plasminogen activator inhibitor levels were found in 37 healthy adolescents with obesity when compared with 16 age-matched controls with healthy weights, resulting in an ex vivo decrease in fibrinolytic capacity in the participants with obesity.37 In the PREVAPIX biomarker substudy, a comprehensive biomarker assessment of hypercoagulability was performed. It was evident that apixaban exerted its role in blunting hypercoagulability in both the obese and nonobese subgroups. A significant difference in hypercoagulability response between obese and nonobese groups that could explain the efficacy in VTE prevention was not observed in the apixaban arm. The small sample size in the biomarker study might have resulted in the lack of statistically significant differences among the biomarkers of hypercoagulability in the obese group when compared with the nonobese group that could have explained the beneficial effects on VTE prevention observed in the obese-apixaban group.
Long-term survivors of pediatric ALL face diverse long-term sequelae related to therapy, including PTS. PTS has a negative impact on the quality of life, can result in chronic pain, functional impairment, and risk for VTE recurrence.38,39 Although most VTE events in the PREVAPIX study were asymptomatic, survivors of pediatric ALL with asymptomatic thrombosis have an incidence of PTS comparable with those with symptomatic thrombosis: 54% after asymptomatic thrombosis compared with 63% incidence for those with symptomatic events.40 These findings highlight the contribution of asymptomatic VTE to PTS in long-term ALL survivors. Identifying subgroups of pediatric patients with ALL/LL at increased risk for VTE and who will benefit from safe and effective primary thromboprophylaxis is of paramount importance to clinicians.
In summary, this subgroup analysis among pediatric patients with obesity treated with apixaban showed a statistically significant (P = .007), 91% relative risk reduction in VTE events when primary prophylaxis with apixaban was given during induction chemotherapy for ALL/LL. Bleeding events were not significantly different among those treated with apixaban when compared with no anticoagulation in the SOC group. No differences in pharmacokinetics and biomarker analyses were observed among the obese and nonobese groups that could have explained the differences observed in VTE prevention. Further analysis in children with healthy weights showed no statistically significant difference for the primary efficacy and secondary safety outcome for those treated with apixaban vs SOC. The primary safety outcome could not be meaningfully evaluated due to the low number of events. The statistically significant efficacy treatment obesity interaction P value of .03 for the primary efficacy endpoint indicates that the treatment effect of apixaban differs significantly between those with obesity and those without. One limitation was the absence of stratification for the participants with obesity in the original overall trial design that might have led to potential confounding factors. Other clinical and/or physiologic differences within the obese group might have not been balanced with the standard randomization trial design; only stratification at the time of randomization would have allowed a more controlled balance of other clinical characteristics within the obese groups randomized to receive apixaban vs no anticoagulation (SOC). The study results show that apixaban is safe and effective in prevention of VTE in pediatric patients with obesity and ALL/LL undergoing induction chemotherapy.
Acknowledgments
The authors acknowledge and thank the children, adolescents, and their families for their participation in PREVAPIX trial.
This study was funded by the Bristol Myers Squibb–Pfizer alliance.
Authorship
Contribution: V.R., S.H.O., L.G.M., A.M., J.L.D., and N.A.F. contributed to the concept, design, and/or analysis of the data and data collection; and all authors contributed to interpretation, critical writing, or revising of the intellectual content, and the final approval of the version to be published.
Conflict-of-interest disclosure: A.M., J.L.D., and N.A.F. are employees of Bristol Myers Squibb (BMS). The institutions of V.R. and S.H.O. received salary support from the Children’s Oncology Group for their roles as study chair and study vice-chair, respectively. E.O. served as a consultant for Jazz Pharmaceuticals; and received consulting fees from Seagen, Inc. L.G.M. received research funding from BMS for the biomarker substudy. The remaining authors declare no competing financial interests.
A complete list of the PREVAPIX-ALL investigators appears in “Appendix.”
Correspondence: Vilmarie Rodriguez, Hematology, Oncology & Bone and Marrow Transplantation, Nationwide Children’s Hospital, 700 Children’s Dr, Columbus, OH 43205; email: vilmarie.rodriguez@nationwidechildrens.org.
