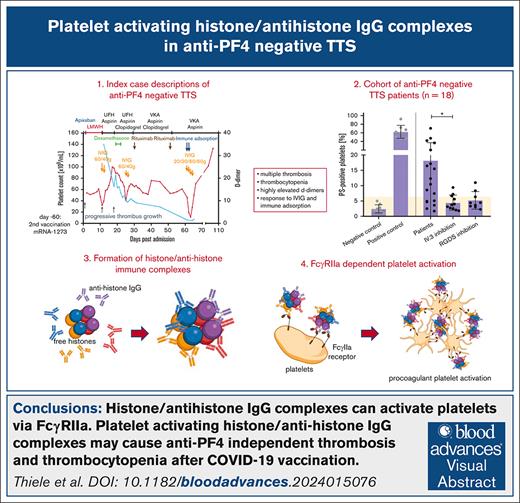
Key Points
Anti-PF4 IgG–negative TTS can develop after mRNA-based COVID-19 vaccination.
Histone/antihistone IgG complexes activating platelets via the FcγIIa receptor are potential mediators in anti-PF4–negative TTS.
Visual Abstract
Thrombosis and thrombocytopenia syndromes (TTS) describe immune-mediated thrombotic adverse reactions after vaccination against COVID-19. Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a well-known subentity of TTS, caused by adenovirus vector–based vaccines. VITT is mediated by anti-platelet factor 4 (PF4) immunoglobulin G (IgG) antibodies, activating platelets via Fc-γ IIa receptors (FcγRIIa). We describe clinical and serological features of 18 patients with anti-PF4/heparin enzyme-linked immunosorbent assay (ELISA)–negative TTS in temporal relationship to messenger RNA (mRNA)–based COVID-19 vaccination. Symptoms began at a median of 7 (range 1 - 61) days after vaccination. Patients showed thrombocytopenia (platelet count 59 × 103/μL; range, 0 to 127 × 103/μL); petechiae (n = 7), venous thromboembolism (n = 11), arterial thrombosis (n = 6), disseminated intravascular coagulation (n = 1), and combined arterial and venous thromboses (n = 1). Twelve sera-induced FcγRIIa-dependent and caspase-independent procoagulant activation of platelets indicated by phosphatidylserine exposure and CD62P expression. We found histones precipitated with IgG fractions of TTS sera. Antibodies binding to histones were found in 8 of 12 platelet-activating sera. Ex vivo–generated histone/antihistone IgG complexes strongly activated platelets via FcγRIIa, whereas antihistone IgG alone did not. Platelet autoantibodies were detected in 7 of 12 sera targeting glycoprotein (GP) IIb/IIIa (n = 5), GPIb/IX (n = 5), and GPIa/IIa (n = 3). However, sera containing platelet anti-GPIIb/IIIa autoantibodies activated also platelets from a patient with Glanzmann thrombasthenia, making it unlikely that these autoantibodies are causative for platelet activation. Finally, 2 of 114 healthy vaccinees developed antihistone antibodies after mRNA-based COVID-19 vaccination. Our data indicate a new subentity of TTS associated with platelet-activating histone/antihistone IgG complexes. Further studies are warranted to characterize the biological and clinical role of post-mRNA–based vaccination antihistone antibodies. The SeCo trial was registered at www.ClinicalTrials.gov as #NCT04370119.
Introduction
During the COVID-19 vaccination programs, rare but severe thrombotic adverse events were reported. Patients developed thrombosis at unusual sites involving cerebral veins and sinuses and/or splanchnic veins combined with thrombocytopenia and high D-dimer levels. Symptoms typically began between 4 to 30 days after vaccination with adenovirus vector–based vaccines ChAdOx1 nCoV-19 COVID-19 Vaccine (Astra-Zeneca),1-3 Janssen COVID-19 Vaccine (Ad26.COV2.S),4,5 and Gam-COVID-Vac (Sputnik V).6 The Brighton collaboration and regulatory agencies named these adverse events thrombosis and thrombocytopenia syndrome (TTS).7
A well-defined subentity of TTS is vaccine-induced immune thrombocytopenia and thrombosis (VITT). VITT is caused by immunoglobulin G (IgG) platelet-activating antibodies directed against the platelet chemokine platelet factor 4 (PF4).1-3 In VITT, anti-PF4 IgG forms immune complexes with PF4, which crosslink Fc-γ IIa receptors (FcγRIIa) on platelets, monocytes, and neutrophils,8,9 inducing strong activation of platelets and other blood cells.
However, in 3% to 11% of patients presenting after COVID-19 vaccination with thromboses and thrombocytopenia, no anti-PF4 antibodies were found.10-12 Such patients observed in our laboratory, had been vaccinated with messenger RNA (mRNA)–based COVID-19 vaccines including BNT162b2 (BioNTech/Pfizer) and mRNA1273 (Moderna). This is in sharp contrast to patients with VITT, who all received adenovirus vector–based vaccines.
Beside anti-PF4 antibodies, other antibodies can induce FcγRIIa-dependent platelet activation. Examples are complexes of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG and the SARS-CoV-2 spike protein, which cause platelet activation after mRNA-based COVID-19 vaccination10; or immune complexes consisting of IgG antibodies of unknown specificity in patients who are severely ill with COVID-19 with thrombosis and thrombocytopenia.13
In this study, we aimed to characterize patients with anti-PF4–negative TTS who had received mRNA-based COVID-19 vaccines, and found that their sera activated platelets via the FcgRIIa in 12 of 18 patients. Among them we identified platelet-activating antihistone antibodies in 8 patients.
Materials and methods
Ethics
Index patients (cases 1 and 2), healthy volunteers (males and females, aged 18-68 years), the patient with Glanzmann thrombasthenia, and participants of the “Screening for COVID-19 and Monitoring of Serological Responses to SARS-CoV-2 in Healthcare Workers study” (SeCo; ClinicalTrials.gov identifier: NCT04370119) provided written and informed consent. For all investigations, ethical approval was provided by the institutional ethical boards (Greifswald: BB 068/20; BB 052/21; BB 014/14; Munich [Ludwig Maximilian University]: no. 20-1067).
Identification of patients with TTS after mRNA vaccination
Patient samples were referred to the Greifswald laboratory between 1 April 2021 and 31 March 2022 for laboratory investigation of thrombosis and thrombocytopenia in temporal relationship to COVID-19 vaccination. Clinical data were retrieved from the patient files (index cases) and the laboratory submission forms. Analyzed were age, sex, vaccine type, and date of vaccination, date of symptom onset, platelet count nadir, and location of thrombosis.
