Nearly half and one-third of patients treated on TT IIIB without ongoing treatment showed no signs of progression at 10 and 15 years.
Three years of VRD maintenance did not improve outcomes for patients with high-risk MM defined by gene expression profiling.
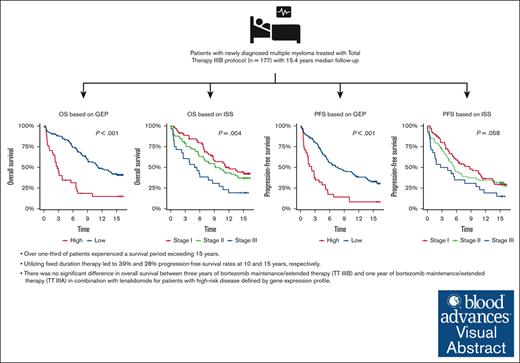
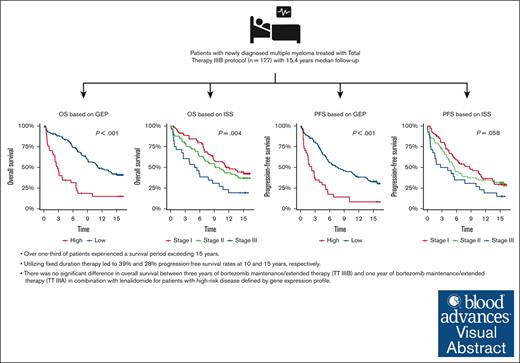
Visual Abstract
The total therapy (TT) IIIB phase 2 study incorporated bortezomib into tandem melphalan–based hematopoietic stem cell transplantation with dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, and etoposide for induction/consolidation and bortezomib, lenalidomide, and dexamethasone (VRD) for maintenance in patients with newly diagnosed multiple myeloma (MM). This updated analysis presents a 15.4-year median follow-up. Of 177 patients, 21% patients had gene expression profile (GEP)–defined high-risk MM. 15-year progression free survival (PFS) was 27.9%. Median PFS was better in GEP–defined low-risk patients at 7.8 years and in International Staging System stage 1 patients at 8.7 years. Overall, median OS was 9.1 years, and 15-year overall survival (OS) was 35.9%. GEP–defined low-risk patients' median OS was 11.2 years, and that of GEP–defined high-risk patients was 2.8 years. There was no difference in OS between TT IIIB and TT IIIA. This study includes the longest follow-up of patients treated with maintenance VRD reported to date. In patients with GEP–defined low-risk, nearly half and one-third of patients without ongoing treatment showed no signs of progression at 10 and 15 years, respectively. One-third of patients survived more than 15 years, but 3 years of VRD maintenance did not improve outcomes for patients with GEP–defined high-risk MM. The study was registered on www.clinicaltrials.gov as #NCT00572169.
Introduction
Multiple myeloma (MM) outcomes have continually improved over the past 2 decades.1 Overall survival (OS) is improved with lenalidomide maintenance therapy after hematopoietic stem cell transplantation (HSCT).2 Patients with high-risk MM generally experience more aggressive disease behavior and poorer outcomes than those with standard-risk MM, and the addition of a proteasome inhibitor (PI) in the maintenance phase is usually suggested for these patients to overcome these worse outcomes. Total therapy (TT) IIIB, an extension of the TT IIIA study,3 used 3 years of bortezomib, lenalidomide, and dexamethasone (VRD) as maintenance for newly diagnosed MM. Primary analysis at a median follow-up of 2 years showed no difference in OS, progression-free survival (PFS), onset of complete response (CR), or CR duration between TT IIIA (1-year of bortezomib, thalidomide, and dexamethasone (VTD) followed by 2 years of thalidomide and dexamethasone [TD]) and TT IIIB.4 This article updates the trial results after a 15-year follow-up to assess the benefit of extended maintenance therapy of VRD on patients’ outcomes.
Methods
Details of the TT IIIB design and primary analysis have been previously published.4 Briefly, TT IIIB was an open-label single-center phase 2 extension trial that recruited patients at the University of Arkansas for Medical Sciences in the period between November 2006 and September 2008. The study was approved by the institutional review board of the University of Arkansas for Medical Sciences and was conducted according to the Declaration of Helsinki.
