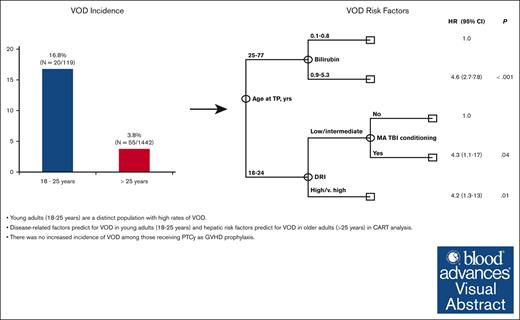
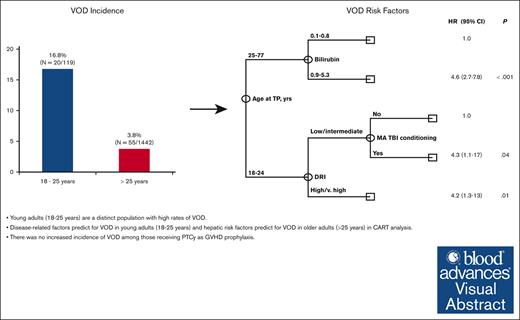
Young adults (aged 18-25 years) are a distinct population with high rates of VOD.
Disease-related factors predict for VOD in young adults (aged 18-25 years), and hepatic risk factors predict for VOD in older adults (aged >25 years).
Visual Abstract
Veno-occlusive disease (VOD) is a rare but potentially life-threatening complication after allogeneic hematopoietic stem cell transplantation (allo-SCT). Although increasing awareness and modern transplant techniques have mitigated risk, the interaction of historic risk factors in the current era with posttransplant cyclophosphamide (PTCy) is unknown. We performed a retrospective single-center analysis of adult patients aged ≥18 years undergoing allo-SCT (N = 1561) using predominately PTCy as graft-versus-host disease (GVHD) prophylaxis (72%). We found a higher rate of VOD at 16.8% (20 of 119) in those aged ≤25 years compared with 3.8% (55 of 1442) in those aged >25 years, with unique predictors of VOD within each cohort. Multivariate classification and regression tree (CART) analysis confirmed age as the primary independent determinant of the rate of VOD. Among patients aged 18 to 25 years, disease risk index (DRI; 31% with high/very high DRI vs 12% low/intermediate DRI; P = .03) and prior lines of chemotherapy (24% with >1 vs 6% with ≤1; P = .03) were the strongest predictors of VOD. Incidence of VOD in patients aged >25 years of age consistently ranged between 3% and 5% across most risk factors evaluated, with only hepatic factors (baseline elevation of bilirubin, aspartate transferase, alanine aminotransferase) or gemtuzumab exposure associated with increased rates of VOD. There was no significant difference in rates of VOD in those receiving PTCy compared with those receiving alternate GVHD prophylaxis. Our data highlight the differences in incidence and predictors of VOD between younger (≤25) and older (>25) adults undergoing allo-SCT.
Introduction
Veno-occlusive disease (VOD), also known as hepatic sinusoidal obstruction syndrome, is a well-known and potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation (allo-SCT). It is mediated by activation and toxic injury to sinusoidal endothelial cells secondary to high-dose chemotherapy, radiation, and hematopoietic SCT causing sinusoidal obstruction.1 The resulting postsinusoidal portal hypertension leads to the clinical syndrome of fluid retention, ascites, weight gain, painful hepatomegaly, and jaundice, which, in severe cases, can progress to multiorgan failure. VOD typically develops within 3 weeks of allo-SCT, although late-onset VOD represents up to 30% of cases.2
The incidence of VOD after allo-SCT varies across studies with median incidence between 10% and 15%, with rates of up to 60% in high risk populations,3-5 although, using Center for International Blood and Marrow Transplant Research data, more recent estimates are <5%.6,7 This marked variability is, in part, related to a lack of a single accepted set of diagnostic criterion, with the Baltimore,8 modified Seattle9 and European Society for Blood and Marrow Transplantation (EBMT)10 criteria most routinely being used, each with different sensitivity and specificity. Furthermore, there is a well described relationship between age and VOD risk, with significantly higher risk in pediatric populations.6 The severity of VOD is highly variable, although mortality associated with severe disease exceeds 80%.1,3 Numerous patient-, disease-, and transplant-related VOD risk factors have been identified.11,12 Modern transplant techniques, including reduced intensity conditioning together with increased awareness of modifiable VOD risk factors, have aided in mitigating the risk of VOD; however, the increasing use of calicheamicin-based antibody drug conjugates in the treatment of acute leukemia may further increase risk because their hepatic toxicity and contribution to VOD risk is well documented.13,14 Furthermore, the increasing use of haploidentical donors and posttransplant cyclophosphamide (PTCy) as graft-versus-host disease (GVHD) prophylaxis in both matched and mismatched donor transplant may further increase risk.12,15 The interaction of modern treatments, transplant approaches, and historic VOD risk factors are unknown.