Patients were included in the mRNA-vaccine cohort, if the following criteria were met:
first, second, or third vaccination against COVID-19 with an mRNA-based vaccine (BNT162b2, Comirnaty, BioNTech/Pfizer, Mainz, Germany; or mRNA-1273, Spikevax; Moderna Biotech, Cambridge, MA);
platelet count nadir of <150 × 109/L; and
new onset of arterial or venous thrombosis; and/or signs of disseminated intravascular coagulation.
Cohort of healthy vaccinees immunized with mRNA-based COVID-19 vaccines
Longitudinal samples of health care workers who participated in the prospective SeCo study from April 2020 until March 2021 and who were vaccinated with at least 2 shots of mRNA-based COVID-19 vaccines were assessed for antihistone antibodies.
Healthy controls
Healthy blood donors without signs and symptoms for TTS and without detectable antibodies against histones provided control sera.
Antibody testing
Anti-PF4/heparin IgG enzyme-linked immunosorbent assay (ELISA) was performed with an in-house test as previously reported.14 This ELISA identifies IgG antibodies against PF4/heparin complexes and PF4 alone.1 Antiplatelet IgG antibodies directed against the glycoproteins (GP) IIb/IIIa, Ib/IX, and Ia/IIa were tested by the monoclonal antibody–specific immobilization of platelet antigens (MAIPA) assay as previously described.15 Direct MAIPA (testing antibodies bound to autologous platelet GP) was performed whenever enough platelets from patients were available. The indirect MAIPA (testing free serum antibodies) was performed when only serum was available. Tests were considered positive, if optical densities were above the threshold of 0.2 optical density units in the indirect and direct MAIPA assay. Antihistone IgG was tested using the antihistone ELISA kit coated with histone proteins H1, H2A, H2B, H3, and H4, according to the manufacturers protocol (Orgentec, Mainz, Germany).
Platelet isolation
Platelets were prepared as previously described.16,17 In brief, blood was drawn by cubital venipuncture and then diluted 1:1 in modified Tyrode buffer (137 mM NaCl, 2.8 mM KCl, 12 mM NaHCO3, 5.5 mM sucrose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH = 6.5); centrifuged at 70g for 35 minutes to generate platelet-rich plasma. Platelet-rich plasma was diluted 1:2 in modified Tyrode buffer supplemented with prostaglandin I2 (0.1 mg/mL) and either albumin (0.1%) or casein (0.01%) followed by centrifugation for 5 to 10 minutes at 1000g at room temperature. The resulting pellets were resuspended in Tyrode buffer (pH 6.5). Platelet counts were assessed using a Sysmex XN-V Series XN-1000V cell counter (Sysmex Deutschland GmbH, Norderstedt, Germany).
Platelet activation assays
For platelet signaling analysis and activation analysis of suspended Glanzmann platelets, platelets were isolated as described earlier, incubated with fluorescent antibodies against CD62P/P-selectin, annexin V, and CD41 (all BioLegend Inc, San Diego, CA) and stimulated with patient-derived serum (dilution 1:1). Hereafter, platelets were fixated using paraformaldehyde (1% final concentration, Sigma) for 10 minutes at room temperature in the dark. Samples were then measured on a BD LSR Fortessa flow cytometer. Gating and analyses of mean fluorescence intensities were performed using FlowJo version 10 (BD Life Sciences, Heidelberg, Germany). Glanzmann platelets were incubated with the sera of patients 1, 2, and 12 (Table 1).
For platelet activation analysis with patients’ sera and artificial histone/antihistone IgG complexes, platelets were purified from anticoagulant citrate dextrose–anticoagulated whole blood from healthy donors who did not take antiplatelet drugs or nonsteroidal anti-inflammatory drugs as previously described.18 These platelets were coincubated with sera from patients with TTS for 10 minutes under stirring (∼300 rpm) at a ratio of 1:1. Activation of washed platelets by patients’ sera was measured by annexin V–allophycocyanin as a marker for phosphatidylserine (PS)-exposure using flow cytometry as previously described.19 Phosphate-buffered saline was used as a negative control, whereas positive controls were platelets costimulated with convulxin and thrombin receptor activation peptide. Shear stress was applied using 2 magnetic spheres per microtiter well on a magnetic stirrer. Tests were considered positive when the percentage of annexin-V–positive platelets exceeded 6.265% (≥3 standard deviations of the mean negative control).
Monoclonal antibody IV.3 was used to block FcγRIIa-mediated platelet activation8,20 and the tetrapeptide Arg-Gly-Asp-Ser (RGDS) was used to block GPIIb/IIIa activation in the presence of patients’ sera. Flow cytometry was performed on a Cytoflex S (Beckman Coulter, Krefeld, Germany).
Further blocking experiments were performed with ciclosporin A (calcineurin inhibitor blocking mitochondrial cyclophilin D and mitochondrial depolarization during procoagulant platelet activation), dasatinib (tyrosine kinase inhibitors), ibrutinib (Bruton kinase inhibitor), U73122 (phospholipase C inhibitor), a specific Syk inhibitor (BI 1002494), and IVIG.
PEG-IgG complex precipitation
Selected patient sera (patients 2, 4, and 11; Table 1) were subjected to polyethylene glycol (PEG) precipitation21,22 and mixed 1:1 with PEG solution (6% [weight per volume] PEG, 100 mM boric acid, 75 mM NaCl, 25 mM sodium tetraborate) and incubated at 4°C overnight. Samples were centrifuged at 2000g and 4°C for 20 minutes. The pellet was washed with 50% PEG solution (diluted in distilled water) and centrifuged again at 2000g and 4°C for 20 minutes. The resulting pellet was resuspended in 100 HEPES sodium dodecyl sulfate buffer (20 mM HEPES, pH 8.0, 1% [weight per volume] sodium dodecyl sulfate) and denatured at 95°C for 2 minutes.
MS
Sample preparation, protein quantification, and tryptic digestion using the SP3 protocol were performed as previously described.23 Briefly, 4 μg of proteins were bound to SP3 beads and digested at 37°C overnight using a trypsin/Lys-C mix (V5071, Promega GmbH, Walldorf, Germany). The eluted peptides were reduced and alkylated with dithiothreitol and iodocaetamide before mass spectrometry (MS) analyses.