Patients with newly diagnosed MM received 2 cycles of VTD-PACE (VTD and a 4-day continuous infusions of cisplatin, doxorubicin, cyclophosphamide, and etoposide) as induction before, and as consolidation therapy after, melphalan–based tandem HSCT, which was followed by 3 years of VRD with monthly cycles of bortezomib 1.0 mg/m2 (days 1, 4, 8, and 11) in year 1 followed by weekly administration in years 2 and 3. For all 3 years, lenalidomide was administered on days 1 to 20 (15 mg), then on days 21 to 28 (5 mg) of each 28-day cycle. Dexamethasone (20 mg) was administered on days 1 to 4 and 8 to 11 of 28-day cycle of year 1 and then weekly with bortezomib in years 2 and 3. Details on treatments administered and dose modifications were previously published.4 Gene expression profile (GEP) of CD138-purified plasma cells was conducted to determine the 70-gene-derived risk score, molecular subgroup designation, and delTP53 status.5,6 The molecular subgroup designations included CCND-1 without CD20 expression (CD-1) or with CD20 expression (CD-2), MAF/MAFB, MMSET/FGFR3, hyperdiploidy, low bone disease, myeloid, and proliferation.5-7 All patients were followed regularly after completion of treatment. The study was registered on ClinicalTrials.gov under identifier NCT00572169.
The maintenance phase of TT IIIB and TT IIIA differed in terms of bortezomib treatment duration—3 years of VRD maintenance (TT IIIB) vs 1-year of VTD followed by 2 years of TD (TT IIIA). The primary aim was to assess clinical endpoints, including PFS, OS, and CR. The sample size calculation of the trial has been previously reported.4 Minimal residual disease (MRD) testing was introduced in December 2012 and was done by-color euroflow and encompassed a minimum of 2 000 000 events to be able to identify 20 aberrant cells, with the lower limit of detection set at 1 × 10−5.
Statistical analyses included predefined plans. Time-to-event analyses used Kaplan-Meier and Cox regression modeling overall and within subgroups. Hazard ratios (HR) assessed treatment effects (95% confidence intervals [CI]). The reverse Kaplan-Meier method was used to estimate the median follow-up.8 Heterogeneity and interaction tests evaluated treatment effects among subgroups using R statistical software (version 4.0.5).9
Results and discussion
In the trial, 177 patients enrolled with median age of 58 years, with 26% of patients >65 years. Refer to Table 1 for patient baseline characteristics.
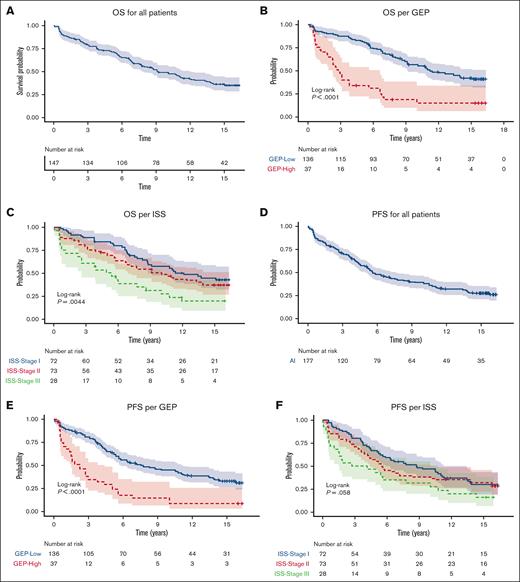
Median PFS was 5.6 years (95% CI, 4.9-8.3). PFS values at 5-years, 10-years, and 15-years were 56.7%, 39.4% and 27.9%, respectively. After a median follow-up of 15.4 years, median OS was 9.1 years (95% CI, 7.7-12.1). The 10-year OS was 49.1% (95% CI, 42-57.5), and 15-year OS was 35.9% (95% CI, 29.1-44.4).