Appendix
List of investigators by country and number of randomized children
Australia: Seong Lin Khaw, Francoise Mechinaud*, The Royal Children's Hospital, Parkville, Victoria (3); Paul James Wood, Peter Downie*, Monash Medical Centre Clayton, Clayton, Victoria (2); Frank Alvaro, John Hunter Children’s Hospital, New Lambton Heights, New South Wales (0); Chris Fraser, Lady Cilento Children's Hospital, South Brisbane, Queensland (7)
Belgium: An Van Damme, Cliniques Universitaires Saint-Luc, Bruxelles (7); Alina Ferster, Hopital Universitaire Des Enfants Reine Fabiola Huderf, Bruxelles (0); Jaques Van Heerden, Antwerp University Hospital, Edegem (3); Veerle Labarque, UZ Leuven, Leuven (7); Veerle Mondelaers, UZ Gent, Gent (1)
Canada: Donna Johnston, Jacqueline Halton*, Children's Hospital of Eastern Ontario, Ottawa, ON (6); Victor Anthony Lewis, Alberta Children's Hospital, Calgary, AB (8); Shayna M Zelcer, Children's Hospital London Health Sciences Centre, London, ON (0); Lisa Goodyear, Janeway Children's Health and Rehabilitation Centre, St. John's, NL (0); Sarah McKillop, Stollery Children's Hospital, Edmonton, AB (2)
Czech Republic: Jaroslav Sterba, Klinika detske onkologie, Fakultni nemocnice Brno (1)
Hungary: Gabor Ottoffy, PTE AOK KK Gyermekgyogy. Klin, Pecs (2); Csongor Kiss, Gyermekhematológiai és Onkológiai Intézet, Debrecen (2); Gabor Kovacs, II. sz. Gyermekgyogyaszati Klinika, Budapest (8)
Korea: Hyoung Jin Kang, Seoul National University Hospital, Seoul (23); Seung Min Hahn, Severance Hospital, Yonsei University Health System, Seoul (11)
Mexico: Norma Candelaria Lopez Santiago, Instituto Nacional De Pediatria, Mexico City, Distrito Federal (2); David Gomez Almaguer, Hospital Universitario Dr. Jose Eleuterio Gonzalez, Monterrey, Nuevo Leon (2); Fernando Sánchez, Hospital Civil De Guadalajara - Nuevo Dr. Juan I. Menchaca, Guadalajara, Jalisco (2)
New Zealand: Siobhan Cross, Christchurch Hospital, Christchurch (0) Poland Tomasz Szczepanski, Oddzial Hematologii i Onkologii Dzieciecej, Zabrze (1); Igor Olejnik, Klinika Transplantacji Szpiku Onkologii i Hematologii Dzieciecej, Wroclaw (0)
Russia: Yulia Dinikina, Federal North West Medical Research Center, St. Petersburg (18); Alexey A Maschan, Center of Pediatric Hematology, Oncology and Immunology, Moscow (3); Timur Tejmurazovich Valiev, N.N. Blokhin National Medical Research Center of Oncology, Moscow (4); Margarita Belogurova, St. Petersburg Clinical & Practice Centre, Saint-Petersburg (1); Margarita Timofeeva, Kirov Research Institute ofHematology and Blood Transfusion, Kirov (0)
United States: Mark Anthony Ranalli, Nationwide Children's Hospital, Columbus, OH (17); Scott Mark Bradfield, Nemours Children's Clinic, Jacksonville, FL (7); Van Thu Huynh, Children's Hospital of Orange County, Orange, CA (0); Anderson B Collier, Gail C Megason*, University of Mississippi Medical Center, Jackson, MS (2); Michael K Richards, Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN (2); Peter M Gordon, University of Minnesota, Minneapolis, MN (0); Glen Lew, Michael A Briones*, Children's Healthcare of Atlanta, Atlanta, GA (33); Albert Kheradpour, Loma Linda University Health, San Bernardino, CA (10); David R Freyer, Children's Hospital of Los Angeles, Los Angeles, CA (19); Peter H Shaw, Gregory Alan Hale*, All Children's Hospital, Saint Petersburg, FL (9); Lisa M McGregor, Penn State Milton S. Hershey Medical Center, Hershey, PA (10); Leonard Charles Bailey, Children’s Hospital of Philadelphia, Philadelphia, PA (11); Emad Salman, Golisano Children’s Hospital of Southwest Florida, Fort Myers, FL (1); Lolie Chua Yu, Children’s Hospital New Orleans, New Orleans, LA (3); Steven James Kuerbitz, Akron Children's Hospital, Akron, OH (1); Stacey Rifkin-Zenenberg, Frances Flug*, Hackensack University Medical Center, Hackensack, NJ (7); Julienne Brackett, Texas Children's Hospital, Houston, TX (4); Patrick Brown, Johns