Liquid chromatography (LC) tandem MS analyses were carried out via an Ultimate 3000 nano-LC system (Thermo Fisher Scientific, MA) connected to a Q Exactive HF mass spectrometer (Thermo Fisher Scientific, MA) operating in data-independent acquisition mode.
Database search using the human reference proteome sequence (Uniprot) and direct data-independent acquisition analysis were performed using the Spectronaut software (Biognosys AG, Schlieren, Switzerland) and Excel (Microsoft, Redmond, WA). Additional analyses and visualizations were conducted using an in-house R pipeline.24
Generation of histone/antihistone IgG complexes and platelet activation
Washed platelets from healthy donors were spiked with 10 μg/mL, 1 μg/mL, or 0.1 μg/mL (final) of recombinant histone dimer (H2A-H2B) or 20 μg/mL, 2 μg/mL, or 0.2 μg/mL (final) recombinant histone octamer (H3.3, both Active Motif, Belgium) or histone storage buffer (0.1 mM Tris, pH 7.4; 20 mM NaCl; 0.01 mM EDTA; 0.1% glycerol; 0.05 mM β-mercaptoethanol) as control. Mouse antihistone 2A and 2B IgG antibodies were pooled and added to samples spiked with histone dimer (H2A-H2B) whereas mouse antihistone 2A, 2B, 3, and 4 IgG antibodies were pooled and added to samples spiked with histone octamer (H3.3) in various concentrations (5 μg/mL, 0.5 μg/mL, or 0.05 μg/mL; all Abcam, United Kingdom). Phosphate-buffered saline was used as buffer control. Samples were incubated for 10 minutes and shear stress was applied using 2 magnetic spheres per microtiter well on a magnetic stirrer. Afterward, samples were labeled using CD62P-PE-Cy5 (Becton Dickinson, NJ) or annexin-V–allophycocyanin, as previously described.19 Inhibition of platelet activation by monoclonal antibody IV.3 was used to block FcγRIIa-mediated platelet activation.
Results
Index case descriptions of anti-PF4–negative TTS
Case 1
A 57-year-old man was hospitalized for worsening of a thrombosis of the right femoral vein, which had initially been found 19 days earlier and treated with apixaban (Figure 1A). The patient received the second dose of mRNA-1273 60 days before the first thrombosis. Patient history includes a kidney transplantation in 2009 because of an IgA nephropathy and COVID-19 infection 6 months earlier. Neutralizing response and cellular immunity against SARS-CoV-2 either by vaccination or natural infection was shown by immunoassay, which quantified the anti–spike-protein-1 receptor-binding domain antibodies with 6035.3 binding antibody units per mL (normal range: 0-7 binding antibody units per mL). On admission, the patient presented with high D-dimers (>35 200 μg/L) and anticoagulation was changed to therapeutic-dose enoxaparin. However, after 10 days, thrombosis further extended into the right common iliac vein and the vein of the transplanted kidney, resulting in graft failure. Treatment was changed to therapeutic-dose unfractionated heparin and aspiration thrombectomy in crossover technique was performed. The low platelet counts of 54 × 109/L identified at hospitalization declined to 29 × 109/L during the patient’s stay. High-dose IVIG was initiated on 2 consecutive days (60 g on day 1, and 40 g on day 2) in assumption of autoantibody-triggered platelet activation. With rising platelet counts (58 × 109/L), aspirin 100 mg/d was added. However, thrombosis extended to the inferior cava vein, to the external iliac and femoral veins on the left (contralateral) side detected by abdominal computed tomography; prompting triple therapy including aspirin, clopidogrel, and activated partial thromboplastin time–adjusted unfractionated heparin as well as high-dose steroids (dexamethasone 40 mg on 5 consecutive days). When the platelet count decreased again at day 25, a new cycle of IVIG was given and anticoagulation was switched to a vitamin-K antagonist. Screening for thrombophilia, underlying autoimmune diseases, and malignancy was negative. In addition, rituximab was given at days 30 and 50. No subsequent thromboembolic events occurred, and the patient was discharged. At day 60 the patient was readmitted because of a new episode of thrombocytopenia (49 × 109/L) and immune adsorption was started at 2 consecutive days with accompanying administration of IVIG at 4 consecutive days, with an increasing platelet count thereafter. Since then, the platelet count stabilized in the normal range (last visit: 169 × 109/L) with a normal kidney allograft function and partial recanalization of the renal vein on ultrasound.
Clinical course of TTSs. Platelet counts (black line) D-dimer levels (blue line) and the clinical course of 2 index patients with anti-PF4–negative TTS after mRNA-based vaccination against COVID-19. (A) Index case 1: 57-year-old male with a history of kidney transplantation in 2009 and a recent COVID-19 infection with progressive thrombosis of the right femoral vein. Despite antithrombotic triple therapy, immunosuppression, and high-dose IVIG, only immunoadsorption was able to control the situation and resulted in a sustained platelet count increase. (B) Index case 2: 37-year-old male with a history of Hodgkin lymphoma in remission and prior episodes of ITP with petechiae, headache, thrombocytopenia, and elevated D-dimer. IVIG was administered to stabilize platelet counts but the patient developed pulmonary embolism and splanchnic vein thrombosis. Therapeutic dose anticoagulation, immunosuppression, and plasma exchange increased platelet counts without further thromboembolic events. LMWH, low-molecular-weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonists.
Clinical course of TTSs. Platelet counts (black line) D-dimer levels (blue line) and the clinical course of 2 index patients with anti-PF4–negative TTS after mRNA-based vaccination against COVID-19. (A) Index case 1: 57-year-old male with a history of kidney transplantation in 2009 and a recent COVID-19 infection with progressive thrombosis of the right femoral vein. Despite antithrombotic triple therapy, immunosuppression, and high-dose IVIG, only immunoadsorption was able to control the situation and resulted in a sustained platelet count increase. (B) Index case 2: 37-year-old male with a history of Hodgkin lymphoma in remission and prior episodes of ITP with petechiae, headache, thrombocytopenia, and elevated D-dimer. IVIG was administered to stabilize platelet counts but the patient developed pulmonary embolism and splanchnic vein thrombosis. Therapeutic dose anticoagulation, immunosuppression, and plasma exchange increased platelet counts without further thromboembolic events. LMWH, low-molecular-weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonists.
