TO THE EDITOR:
Since 2022, large B-cell lymphoma with IRF4-rearrangment (LBCL-IRF4+) has been recognized as a definite entity by the World Health Organization (WHO) classification of hematolymphoid tumors.1,2 It is characterized by a follicular and/or diffuse growth pattern as well as a strong expression of IRF4, which is due to translocation of IRF4 with an immunoglobulin gene.1,3-6 Essential and desirable diagnostic criteria are set by the WHO, including clinical, morphological, immunophenotypical, and molecular-genetic parameters.1,2
Given that most patients with LBCL-IRF4+ are children and young people, the rarity of this lymphoma, and lack of any prospective trials, most data on LBCL-IRF4+ come from small reports.7-12 Thus, 2 of the largest childhood non-Hodgkin lymphoma (NHL) consortia, the European Intergroup for Childhood NHL and the international Berlin-Frankfurt-Münster Group, designed a retrospective multinational study of LBCL-IRF4+ in children and young people. Here, we present data from this series, which, to our knowledge, is the largest reported to date.
Between December 2022 and November 2023, we performed an international survey of LBCL-IRF4+ cases diagnosed between 2010 and 2022 from 15 European Intergroup for Childhood NHL and/or International Berlin-Frankfurt-Münster Study Group members but only included patients with centrally reviewed histopathology. Questionnaires were sent out to obtain data on demographics, histopathology, treatment, and outcome.3,11 A total of 75 patients aged ≤21 years were identified, including 10 patients for whom only initial characteristics and basic therapy components were collected because they were still included in an ongoing trial (NCT03206671). Diagnosis of LBCL-IRF4+ was based on the WHO criteria, which emphasize that a cytogenetically cryptic translocation of IRF4 can be detected by molecular-genetic methods in most cases.1,2 Staging procedures and protocols are described elsewhere.13-20 Patients were included in national studies and/or registries and treated with informed consent from their legal guardians. Studies were conducted in accordance with the Declaration of Helsinki, and approval was delivered by the ethics committees and/or institutional review boards. Event-free survival (EFS) was calculated from the date of diagnosis to the date of the first event. Events considered included relapse, progression, or death. Overall survival (OS) was defined as the time from diagnosis to death from any cause or the date of last follow-up. EFS and OS were estimated by the Kaplan-Meier method.
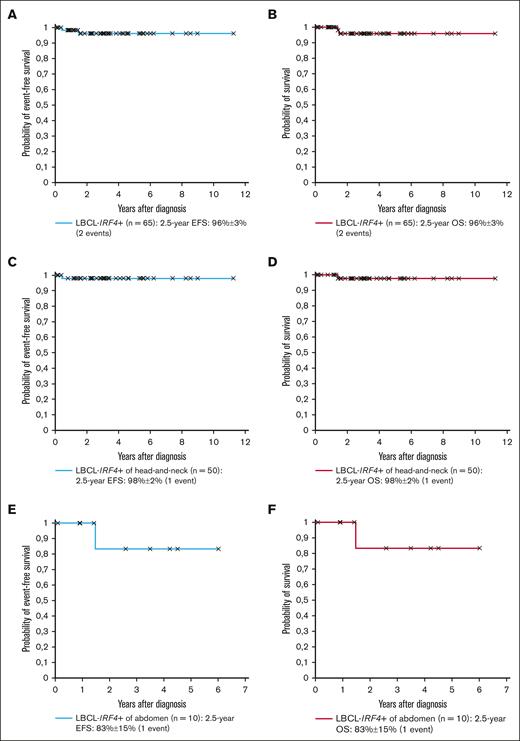
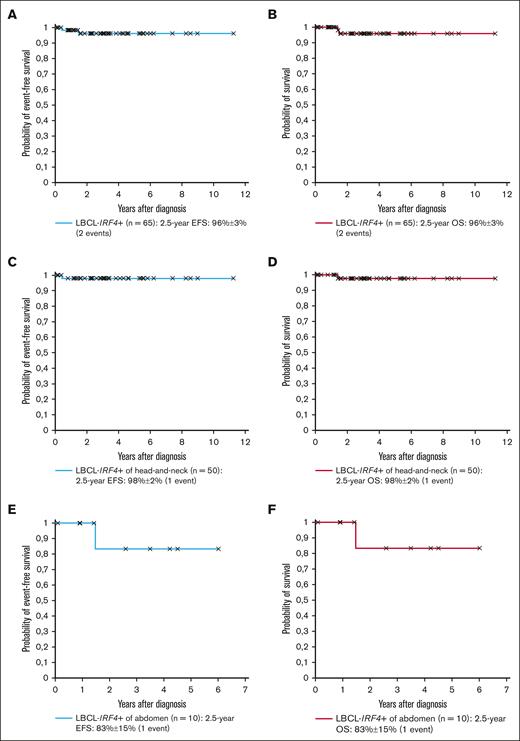
The study cohort is shown in Tables 1 and 2. The male-to-female ratio was 1:1, and the median age 10.0 years. Four patients (5%) had a preexisting immunodeficiency. Three histological growth patterns were described: (1) follicular (n = 14; 19%); (2) diffuse (n = 47; 62%); and (3) follicular and diffuse (n = 12; 16%); and it was not available in 2 cases (3%). An IRF4-rearrangement was detected by molecular-genetic methods in 73 patients (97%), whereas, for the remaining 2 patients (3%), the diagnosis was established by strong IRF4-positivity through