Hopkins University, Baltimore, MD (17); Jessica L Boklan, Phoenix Children's Hospital, Phoenix, AZ (9); Mariko Sato, University of Iowa Hospitals and Clinics, Iowa City, IA (0); Stuart H Gold, North Carolina Cancer Hospital, Chapel Hill, NC (11); Ashok Raj, Novak Center for Children's Health, Louisville, KY (3); James D Cooper, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA (1); Barbara Jean Bambach, Women and Children's Hospital of Buffalo, Buffalo, NY (11); Judy Linn Felgenhauer, Providence Sacred Heart Medical Center and Children's Hospital, Spokane, WA (8); Thomas B Russell, Wake Forest Baptist Health, Winston-Salem, NC (0); Steven W Pipe, University of Michigan Cancer Center, Ann Arbor, MI (6); Jessica C Hochberg, New York Medical College, Valhalla, NY (24); Wendy Leigh Woods-Swafford, Blank Cancer and Blood Disorders Center, Des Moines, IA (5); Anne-Marie Langevin, University of Texas Health Science Center San Antonio, San Antonio, TX (1); Mira Ann Kohorst, Vilmarie Rodriguez*, Mayo Clinic, Rochester, MN (9); Narayana Gowda, St. Mary’s Medical Center, West Palm Beach, FL (3); Evangeline Joy Brown, Jeffrey Schwartz*, Sacred Heart Hospital, Pensacola, FL (0); Guy Howard Grayson, Scott & White Memori al Hospital and Clinic, Temple, TX (1); Adam Esbenshade, Vanderbilt University Medical Center, Nashville, TN (9); Richard Drachtman, Robert Wood Johnson University Hospital, New Brunswick, NJ (6); Emi Caywood, Nemours A. I. duPont Hospital for Children, Wilmington, DE (40); Jagadeesh Ramdas, Geisinger Medical Center, Danvville, PA (17); Angela Rose Girvin, Jeffrey Robert Andolina*, University of Rochester Medical Center, Rochester, NY (4); Craig David Lotterman, Ochsner Medical Center, New Orleans, LA (7); Michael Scott Isakoff, Connecticut Children's Medical Center, Hartford, CT (6); Robert Gordon Irwin, Mary Bridge Children Health Center, Tacoma, WA (0); Jason M Fixler, Sinai Hospital of Baltimore, MD (0); Eugenia Chang, St Luke's Regional Medical Center, Boise, ID (2); Shannon Mitchell Cohn, Amy C Fowler*, Virginia L Harrod*, Children's Blood and Cancer Center, Austin, TX (2); Jacob Troutman, Felipe S Bautista Otanez*, Lydia Alberta K Boateng*, Lehigh Valley Health Network, Allentown, PA (7); Paul D HarkerMurray, Medical College of Wisconsin, Milwaukee, WI (4); Lauren Weintraub, Alexander Gozman*, Vikramjit S Kanwar*, Kenneth Gerald Lucas*, Albany Medical Center, Albany, NY (1); Sanjay Ahuja, Yousif Matloub*, University Hospitals, Cleveland, OH (6); Hilda H Ding, Rady Children's Hospital - San Diego, San Diego, CA (16); Anna Beata Pawlowska, Weili Sun*, City of Hope, Duarte, CA (2); Douglas Cipkala, St Vincent Hospital, Indianapolis, IN (0); Haydar Frangoul, Children's Hospital at TriStar Centennial, Nashville, TN (1); Nkechi Ifeoma Mba, Driscoll Children's Hospital, Corpus Christi, TX (3); Jay Michael Balagtas, Lucile Packard Children's Hospital (LPCH), Palo Alto, CA (0); Maureen M O'Brien, Cincinnati Children's Hospital Medical Center, Cincinnati, OH (0); William B Slayton, Shands Hospital at University of Florida, Gainesville, FL (2); Jhon Guerra Moreno, HIMA San Pablo Hospital, Caguas, PR (0); Ramamoorthy Nagasubramanian, Nemours Children's Clinic – Orlando, Orlando, FL (3); Alissa Kahn, St. Joseph's Children's Hospital, Paterson, NJ (0); Nina Kadan-Lottick, Smilow Cancer Hospital at Yale New Haven, New Haven, CT (0); James Thomas Curry Badgett, University of Kentucky Markey Cancer Center, Lexington, KY (1); Philip Michael Monteleone, SUNY Upstate Medical University, Syracuse, NY (2); Sushmita Nair, Navicent Health Physician Group, Macon, GA (0)
∗Previous Principal Investigator.
References
Author notes
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html. Data are available on request from the corresponding author, Vilmarie Rodriguez (vilmarie.rodriguez@nationwidechildrens.org).
The full-text version of this article contains a data supplement.