Case 2
Three days after his second vaccination with BNT162b2, a 37-year-old man was admitted with petechiae and headache, and a platelet count of 8 × 109/L (Figure 1B). SARS-CoV-2 polymerase chain reaction of a nasopharyngeal swab was negative. The patient’s history included Hodgkin lymphoma in remission and recurrent episodes of immune thrombocytopenia 8 years before. Because of strongly elevated D-dimer (>12 mg/L) with a low platelet count suggestive of TTS, IVIG was given but no corticosteroids because of suspicion of reactivation of Epstein-Barr virus infection. Platelet counts increased thereafter, but a few days later the patient developed pulmonary embolism. Despite starting anticoagulation with therapeutic dose apixaban, the patient developed extensive splanchnic vein thrombosis 9 days later. Local thrombolysis with recombinant tissue plasminogen activator was initiated. Although initially successful, abdominal vein thrombosis recurred. After a second course of recombinant tissue plasminogen activator the patient developed severe abdominal bleeding but recovered after closure of an arterial-venous fistula in the liver. For suspected immune thrombocytopenia, the patient received plasmapheresis and another cycle of treatment with IVIG. Under anticoagulation with argatroban, platelet counts stabilized without further thromboembolic events.
Case series of anti-PF4–negative TTS
Blood samples of 18 patients (Table 1; including index patient 1 and 2) with documented thrombocytopenia and venous and/or arterial thrombosis after mRNA-based vaccination against COVID-19 were referred for investigation of antibody-mediated adverse events. All patients tested negative for anti-PF4 IgG. Median age was 58 years (range, 13-83), and symptoms occurred at a median of 7 days after vaccination (range, 1 to >60). However, only 7 of 18 patients showed an onset of symptoms within the typical time window for VITT, that is, days 5 to 20.11,25
Patients developed venous thromboembolism (n = 11), arterial thromboses (n = 6), and disseminated intravascular coagulation (n = 1). One patient had venous and arterial thromboses. Notably, 3 of 18 patients had cerebral venous sinus thrombosis, and 1 patient had splanchnic vein thrombosis (index patient 2). For 7 of 18 patients, additional bleeding symptoms were reported. Mean platelet counts at nadir were 59 × 109/L with a range of 0 to 127 × 109/L.
High proportion of anti-PF4–negative TTS sera activate platelets via FcγRIIa
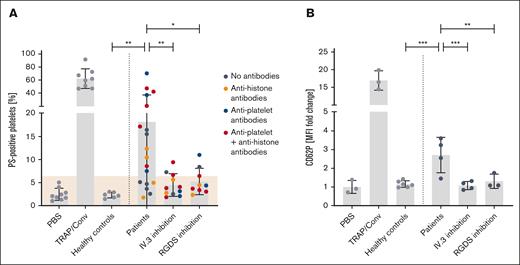
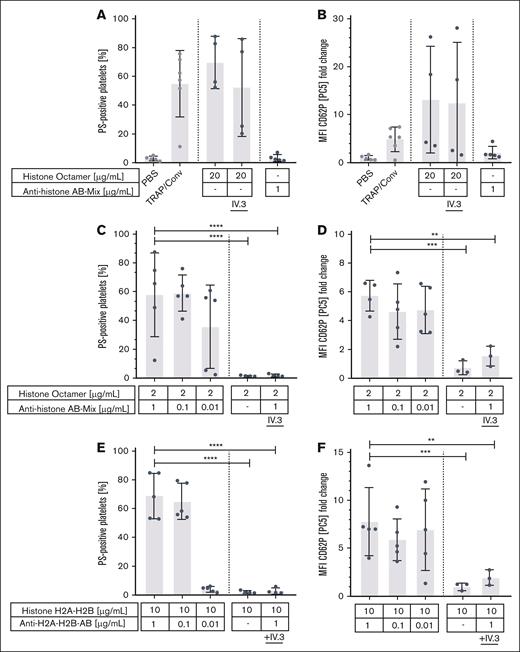
Based upon the clinical observation of thrombosis and thrombocytopenia with an improvement after IVIG administration, we hypothesized that anti-PF4–negative TTS might also be caused by immune complex induced platelet activation. We therefore coincubated platelets from healthy volunteers with sera from patients with TTS. Of 18 sera, 12 strongly activated platelets by inducing PS exposure and CD62P expression. This was inhibited by the monoclonal antibody IV.3 (inhibiting FcγRIIa-mediated platelet activation) and by RGDS (inhibiting GPIIb/IIIa mediated platelet activation; Figure 2; supplemental Figures 1 and 2).
Platelet activation pattern induced by TTS sera. Platelet activation induced by sera from 18 patients with anti-PF4/heparin ELISA–negative TTS. Control sera (n = 6) were obtained from healthy blood donors. Platelets of healthy donors were washed and incubated with the patients’ sera. Each dot represents the mean of at least 3 experiments. (A) Procoagulant platelet formation and (B) CD62P expression was assessed in flow cytometry. Activation was blocked by the monoclonal antibody IV.3, which inhibits platelet activation via FcγRIIa and by RGDS (blocks signaling of GPIIb/IIIa). Cutoff was defined as mean + 3 standard deviations of negative control (shown in beige). Negative control: phosphate-buffered saline; positive control: 20 μM thrombin receptor activating peptide + 100 ng/mL convulxin. Statistics were performed using an ordinary 1-way analysis of variance followed by an uncorrected Fisher least significant difference test, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; TRAP/Conv, thrombin receptor activating peptide + convulxin.
Platelet activation pattern induced by TTS sera. Platelet activation induced by sera from 18 patients with anti-PF4/heparin ELISA–negative TTS. Control sera (n = 6) were obtained from healthy blood donors. Platelets of healthy donors were washed and incubated with the patients’ sera. Each dot represents the mean of at least 3 experiments. (A) Procoagulant platelet formation and (B) CD62P expression was assessed in flow cytometry. Activation was blocked by the monoclonal antibody IV.3, which inhibits platelet activation via FcγRIIa and by RGDS (blocks signaling of GPIIb/IIIa). Cutoff was defined as mean + 3 standard deviations of negative control (shown in beige). Negative control: phosphate-buffered saline; positive control: 20 μM thrombin receptor activating peptide + 100 ng/mL convulxin. Statistics were performed using an ordinary 1-way analysis of variance followed by an uncorrected Fisher least significant difference test, ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; TRAP/Conv, thrombin receptor activating peptide + convulxin.