We aim to describe the incidence and risk factors of VOD in a modern cohort of adult patients undergoing allo-SCT at a high-volume single center, including using predominately PTCy as GVHD prophylaxis.
Methods
We conducted a retrospective, single center, chart-based study of consecutive adult patients (aged ≥18 years) undergoing allo-SCT at MD Anderson Cancer Center between 1 January 2017 and 31 December 2021. VOD was defined using the classic VOD EBMT criteria with total bilirubin level of ≥2 mg/dL and 2 of 3 additional criteria including weight gain of >5%, hepatomegaly and/or ascites within ≤21 days after allo-SCT,10 or if patients received defibrotide treatment for VOD based on clinical judgment of the treating physician. Patients diagnosed with late-onset VOD (>21 days after allo-SCT) were not included in this study. Practice at our institution is for all patients undergoing allo-SCT to receive ursodiol as VOD prophylaxis. No patients in this study received defibrotide for VOD prophylaxis. Baseline pretransplant aspartate transferase (AST), alanine aminotransferase (ALT), and bilirubin were the most recent available value between day −30 before allo-SCT and the start of conditioning. The MD Anderson institutional review board approved this study (protocol #2020-0683), which was conducted in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act.
Statistical analysis
The primary end points were the incidence and risk factors for VOD occurring within the first 21 days after transplant. Characteristics according to VOD status were compared using χ2 test for categorical variables and the Wilcoxon rank-sum test for continuous variables. The incidence of VOD was estimated using the cumulative incidence method considering death before VOD diagnosis as a competing risk. Risk factors for VOD were evaluated in univariate analysis using Fine and Grey regression analysis to account for death without VOD as a competing risk. Multivariate analysis was performed using classification and regression tree (CART) analysis to evaluate independent effects accounting for potential interaction effects. CART analysis is a form of supervised machine learning that performs recursive partitioning to identify significant predictors of outcomes. All factors found to be statistically significant in univariate analysis were included in the CART analysis. Statistical significance was based on the .05 error level. Analyses were performed using STATA (StataCorp. 2019; Stata Statistical Software: Release 16. College Station, TX; StataCorp LLC.)
Results
Baseline characteristics
A total of 1561 patients underwent allo-SCT between 1 January 2017 and 31 December 2021. Median age was 56 years (range, 18-77 years) and 941 patients (60%) were male. The majority of patients had acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS; n = 941; 60%), ALL (n = 207; 13%), and myeloproliferative neoplasms (n = 182; 12%). Overall, 699 patients (45%) received >1 line of therapy before transplant. Most patients had matched related (n = 436; 28%) or unrelated (n = 747; 48%) donors, and 332 patients (21%) underwent haploidentical transplantation. Patients primarily received peripheral blood grafts (n = 1164; 75%). Forty-nine (3%) patients received inotuzumab ozogamicin (InO)-containing regimens before allo-SCT, and 30 (2%) were exposed to gemtuzumab ozogamicin (GO). In total, 794 (51%) patients received myeloablative conditioning (MAC), with 106 (7%) receiving myeloablative total body irradiation (TBI). PTCy was used as GVHD prophylaxis in 1130 patients (72%). The majority (98%) of the 1130 patient who received PTCy as GVHD prophylaxis received the standard schedule of cyclophosphamide 50 mg/kg on days 3 and 4 after SCT infusion; 23 patients, all aged >25 years, received decreased doses of cyclophosphamide on alternative schedules based on physician discretion (n = 16, cyclophosphamide 30 mg/kg on days 3 and 4 after SCT infusion) or as part of a GVHD prophylaxis clinical trial (n = 7, cyclophosphamide 30 mg/kg or 50 mg/kg on day 3 after SCT infusion). Of those with VOD, 51% received treatment with defibrotide. There was a significant difference in the median age of patients diagnosed with VOD compared with those without VOD (46 years vs 56 years, respectively; P = .001). This prompted further examination of the incidence of VOD within different age groups, in which we identified a VOD rate of 16.8% (20 of 119) in those aged ≤ 25 years compared with 3.8% (55 of 1442) in those aged >25 years (Table 1). Of patients aged ≤25 years and those aged >25 years diagnosed with VOD, 4 (4 of 20; 20%) and 11 (11 of 55; 20%) cases, respectively, were included based on treatment with defibrotide at the discretion of the treating physician, whereas the remaining cases met classical EBMT VOD criteria. Given these findings, risk factor analysis was stratified according to age (Table 2).