Long-term survival outcomes according to disease risk
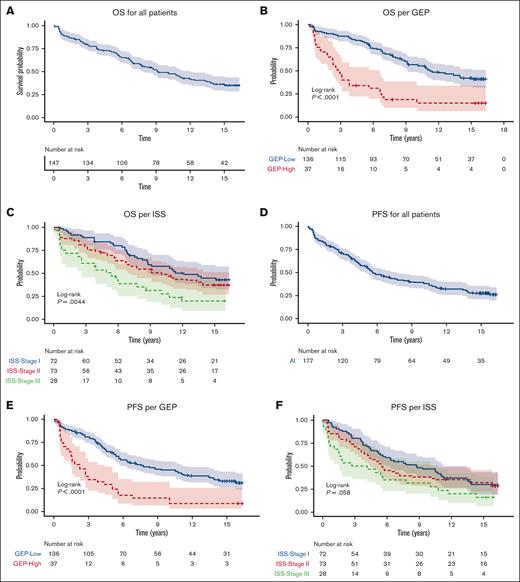
Median PFS was 7.8 years (95% CI, 5.6-11.4) and 2.2 years (95% CI, 1.5-4.4) for GEP-defined low-risk and high-risk, respectively. 10-year PFS for International Staging System (ISS) stage 1 was 8.7 years (95% CI, 5.6-13.4) and 3.5 years (95% CI, 1.5-10.8) for patients with ISS stage III. The 10-year PFS was 45.1% (95% CI, 37.2-54.6) for GEP–defined low-risk patients and 14.6% (95% CI, 6.5-32.5) for GEP–defined high-risk patients. The 15-year PFS was 33.2% (95% CI, 25.9-42.7) for GEP–defined low-risk patients (Figure 1).
PFS and OS for all patients, per GEP and per ISS. (A) OS for all patients (B) OS per GEP (C) OS per ISS (D) PFS for all patients (E) PFS per GEP (F) PFS per ISS.
PFS and OS for all patients, per GEP and per ISS. (A) OS for all patients (B) OS per GEP (C) OS per ISS (D) PFS for all patients (E) PFS per GEP (F) PFS per ISS.
Median OS was 11.2 years (95% CI, 9.11-NR) in GEP–defined low-risk patients and 2.8 years (95% CI, 2.3-6.6 years) in GEP–defined high-risk patients. The 10-year OS was 56.6% (95% CI, 48.6-66) for GEP–defined low-risk patients and 18.7% (95% CI, 9.3-37.8) for GEP–defined high-risk patients. The 15-year OS was 42.2% (95% CI, 34.2-52.1) for GEP–defined low-risk patients. The 10-year OS was 57.1% (95% CI, 46.1-70.7) for patients with ISS stage 1 and 30.8% (95% CI, 17.4-54.3) for those with ISS stage 3 (Figure 1).
Multivariate analysis demonstrated worse OS outcomes with GEP–defined high-risk disease (HR, 2.4; 95% CI, 1.4-4.2; P = .002) and immunoglobulin A subtype (HR, 2; 95% CI, 1.2-3.3; P = .012).
Peripheral neuropathy (PN) was reported in 26% (n = 46) of patients with highest PN grade as grade 1 (39%) or grade 2 (59%). Grade 3 PN occurred in 1 patient. PN was managed by dose reduction in most cases with bortezomib discontinuation related to PN in 6% of patients. Of 177 patients, 10 patients (5.6%) developed secondary hematological malignancy (2 acute myeloid leukemia, 6 myelodysplastic syndrome, 1 acute lymphoblastic leukemia, and 1 non-Hodgkin lymphoma). Details on secondary malignancies will be published in the future.10
One vs 3 years of bortezomib during maintenance phase
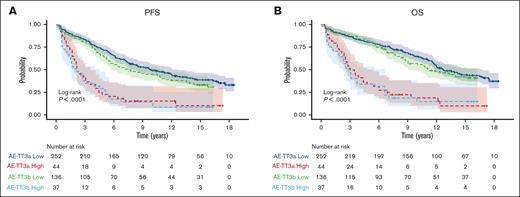
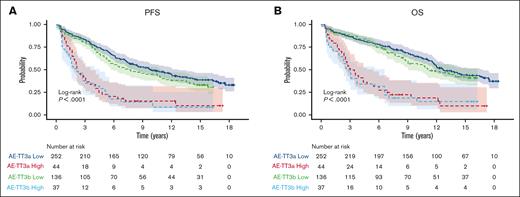
Three-years of bortezomib during maintenance (TT IIIB) did not result in improvement of OS compared to 1-year of bortezomib during maintenance (TT IIIA) (HR, 1.2; 95% CI, 0.9-1.5; P = .166). Of note, TT IIIB included a higher incidence of certain adverse factors such as albumin levels below 3.5 g/dL (45% vs 26%; P < .001) and B2M levels ≥3.5 mg/L (57% vs 45%, P = .012). Consequently, there was a higher prevalence of ISS stages 2 and 3 in TT IIIB compared with TT IIIA (70% vs 55%, P < .001). Furthermore, the GEP–defined high-risk category was more frequent (22% vs 15%, P = .038), and the GEP–defined CD-1 molecular subgroup was also overrepresented in TT IIIB (11% vs 5%, P = .037). PFS was worse in TT IIIB than in TTIIIA (HR, 1.3; 95% CI, 1.0-1.6; P = .048]) (Figure 2).