Histones are precipitated with IgG of patients with anti-PF4–negative TTS
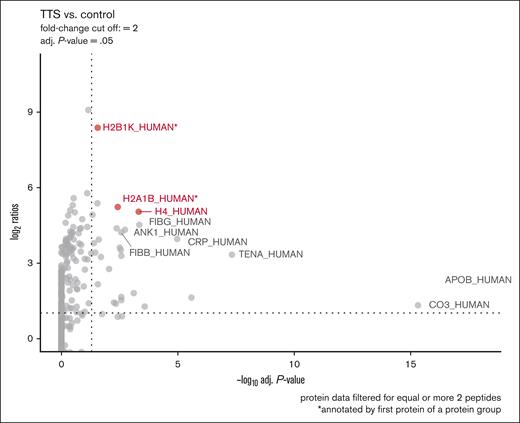
To further identify the potential binding partners of platelet-activating antibodies, we applied PEG IgG complex precipitation to enrich IgG complexes of patients. We used the sera of patients 2, 4, and 11 (Table 1; selected by sufficiently available amount of serum); and 3 healthy controls and performed LC electron-spray ionization tandem MS analysis of precipitated IgG complex fractions. Strikingly, protein groups containing histones H2B1K, H2A1B, and H4 showed a higher abundance in IgG complex precipitates of all 3 patients than controls (Figure 3).
Identification of histones as a target protein of immune complexes. Proteomic analysis of PEG-precipitated sera. Three sera from patients with anti-PF4–negative TTS (patients 2, 4, and 11; Table 1) were compared with 3 sera from healthy control donors, each subjected to PEG precipitation. Proteins were determined and quantified using LC electron-spray ionization tandem MS and results depicted in a volcano plot. Histones H2B type 1B (H2A1B), type 1-A H2B type 1-K (H2B1K); and H4 were found with the highest ratios (y-axis) among the significantly enriched proteins. ∗Highest ranked identification in LC electron-spray ionization tandem MS. adj., adjusted.
Identification of histones as a target protein of immune complexes. Proteomic analysis of PEG-precipitated sera. Three sera from patients with anti-PF4–negative TTS (patients 2, 4, and 11; Table 1) were compared with 3 sera from healthy control donors, each subjected to PEG precipitation. Proteins were determined and quantified using LC electron-spray ionization tandem MS and results depicted in a volcano plot. Histones H2B type 1B (H2A1B), type 1-A H2B type 1-K (H2B1K); and H4 were found with the highest ratios (y-axis) among the significantly enriched proteins. ∗Highest ranked identification in LC electron-spray ionization tandem MS. adj., adjusted.
A high proportion of patients with anti-PF4–negative TTS have antihistone antibodies
Next, we tested the sera of 18 patients with anti-PF4–negative TTS for antihistone IgG. In 11 of 18 patients, antihistone IgG were present (including 1 patient with a borderline result of 39.2 U/mL; cutoff ELISA: 40 U/mL). Eight of 11 sera also induced procoagulant platelet activation.
Four sera also activated platelets, but antihistone IgG were lacking (n = 2) or no serum was available for antihistone IgG testing (n = 2). Three sera neither activated platelets nor had detectable antihistone antibodies (1/3 sera were not available for antihistone antibody testing; Table 1).
Anti-PF4–negative TTS sera and histone/antihistone IgG complexes induce procoagulant platelet activation via FcγRIIa
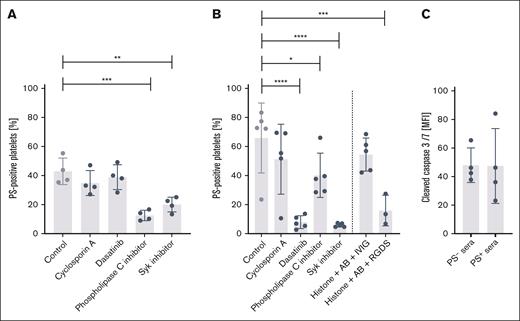
To elucidate further underlying prothrombotic mechanisms, we investigated platelet signaling cascades downstream of FcγRIIa. Indeed, we found that PS exposure of healthy platelets in response to patient sera was mediated through phospholipase Cγ and dependent of extracellular calcium influx. We also found that inhibition of Syk, a Src-family kinase acting downstream of FcγRIIa, but not cyclophilin D, c-Src, or Bruton kinase, which have been described previously to be implicated in procoagulant platelet formation, reduced plasma-induced PS.16,26-28 No difference in caspase activation was detected when platelets were incubated with platelet-activating and nonactivating TTS sera. These findings indicate that procoagulant platelet formation but not caspase-mediated platelet apoptosis is induced by anti-PF4–negative TTS sera through FcγRIIa-mediated signaling (Figure 4).
Signaling pathways involved in immune complex–mediated procoagulant platelet activation. Platelet activation depends on extracellular calcium and is partially mediated through Syk signaling. (A) Platelets from n = 4 healthy donors were exposed to TTS sera after preincubation with the indicated inhibitors and blocking agents. (B) Platelets from n = 4 healthy donors were exposed to 2 μg/mL histone octamer and a mix of the corresponding mouse antihistone antibodies (0.1 μg/mL each) after preincubation with the indicated inhibitors and blocking agents. Statistics were performed using an ordinary 1-way analysis of variance followed by an uncorrected Fisher least significant difference test. Controls are the uninhibited conditions with TTS sera (A) and generated histone/antihistone complexes (B) ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (C) Quantification of cleaved caspase 3/7 activity after treatment with platelet activation–inducing or noninducing plasma samples (n = 4 per group) as determined by flow cytometry. Viable platelets were assessed without paraformaldehyde fixation after incubation with TTS sera inducing PS exposure (PS+) compared with sera from patients with TTS and/or immune thrombocytopenia that do not induce PS exposure (PS−). Gating and analyses of MFIs were performed using FlowJo version 10 (BD Biosciences). AB, antibody; MFI, mean fluorescence intensity.
Signaling pathways involved in immune complex–mediated procoagulant platelet activation. Platelet activation depends on extracellular calcium and is partially mediated through Syk signaling. (A) Platelets from n = 4 healthy donors were exposed to TTS sera after preincubation with the indicated inhibitors and blocking agents. (B) Platelets from n = 4 healthy donors were exposed to 2 μg/mL histone octamer and a mix of the corresponding mouse antihistone antibodies (0.1 μg/mL each) after preincubation with the indicated inhibitors and blocking agents. Statistics were performed using an ordinary 1-way analysis of variance followed by an uncorrected Fisher least significant difference test. Controls are the uninhibited conditions with TTS sera (A) and generated histone/antihistone complexes (B) ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. (C) Quantification of cleaved caspase 3/7 activity after treatment with platelet activation–inducing or noninducing plasma samples (n = 4 per group) as determined by flow cytometry. Viable platelets were assessed without paraformaldehyde fixation after incubation with TTS sera inducing PS exposure (PS+) compared with sera from patients with TTS and/or immune thrombocytopenia that do not induce PS exposure (PS−). Gating and analyses of MFIs were performed using FlowJo version 10 (BD Biosciences). AB, antibody; MFI, mean fluorescence intensity.