Incidence and predictors of VOD in patients aged ≤25 years
Within the cohort of patients aged ≤25 years, disease risk index (DRI) was the strongest predictor of VOD. Patients with a high/very high DRI had a higher rate of VOD than those with low/intermediate DRI (31% vs 12%; P = .03). Consistent with this finding, patients ≤ aged ≤25 years receiving >1 prior line of chemotherapy had a higher incidence of VOD than those receiving only 1 line of therapy before allo-SCT (24% vs 6%; P = .03). In patients aged ≤25 years with AML/MDS, 3 received GO and 2 of 3 (67%) patients developed VOD. In contrast, 10% of patients with AML/MDS who did not receive GO developed VOD (67% vs 10%, P = .05). Median GO cumulative dose was 2.1 mg/m2 (range, 2.1-3 mg/m2) in patients aged ≤25 years who received GO as part of their prior therapies. Several previously documented risk factors showed a trend for an association with VOD rate in the younger cohort including a hematopoietic cell transplantation–specific comorbidity index score of >3 (23% vs 12%; P = .1), myeloablative TBI compared with no myeloablative TBI (26% vs 15%; P = .2), and patients with ALL and exposure to InO compared with those with ALL not receiving InO (33 vs 21, P = .1). Median cumulative InO dose among those aged ≤25 years receiving InO before allo-SCT was 1.5 mg/m2 (range, 0.6-4.15 mg/m2). Similarly, time from diagnosis to transplant of >12 months compared with < 12 months trended toward high rate of VOD (23% vs 11%; P = .09). These trends did not reach statistical significance. There was no difference in the incidence of VOD in patients aged ≤25 years receiving PTCy as GVHD prophylaxis compared with those receiving other GVHD prophylaxis regimens (22% vs 12%; P = .2). Elevations in AST, ALT, or bilirubin before allo-SCT were not associated with an increased incidence of VOD in the younger patient cohort.
Incidence and predictors of VOD in patients aged >25 years
Interestingly, VOD incidence in patients aged >25 years consistently ranged between 3% and 5% across most risk factors evaluated. Contrary to that observed in younger adults, there was no difference in incidence of VOD observed in those aged >25 years with a high/very high DRI compared with those with a low/intermediate DRI (4% vs 4%, P = .9) and patients receiving >1 prior line of chemotherapy compared with those receiving only 1 line of therapy (4% vs 4%, P = .6). Within the >25-years-of-age cohort, hepatic risk factors including elevated baseline bilirubin, AST, ALT, and GO exposure were associated with increased rate of VOD. VOD incidence with baseline bilirubin within normal limits (WNL, greater than the upper limit of normal (>ULN) to 1.5× ULN, and > 1.5× ULN was 3%, 14%, and 16%, respectively (P < .001). VOD incidence with baseline ALT WNL, >ULN to 2.5× ULN, and >2.5× ULN was 4%, 3%, and 8%, respectively (P = .02). Similarly, rates of VOD with baseline ALT WNL, >ULN to 2.5× ULN, and >2.5× ULN were 3%, 4%, and 27%, respectively (P ≤ .001). In patients aged >25 years with AML/MDS, receiving GO was associated with a higher incidence of VOD compared with patients with AML/MDS not exposed to GO (15% vs 3%; P = .01). Median cumulative dose of GO was 2.7 mg/m2 (range, 0.6-3.9 mg/m2). Those with body mass index of ≤20 had higher rates of VOD compared than those with a body mass index of >20 (10% vs 3%; P > .001). There was no difference in those aged >25 years, with VOD incidence of 3% in 1070 patients exposed to PTCy compared with 5% in those using alternate GVHD prophylaxis (P = .1). Similarly, there was no increased risk of VOD in those receiving InO before allo-SCT, with a median InO cumulative dose of 1.5 mg/m2 (range, 0.6-3.9 mg/m2) in patients aged >25 years exposed to InO. Notably, conditioning type or intensity and myeloablative TBI exposure were not associated with elevated incidence of VOD.