immunohistochemistry and typical histopathology as allowed by the WHO criteria.1,2 For 12 patients, an IRF4::IGH fusion gene was reported while not available for the remainder. BCL2- and MYC-rearrangements were absent in all patients analyzed, whereas for 3 of 38 patients (8%) analyzed, BCL6 was rearranged. After a median follow-up of 2.84 years, the 2.5-year EFS and OS of the 65 patients with LBCL-IRF4+, for whom outcome data were available, was 96% ± 3% for both outcomes (Figure 1A-B).
Outcome of patients with LBCL-IRF4+. (A-B) EFS and OS of the 65 patients with LBCL-IRF4+; (C-D) EFS and OS of the 50 head-and-neck patients with LBCL-IRF4+; (E-F) EFS and OS of the 10 abdominal patients with LBCL-IRF4+.
Outcome of patients with LBCL-IRF4+. (A-B) EFS and OS of the 65 patients with LBCL-IRF4+; (C-D) EFS and OS of the 50 head-and-neck patients with LBCL-IRF4+; (E-F) EFS and OS of the 10 abdominal patients with LBCL-IRF4+.
The site of involvement was the head-and-neck region in 57 patients (76%), with 43 (75%) including a pharyngeal location. Histopathology showed a follicular, diffuse, and follicular and diffuse pattern in 13, 34, and 9 of the 56 patients (not available for 1), respectively. No patient had lactate dehydrogenase (LDH) levels ≥2× upper limit of normal. Twenty-six patients had stage I (46%), and 31 (54%) had stage II disease. Sixteen of 57 patients (28%) had a complete resection, with 2 patients undergoing watchful waiting and 14 receiving chemotherapy (3 with rituximab). All other 41 patients (72%) without a complete resection received chemotherapy (4 with rituximab). Only 1 patient relapsed after 5 months of watchful waiting and died while in complete lymphoma remission from a viral infection after allogeneic stem cell transplantation, which was performed due to the underlying immunodeficiency. Both 2.5-year EFS and OS were 98% ± 2% for the 51 patients with head-and-neck LBCL-IRF4+ (6 without outcome data; Figure 1C-D).
The site of involvement was the abdomen for 14 patients (19%), with 13 (93%) involving the gastrointestinal tract. Histopathology showed a follicular, diffuse, and follicular and diffuse pattern in 1, 9, and 3 of the 14 patients (not available for 1), respectively. Only 1 patient had LDH levels ≥2× upper limit of normal. Stage II (50%) and stage III (50%) disease was present in 7 patients each. Although 3 of 14 (21%) had a complete resection, all patients received chemotherapy (4 with rituximab). No patient relapsed. The only patient who died had ataxia telangiectasia and succumbed due to interstitial lung disease. Both 2.5-year EFS and OS were 83% ± 15% for all 10 patients with abdominal LBCL-IRF4+ (4 without outcome data; Figure 1E-F).
Among the remaining 4 patients (5%), sites of involvement included head-and-neck and abdomen (n = 1), liver only (n = 1), and lymph nodes only outside the head-and-neck region (n = 2). Histopathology showed a diffuse pattern in all 4 cases. Three patients had stage III and 1 patient stage II disease. Although the latter had no complete resection of the involved lymph nodes, watchful waiting was pursued. The other 3 patients received chemotherapy and rituximab, and all 4 patients have survived event free.