Next, we tested the ability of ex vivo–generated histone/antihistone immune complexes to activate platelets. Immune complexes were formed by recombinant human histone octamers or histone dimers (H2A-H2B) with mouse anti-human histone antibodies of the IgG subtype 1 (which are able to activate human platelets29). Very high concentrations of histone octamers alone (20 μg/mL) activated platelets independent of antihistone antibodies and independent of the FcγRIIa. In contrast, histone octamers and dimers (H2A/H2B) together with antihistone H2A, H2B, H3, and H4-antibodies dose dependently activated healthy human platelets inducing PS exposure and CD62P expression. This was FcγRIIa dependent and inhibited by IV.3. Histone octamers at low concentrations (≤2 μg/mL) or histone dimers at any concentration did not activate platelets in the absence of antihistone antibodies (Figure 5).
Platelet activation induced by histones, antihistone antibodies, and histone/antihistone immune complexes. PS exposure and CD62P expression of washed platelets incubated with high concentrations of histone octamers and antihistone antibodies alone (A-B); with various concentrations of histone octamers mixed with antihistone antibodies (B-C); and histone dimers H2A/H2B with a mix of the corresponding antihistone antibodies (D-E). PS exposure was assessed after annexin-V staining and CD62P expression in flow cytometry. Immune complexes were produced by incubating human histones and mouse antihistone antibodies dose dependently. High concentrations of histone octamers were able to activate platelets. This was independent of FcγRIIa because platelet activation could not be blocked by the monoclonal antibody IV.3 (which inhibits platelet activation via FcγRIIa) (A-B). In contrast, 10-fold lower concentrations of histone octamers were only able to activate platelets after incubation with the corresponding antihistone antibodies. This could be blocked by the monoclonal antibody IV.3 showing that activation by immune complexes was FcγRIIa dependent (B-C). The same was observed for histone dimers H2A/H2B incubated with the corresponding antihistone antibodies ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; TRAP/Conv, thrombin receptor activating peptide + convulxin.
Platelet activation induced by histones, antihistone antibodies, and histone/antihistone immune complexes. PS exposure and CD62P expression of washed platelets incubated with high concentrations of histone octamers and antihistone antibodies alone (A-B); with various concentrations of histone octamers mixed with antihistone antibodies (B-C); and histone dimers H2A/H2B with a mix of the corresponding antihistone antibodies (D-E). PS exposure was assessed after annexin-V staining and CD62P expression in flow cytometry. Immune complexes were produced by incubating human histones and mouse antihistone antibodies dose dependently. High concentrations of histone octamers were able to activate platelets. This was independent of FcγRIIa because platelet activation could not be blocked by the monoclonal antibody IV.3 (which inhibits platelet activation via FcγRIIa) (A-B). In contrast, 10-fold lower concentrations of histone octamers were only able to activate platelets after incubation with the corresponding antihistone antibodies. This could be blocked by the monoclonal antibody IV.3 showing that activation by immune complexes was FcγRIIa dependent (B-C). The same was observed for histone dimers H2A/H2B incubated with the corresponding antihistone antibodies ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001. MFI, mean fluorescence intensity; PBS, phosphate-buffered saline; TRAP/Conv, thrombin receptor activating peptide + convulxin.
In addition, procoagulant platelet activation was enhanced by addition of low concentrations of histones to sera containing antihistone IgG. On the contrary, immune complexes of bovine serum albumin (BSA) and anti-BSA IgG did not activate platelets. These data demonstrate that histone/antihistone IgG immune complexes but not randomly assembled complexes such as BSA/anti-BSA IgG are able to activate platelets (supplemental Figures 3 and 4).
Platelet autoantibodies do not induce platelet activation
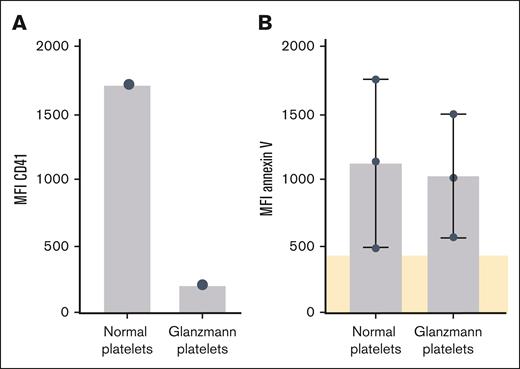
We previously detected platelet autoantibodies after vaccination against COVID-19 with adenovirus vector–based vaccines.30 We also found platelet autoantibodies in the sera of 7 of 18 patients with anti-PF4–negative TTS by MAIPA: 5 patients had anti-GPIIb/IIIa autoantibodies, 5 patients had anti-GPIb/IX, and 3 patients had anti-GPIa/IIa autoantibodies (Table 1). Of 7 sera containing antiplatelet autoantibodies, 6 also induced procoagulant platelet activation. However, sera containing anti-GPIIb/IIIa autoantibodies also activated platelets of a patient with Glanzmann thrombasthenia, a hereditary condition in which platelets do not express the GPIIb/IIIa receptor.31 Furthermore, a representative serum of a patient with typical immune thrombocytopenia containing autoantibodies against GPIIb/IIIa did not induce procoagulant platelet formation in a thrombus formation experiment. Thus, it seems unlikely that platelet autoantibodies induced procoagulant platelet activation in our experiments. (Figure 6; supplemental Figure 5).
Activation response of GPIIbIIIa-deficient platelets to TTS sera. CD41 expression and platelet activation of Glanzmann platelets in response to sera from patients with TTS. (A) CD41 measured on normal platelets and platelets from a patient with Glanzmann thrombasthenia. (B) Patients’ sera containing or lacking platelet autoantibodies with GPIIb/IIIa specificity were incubated with normal platelets and platelets from a patient with Glanzmann thrombasthenia. Platelet activation was assessed by testing PS exposure after annexin-V staining in flow cytometry, cutoff was defined as mean of negative sera (shown in beige).