CART multivariate analysis
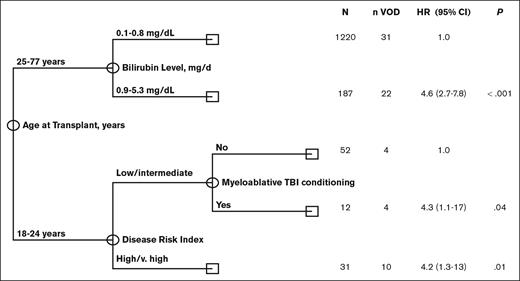
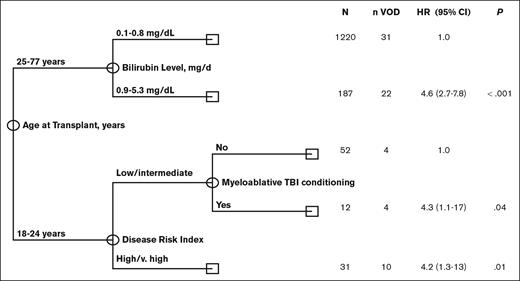
The low incidence of VOD in the >25-years-age cohort precluded the assessment of the independent impact of age using multivariate Fine and Grey regression analysis. In addition, univariate analysis revealed that risk factors for VOD differed significantly among young adults, aged 18 to 25 years, and older adults aged >25 years, further limiting the statistical power to perform such regression analyses. Given these limitations, we performed multivariate analysis using CART analysis that is more suitable for the identification of independent prognostic factors in the presence of an interaction effect. The results of the CART analysis were consistent with and confirmed the results obtained in univariate analysis. Specifically, CART analysis confirmed that age at transplant was the most significant predictor of VOD rate, with patients aged <25 years old having a significantly higher rate of VOD (hazard ratio [HR], 5; 95% confidence interval [CI], 2.9-8.5; P < .001). This is consistent with the findings of the univariate analysis categorizing age of ≤25 years as a cutoff value. Among patients aged <25 years, CART analysis confirmed that high/ very high DRI (HR, 4.2;, 95% CI 1.3-13; P = .01) was associated with a significantly increased rate of VOD. In addition, it revealed that, the rate of VOD was equally high in patients with low/intermediate DRI who received myeloablative conditioning with TBI (HR, 4.3; 95% CI, 1.1-17; P = .04). Among patients aged ≥25 years, CART analysis showed bilirubin of >0.8 mg/dL (HR, 4.6; 95% CI, 2.7-7.8; P < .001) to be associated with a significantly high rate of VOD. Notably, none of the remaining markers of liver function were shown to add prognostic value in the older subset of patients (Figure 1).
Multivariate classification and regression tree (CART) analysis for VOD risk.
Discussion
In this study, we describe the incidence and risk factors of VOD in a large contemporary population of patients undergoing allo-SCT for primarily malignant disease at a single center using predominately PTCy as the GVHD prophylaxis. The small sample size of patients with VOD within each variable of interest limited the ability to detect statistically meaningful difference in VOD incidence however several trends emerged. We identified that younger adults (aged ≤25 years) represent a distinct adult population with considerably increased rates of VOD compared with their older counterparts, which are exacerbated by disease- and treatment-related factors (DRI and number lines of therapy). In contrast, patients aged >25 years had low rates of VOD even in the presence of historical predictors of VOD, with only hepatic risk factors identified as increasing baseline VOD risk.