To our knowledge, this report including 75 patients with LBCL-IRF4+ represents by far the largest series of LBCL-IRF4+ in children and young people reported to date.3,7-10,12,21-23 Our results show that LBCL-IRF4+ affects both sexes equally and is associated with a median age of 10.0 years, normal LDH levels, an absence of stage IV disease, and, possibly, with an underlying immunodeficiency.9,10 Due to the latter observation, we believe that prospectively systematic screening for an underlying predisposition shall be performed for all patients with LBCL-IRF4+ in future clinical trials.
We, to our knowledge, for the first time, show the relative distribution of cases between the head-and-neck and the gastrointestinal location, with 76% occurring in the former location, and almost all purely follicular cases are confined to the head-and-neck region. EFS rates of 96% ± 3% for the whole cohort, 98% ± 2% for the head-and-neck, and 83% ± 15% for the abdominal tumors are comparable with what has been reported in the literature.3,7-10,12,21-23 If the 4 patients with an immunodeficiency are excluded, outcome for LBCL-IRF4+ was excellent with no events in our cohort.
Although 25% of cases had a primary complete resection of their tumor (n = 19; 16 head-and-neck and 3 abdominal tumors), only 2 of them underwent watchful waiting, reflecting the obvious cautionary approach of treating physicians and lack of separate treatment recommendations for LBCL-IRF4+. In addition, only 6 of 19 patients (32%) with complete resection had a purely follicular LBCL-IRF4+, which, due to its clinical and biological closeness to pediatric-type follicular lymphoma, could have indicated a watchful waiting approach, but this was only done in 1 patient.11,12,24-26
Taking our results into account, we could neither confirm that watchful waiting is already performed in completely resected disease (n = 2) nor that patients with LBCL-IRF4+ can be cured with approaches other than standard-of-care B-cell non-Hodgkin lymphoma chemotherapy (Tables 1 and 2; supplemental Figure 1). Nevertheless, due to the extraordinarily good prognosis, we believe that LBCL-IRF4+ could work as prototype of an ultrarare malignant mature B-cell neoplasm for which treatment is not stratified by stage, but histopathology alone, allowing for patients with stage I to III disease to be included in the lowest-risk arms in which treatment could be deescalated in a nonrandomized (due to its rarity) manner in prospectively controlled trials. Such trials might also test watchful waiting for (in)completely resected, purely follicular LBCL-IRF4+ of the head-and-neck region.12,24-27
Our study has limitations including its retrospective nature, with potential for reporting bias, and the small patient numbers. However, given the rarity of this entity, these data are likely to be the best available to determine the prognosis of LBCL-IRF4+ in children and young people and to develop future therapy studies. They will also allow for us assessing the true overall incidence of this rare LBCL entity among childhood NHL and, in particular, comparing its biology and clinical behavior with pediatric-type follicular lymphoma and other diffuse LBCL.
Acknowledgments: The authors thank all participating institutions and physicians for their support of the study.
This European Intergroup for Childhood NHL and i-BFM article was written on behalf of the Berlin-Frankfurt-Münster (BFM) Study Group (Austria, Germany, Czech Republic), Associazione Italiana Ematologia Oncologia Pediatrica, Société Française de Lutte contre les Cancers et Leucémies de l'Enfant, Belgian Society of Pediatric Hematology and Oncology, Dutch Childhood Oncology Group, Hungarian Pediatric Oncology Network, Japan's Children’s Cancer Group, Hong Kong Pediatric Hematology and Oncology Study Group, Israel's Society of Pediatric Hematology and Oncology, Spanish Society of Pediatric Hematology and Oncology, and 3 single institutions from Australia (Perth), Canada (Toronto), and Russia (Moscow).
This study was supported by the Deutsche Kinderkrebshilfe to conduct the NHL-BFM registry 2012 (W.W. and B.B.), the St. Anna Children’s Cancer Research Institute (A.A.), and Japan Agency for Medical Research and Development, grant/award numbers 18ck0106434 and 20ck0106635h0001 (D.H.).
Contribution: M.H., A.A., M.P., B.B., and W.W. designed and planned the study; A.A. and M.H. wrote the manuscript; M.H., A.A., and H.v.M. were in charge of data pooling, data checking, and statistical analysis; and all authors were principal or coinvestigators in their study groups and institutions, coordinated the national trials and/or registries in their countries, provided study materials, recruited patients, and read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andishe Attarbaschi, St. Anna Children’s Hospital, Kinderspitalgasse 6, 1090 Vienna, Austria; email: andishe.attarbaschi@stanna.at.
References
Author notes
Original data available upon reasonable request from the corresponding author, Andishe Attarbaschi (andishe.attarbaschi@stanna.at).
The full-text version of this article contains a data supplement.