Activation response of GPIIbIIIa-deficient platelets to TTS sera. CD41 expression and platelet activation of Glanzmann platelets in response to sera from patients with TTS. (A) CD41 measured on normal platelets and platelets from a patient with Glanzmann thrombasthenia. (B) Patients’ sera containing or lacking platelet autoantibodies with GPIIb/IIIa specificity were incubated with normal platelets and platelets from a patient with Glanzmann thrombasthenia. Platelet activation was assessed by testing PS exposure after annexin-V staining in flow cytometry, cutoff was defined as mean of negative sera (shown in beige).
Vaccinees can develop antihistone antibodies after repeated mRNA-based COVID-19 vaccination
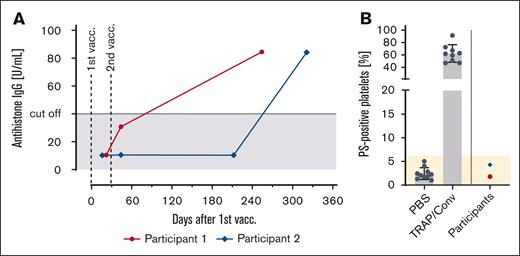
Finally, we tested repeated samples of 114 health care workers participating in the SeCo study (mean age, 44 years; 85 women) for the presence of antihistone antibodies after vaccination. We identified 2 of 114 patients, who developed antihistone antibodies in temporal relationship to vaccination. One had a positive test 15 days after the second vaccination, and the other 293 days after the second vaccination. Both participants tested negative for anti-PF4 IgG and had no history of any autoimmune disease. These sera testing positive for antihistone antibodies, however, did not activate platelets by means of PS exposure (Figure 7).
Development of antihistone IgG in temporal relationship to mRNA vaccination. (A) Antihistone IgG titers in 2 of 144 vaccinated participants of the SeCo study, who received 2 shots of mRNA-based COVID-19 vaccines. (B) Platelet activation analysis with the sera of vaccinees who tested positive for antihistone IgG. Platelets of healthy donors were washed and incubated with the vaccinees’ sera. Each dot represents the mean of at least 3 tests. No procoagulant platelet formation was detected by testing PS exposure after annexin-V staining in flow cytometry. Negative control: phosphate buffered saline; positive control: 20 μM thrombin receptor activating peptide + 100 ng/mL convulxin. PBS, phosphate-buffered saline; TRAP/Conv, thrombin receptor activating peptide + convulxin; vacc., vaccination.
Development of antihistone IgG in temporal relationship to mRNA vaccination. (A) Antihistone IgG titers in 2 of 144 vaccinated participants of the SeCo study, who received 2 shots of mRNA-based COVID-19 vaccines. (B) Platelet activation analysis with the sera of vaccinees who tested positive for antihistone IgG. Platelets of healthy donors were washed and incubated with the vaccinees’ sera. Each dot represents the mean of at least 3 tests. No procoagulant platelet formation was detected by testing PS exposure after annexin-V staining in flow cytometry. Negative control: phosphate buffered saline; positive control: 20 μM thrombin receptor activating peptide + 100 ng/mL convulxin. PBS, phosphate-buffered saline; TRAP/Conv, thrombin receptor activating peptide + convulxin; vacc., vaccination.
Discussion
This study identifies platelet-activating histone/antihistone IgG complexes as a potential cause of anti-PF4–negative TTS after COVID-19 vaccination. We identified 18 patients with anti-PF4–negative TTS with thrombocytopenia and thrombosis. The case vignettes of 2 index patients demonstrate high D-dimer levels, uncontrolled hypercoagulability almost refractory to anticoagulants and antiplatelet therapy, and multiple thromboses (Figure 1). These clinical descriptions of TTS are reminiscent to the clinical picture of VITT.
However, only 7 of 18 patients showed an onset of symptoms in the typical time window between days 5 and 20,11,25 whereas the other 11 patients had a much shorter or longer onset of symptoms. Furthermore, and also in contrast to VITT,11,25,32 only 5 of 18 patients became symptomatic after their first vaccination, whereas the other patients developed symptoms after their second or third dose.
The fact that 3 patients had cerebral venous sinus thrombosis, and 1 had splanchnic vein thrombosis raised the question whether immune complex–mediated platelet activation could play a role in these patients with TTS similar to the situation in VITT. Strikingly, 12 of 18 sera activated platelets and even triggered procoagulant platelets as shown by high PS exposure and P-selectin expression (Figure 2). This activation depended on FcγRIIa, which is pathognomonic for immune complex–driven platelet activation.33
One candidate for inducing procoagulant platelets in anti-PF4–negative TTS are antihistone antibodies, which we identified by PEG immune complex precipitation (Figure 3). This method has limitations because not only immune complexes but also other large molecules could be precipitated. However, consistent with a role of histone–antihistone immune complexes in PF4-negative TTS, we identified antihistone IgG in 11 of 18 patients and most sera with antihistone antibodies also induced procoagulant platelet activation (Table 1).
PS exposure induced by TTS sera can occur in procoagulant platelets or in aged platelets undergoing apoptosis.34,35 We found that both extracellular calcium influx, PLCγ, and Syk kinase activity downstream of FcγRIIa were essential for TTS serum-induced procoagulant platelet activation, whereas caspase activation and thus classical platelet apoptosis pathways were not involved (Figure 4). This indicates that procoagulant platelet activation rather than apoptosis plays a role in the pathogenesis of anti-PF4–negative TTS, which was also shown for other immunothromboses such as VITT.36 In line, previous reports have highlighted the role of procoagulant platelets in arterial thrombus formation and correlated systemic elevations in procoagulant platelet counts with a hypercoagulable state, thrombosis, and mortality.13,37-40
In addition, ex vivo generated histone-IgG complexes strongly activate platelets in an FcγRIIa dependent manner, whereas antihistone IgG antibodies alone did not. It is known from previous studies, that free histones alone can activate platelets via Toll-like receptors 2 and 4.41 We confirm these findings but only for very high concentrations of histone octamers of 20 μg/mL (Figure 5). Platelet activation by histones alone is also independent of FcγRIIa because it could not be blocked by the monoclonal antibody IV.3. In contrast, much lower concentrations of histone dimers and octamers (≤2 μg/mL) together with antihistone IgG strongly activated platelets via FcγRIIa. These observations strongly suggest that IgG antibodies and histones must form an immune complex to activate platelets via FcγRIIa, and add to the hypothesis that these immune complexes are capable of inducing TTS. Furthermore, platelet activating sera will need to contain histone/antihistone IgG complexes rather than the antibodies alone.