Young adults with cancer represent a patient population with unique biology and distinct challenges,16 and it is well described that VOD disproportionately effects pediatric patients.6 To account for the difference in the incidence, predisposition and presentation of VOD in younger patients, the EBMT published the first pediatric-specific diagnostic criteria (pEBMT) for VOD17 distinct from the EBMT revised diagnostic criteria for VOD published several years prior.10 Among other key difference, the pEBMT criteria recognized the increased incidence of anicteric VOD among pediatric and adolescent and young adult patients.5,17,18 Neither the pEBMT or EBMT criteria specifies age cutoffs for which these diagnostic criteria should be applied; however, a recent international expert position statement on children, adolescents, and young adults with VOD included young adults up to the age of 25 years alongside pediatric patients.17 The panel recommended the pEBMT criteria be used in those aged ≤25 years; however, to our knowledge, there are no direct comparisons of VOD in younger adults aged 18 to 25 years to either younger pediatric (aged <18 years) or older adult patients (aged >25 years).
Data on pediatric and adolescent and young adult patients aged up to 25 years old (median, 17 years) from our center have recently been published using the new pEBMT criteria.19 The authors found a VOD incidence of 15.9% within their study population, with a median age of 20 years vs 16 years of patients who developed VOD compared with those who did not, respectively. Although we used the classical VOD criteria for our study, the relatively high rate of VOD seen in our younger adult population is consistent with this report. The higher incidence of VOD in younger patients can be attributed to several factors. There is an increased prevalence of diseases in childhood that are known to have higher risk of VOD, including congenital macrophage activation syndromes5,20 and patients with thalassemia with hepatomegaly and iron overload.21,22 Immaturity of the liver and smaller caliber hepatic venules may also contribute to the heightened VOD risk,9,22 although these factors are unlikely to explain differences between younger and older adults seen in our study. Age-related changes in pharmacokinetics and pharmacodynamics likely play a role, as supported by the predominant risk factor being the number of therapy lines in younger adults; however, an exact mechanism remains unclear. Interestingly, a previous report found that the use of pharmacokinetic (PK) monitoring of busulfan dosing raised VOD risk.6 PK-guidance is associated with higher busulfan exposure23 and busulfan pharmacokinetics vary widely in pediatric patients.24 All patients in this study receiving busulfan underwent PK-guided dosing in both MAC and reduced-intensity conditioning (RIC) regimens. Although these findings raise questions, PK-guided busulfan dosing has been shown to improve outcomes at our institution25 and others.26 Further studies are needed to elucidate the relationship between PK-guided dosing of busulfan, rare events such as VOD, and potential age-related differences. Despite the absence of a clear pathophysiological mechanism, our study emphasizes the importance of recognizing young adults aged <25 years as a distinct adult population with increased risk of VOD akin to pediatric patients.
Despite increasing recognition, young adults (aged ≤25 years) are often treated at adult centers in which providers may be more familiar with adults rather than pediatric clinical practice guidelines and recommendations for transplant-related complications. Given the poor outcomes associated with VOD, including in young adults, it raises the question of whether more sensitive VOD diagnostic criteria, such as the pEBMT criteria, should be preferentially used for younger adults to allow for earlier recognition and intervention. In the defibrotide expanded access T-IND study, early initiation of treatment with defibrotide was associated with better survival.27,28 Similarly, in a prospective evaluation of the pEBMT criteria for VOD, earlier diagnosis facilitated by the increased sensitivity of the pEBMT criteria improved patient outcomes by allowing earlier treatment in pediatric patients.29 Furthermore, in the recent DEFIFrance registry study examining the effectiveness and safety of defibrotide for VOD, patients with less severe disease receiving defibrotide for treatment had better survival than those with severe or very severe disease.30 Although it is not clear whether patients with nonsevere VOD would progress in severity, these finding combined with the high reported mortality of VOD even in patients with nonsevere disease31 have resulted in recommendations to initiate therapy for VOD promptly even with moderate disease11 rather than being delayed until severity worsens. Consideration should be given to using more sensitive diagnostic criteria, such as the pEBMT criteria, for young adults aged ≤25 years in order to intervene earlier in the disease process, potentially improving patient outcomes.
The increased risk of VOD associated with InO13 and GO14 in those undergoing allo-SCT is well established. In the pivotal INNOVATE trial of InO compared with standard of care as salvage therapy for relapsed/refractory ALL, 22% of those randomized to InO who underwent allo-SCT developed VOD.13 Similarly, early observation studies of GO followed by allo-SCT within 3 months demonstrated a VOD rate of 15% to 40%.14 Expert guidelines have since been published outlining recommendations on the prevention, monitoring, and management of VOD in the setting of InO or GO use32,33; however, the impact of these strategies are not well studied. In our population, GO use increased the risk of VOD regardless of patient age, whereas there was no apparent increased incidence of VOD in patients receiving InO. Of note, these results may, in part, be related to the generally lower doses of InO used at our center in which InO is used in combination with other chemotherapy.34,35 Nonetheless, these results are reassuring given that InO is increasingly being incorporated in both the upfront and relapsed setting for ALL.
There is a paucity of data surrounding the risk of VOD associated with PTCy as GVHD prophylaxis. Given the association between alkylating agents used in conditioning regimens with VOD, there have been concerns that using cyclophosphamide as GVHD prophylaxis may heighten VOD risk. This question is particularly relevant given the increasing use of PTCy in matched donor transplant in addition to mismatched donor transplant. PTCy has been shown to reduce the risk of GHVD in HLA-matched donor allo-SCT with both RIC and MAC regimens. Furthermore, the recently published phase 3 CTN 1703 trial comparing PTCy-based GVHD prophylaxis with conventional prophylaxis in RIC allo-SCT showed higher GVHD-free, relapse-free survival, or progression-free survival without increased risk of relapse or death36 and will undoubtedly lead to an uptake in PTCy use vs conventional GVHD prophylaxis. The majority of patients in our cohort received PTCy-based GVHD prophylaxis, with an incidence of VOD of 4.3% and no evidence of an increased risk of VOD. These results are encouraging and should reassure clinicians regarding the safety of PTCy although, ideally, these findings should be explored in prospective studies.
This study has several limitations inherent to its single-center, retrospective nature. Although PTCy was used in most patients, we do not have data as to why alternate GVHD prophylaxis was used in some cases. Similarly, choice of pretransplant therapy and conditioning intensity is heavily dependent on patient fitness including perceived likelihood of posttransplant complications including VOD. Furthermore, we focused on classic VOD criteria, including the requirement of elevated bilirubin, within 21 days of transplant, to facilitate identification of patients through our chart review. We recognize that our approach missed anicteric and late-onset VOD; any difference in late-onset VOD between younger and older adults is beyond the scope of this study. Nonetheless, to the best of our knowledge, this analysis represents the largest study to date examining classic VOD incidence and risk factors in a contemporary cohort of patients with predominately PTCy as GVHD prophylaxis.
In summary, our data highlight that young adult patients (aged 18-25 years) undergoing allo-SCT for predominately malignant disease are a distinct adult population with high rates of VOD akin to pediatric patients with unique predictors of VOD compared with their older adult counterparts. Adult patients aged >25 years of age have consistently low rates of VOD with baselined bilirubin predicting risk. There was no observed increased incidence of VOD among those receiving PTCy as GVHD prophylaxis. Ideally, future studies of VOD risk, prevention, and treatment in adults should include analysis specifically on this high-risk young adult population.
Authorship
Contribution: P.K. conceived and designed the study; C.M. and W.W. collected and assembled the data; C.M., W.W., R.M.S., and P.K. analyzed and verified the data; C.M., R.M.S., and P.K. wrote the article; and all authors interpreted the data, approved the article, and are accountable for publication.
Conflict-of-interest disclosure: P.K. reports consultancy for Pfizer and Jazz Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Partow Kebriaei, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; email: pkebriae@mdanderson.org.
References
Author notes
Data are available on request from the corresponding author, Partow Kebriaei (pkebriae@mdanderson.org).
The full-text version of this article contains a data supplement.