Histones are positively charged proteins and can expose repetitively the same epitopes by forming tetramers or octamers.42 These tetramers or octamers apparently bind corresponding IgG antibodies in a way that their free Fc parts cluster and activate FcγRIIa on platelets, in analogy to anti-PF4–IgG and PF4/heparin–IgG complexes found in VITT and in heparin-induced thrombocytopenia. Multimerization may therefore be prone histones and corresponding IgG antibodies to form platelet-activating immune complexes.
Antihistone antibodies are well known as autoantibodies in systemic lupus erythematosus.43 Potentially, patients who develop anti-PF4–negative TTS after COVID-19 vaccination have a predisposition for the formation of antihistone autoantibodies. An underlying autoimmune condition might also explain the relatively high prevalence of antiplatelet autoantibodies in our patient cohort, supporting a recent study of Meier et al44 who found 19% of patients with clinically suspected VITT testing positive for anti-platelet antibodies. Furthermore, Petito et al45 observed a high frequency of antiplatelet autoantibodies in recipients of COVID-19 vaccines together with increased circulating platelet–derived microvesicles and soluble P-selectin and blood clotting activation, suggesting a prothrombotic potential of these autoantibodies. This study does not rule out that antiplatelet autoantibodies also contribute to anti-PF4–negative TTS. Interestingly, we found the strongest platelet activation for sera containing both antihistone IgG and platelet autoantibodies (supplemental Figure 1).
However, our experiments using PF4-negative TTS sera containing anti-GPIIb/IIIa autoantibodies, and platelets of a patient with Glanzmann thrombasthenia, lacking expression of the platelet receptor GPIIb/IIIa show, at least for anti-GPIIb/IIIa antibodies, that these autoantibodies alone cannot be responsible for inducing procoagulant platelet activation (Figure 6; supplemental Figure 5).
We identified at least 2 patients (patients 11 and 12; Table 1; supplemental Figure 1) whose sera induced procoagulant platelets via FcγRIIa activation in whom we could neither detect anti-PF4 IgG nor antihistone antibodies. However, in patient 11, histones were also precipitated by PEG with the IgG fraction. Potentially, antihistone IgG were not detected in this patient because of limitations of the ELISA, or other platelet-activating immune complexes may still exist.
Finally, not all antihistone antibodies are pathologic. We found 2 of 114 healthy vaccinees who seroconverted for antihistone antibodies during the follow-up phase of our prospective study with health care workers (Figure 7). Their sera did not activate platelets. Whether these antibodies have been induced by vaccination or occurred by coincidence remains unresolved.
An interesting additional observation is that inhibition of GPIIb/IIIa by the tetrapeptide RGDS (a tirofiban analog) effectively inhibited formation of procoagulant platelets in vitro (Figure 2). GPIIbIIIa blockade results in an inhibition of calcium channels46 interfering with intracellular calcium levels in platelets, a prerequisite for PS exposure. Tirofiban might therefore be a therapeutic option for patients with TTS.
Based on our findings, we hypothesize a mechanism of anti-PF4–negative TTS: mRNA-based COVID-19 vaccinations (or other proinflammatory processes) trigger activation of macrophages, monocytes,47 and granulocytes. Thereby, histones are released inducing the formation of antihistone (auto-)antibodies (first hit). A second vaccination (or other process) again induces new histone release boosting antibody synthesis and leading to the rapid formation of immune complexes between circulating histones and corresponding antihistone antibodies (second hit). These complexes activate platelets resulting in TTS. Importantly, we cannot exclude that other vaccinations are able to induce histone/antihistone IgG complexes, too. Hence, the mechanisms and potential cofactors will need to be addressed in upcoming studies.
In summary, anti-PF4–negative TTS can occur after mRNA–based COVID-19 vaccination. Histone/antihistone IgG complexes are interesting candidates to induce procoagulant platelet activation comparable with anti-PF4/PF4 IgG complexes in VITT.
Acknowledgments
The authors thank Jessica Fuhrmann, Julia Klauke, and Katrin Schoknecht for technical assistance; Christian Hentschker for measuring of the mass spectrometry samples; and Stephan Michalik for providing and maintaining the in-house R pipeline.
This study was funded by the Deutsche Forschungsgemeinschaft grants TH 2320/3-1 and GR 2232/9-1.
Authorship
Contribution: T.T., S.H., and M.E. developed the concept; L.H., C.F., L.L., J.A., K.L., T.H., and M.H. treated and described the patients; S.H., J.R., R.K., L.D.F., L.N., A.-C.W., D.R., and J.W. performed laboratory analyses; L.S. and U.V. performed the proteomic study; L.U., K.B., and N.O.H. performed the SeCo study; M.E., S.H., A.G., and T.T. analyzed the results and wrote the manuscript; and all authors have revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: T.T. reports grants from Deutsche Forschungsgemeinschaft during the conduct of the study (TH 2320/3-1); personal fees and other from Bristol Myers Squibb (BMS), Pfizer, and Chugai Pharma; personal fees from Bayer and Novartis; and travel support and honoraria from Novo Nordisk, Daichii Sankyo, and Leo Pharma, outside the submitted work. A.G. reports grants from Deutsche Forschungsgemeinschaft (GR 2232/9-1) and nonfinancial support from Aspen, Boehringer Ingelheim, Merck & Co, Inc (MSD), BMS, Paringenix, Bayer Healthcare, Gore Inc, Rovi, Sagent, and BioMarin/Prosensa; personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, and Instrumentation Laboratory; and nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, and Gesellschaft für Thrombose und Hämostaseforschung e.V., outside the submitted work. J.A. reports research grants from Shire and CSL Behring; honoraria from Werfen, Stago, Siemens, Sysmex, Roche, Baxter, CSL Behring, and Sobi; and acts on advisory boards for Sobi and Novo Nordisk, outside the submitted work. C.F. reports research grants from Sobi and Biotest AG, and honoraria fees from Amgen, CSL Behring, Bayer, BioMarin, Daichii Sankyo, Sobi, Novartis, Novo Nordisk, Pfizer, and Takeda, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Thomas Thiele, Institut für Transfusionsmedizin, Universitätsmedizin Greifswald, 17487 Greifswald, Germany; email: thomas.thiele@med.uni-greifswald.de.
References
Author notes
M.E. and S.H. are joint first authors.
Data are available on request from the corresponding author, Thomas Thiele (thomas.thiele@med.uni-greifswald.de).
The full-text version of this article contains a data supplement.