PFS and OS by GEP comparing TT IIIA and TT IIIB. (A) PFS, (B) OS of GCB patients.
PFS and OS by GEP comparing TT IIIA and TT IIIB. (A) PFS, (B) OS of GCB patients.
MRD
Data on MRD were available for 99 patients (60%) of enrolled patients. Median time for first MRD testing was 5.5 years after enrollment. On average, each patient underwent 6 MRD tests (range, 1-16). Multivariable analysis demonstrated that achieving MRD negativity at any time during treatment and follow-up, even for patients who subsequently relapsed, was associated with improved OS (HR, 0.61; 95% CI, 0.42-0.88; P = .008]). In patients with no evidence of progression of at least 10 years with MRD data (n = 44), 86.4% of patients were MRD negative while in patients with no evidence of progression of at least 15 years with MRD data (n = 26), 69.2% of patients were MRD negative.
This study demonstrates significant improvement in outcomes for patients with MM due to the introduction of immunomodulatory drugs, PIs, and HSCT. One-third of patients survived for over 15 years. Fixed duration therapy in TT IIIB resulted in 39.4% achieving 10-year and 27.9% achieving 15-year disease control without progression. In patients with GEP–defined low-risk, nearly half and one-third of patients without ongoing treatment showed no signs of progression at 10 and 15 years, respectively. These data show that an operational cure as defined by 15-year PFS is feasible with a comprehensive treatment approach even before the introduction of anti-CD38 monoclonal antibodies such as daratumumab. An obvious caveat is that not all patients are able to undergo such an intensive treatment regimen. Our study is limited by the fact it is a phase 2 extension study and lacks a control arm.
MRD negativity was associated with a better prognosis, consistent with findings from previous trials,11,12 however our study did not have data on early MRD testing and was limited for patients who survived until the time of introduction of MRD testing. The incidence of secondary hematological cancers during long-term follow-up was relatively low at 5.6%.
Extended maintenance therapy with an immunomodulatory drug and PI for 3 years did not improve OS in patients with high-risk MM. These data suggest that presently recommended maintenance treatments for high-risk MM do not suffice. New strategies are needed to improve outcomes for patients with high-risk MM. Early results of OPTIMUM (MUKnine) study which used anti-CD38 antibody showed promising results at 30 months’ follow-up with PFS of 77% and OS 83.5%.13 Our study provides valuable long-term follow-up data which is limited in MM clinical trials14 and highlights the importance of analyzing long-term outcomes in MM clinical trials to fully appreciate the impact of therapy.
Acknowledgments
The authors thank all the patients who participated in this clinical trial.
This work was supported by National Cancer Institute grant CA 55813.
Authorship
Contribution: S.A.H., C.D.S., S.T., B.B., M.Z., and F.v.R. were involved in conception and design; S.A.H., C.D.S., S.T., C.B., R.S., S.P., D.A., M.C.-F., G.T., J.D.S. Jr, F.Z., J.S., B.B., M.Z., and F.v.R. were involved in provision of study materials or patients; S.A.H., C.B., R.S., and S.P. were involved in collection and assembly of data; and all authors were involved in data analysis and interpretation, manuscript writing, final approval of manuscript, and are accountable for all aspects of the work.
Conflict-of-interest disclosure: The authors report no competing financial interests.
Correspondence: Samer Al Hadidi, FACP Myeloma Center, Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, 449 Jack Stephens Dr, Little Rock, AR 72205; email: salhadidi@uams.edu.
References
Author notes
∗F.v.R. and M.Z. contributed equally.
Portions of this manuscript presented at the annual meeting of the American Society of Clinical Oncology, Chicago, IL, 2-5 June 2023.
Individual participant data are available, including data dictionaries, for approved data sharing requests. Individual participant data that underlie the results reported in this article will be shared after deidentification and normalization of information (text, tables, figures, and appendices). The statistical analysis plan will be provided upon request. Beginning 3 months after publication of this article and ending 5 years after, anonymized data will be available to researchers who provide a completed Data Sharing Agreement that describes a methodologically sound proposal for the purpose of the approved proposal. Proposals should be directed to salhadidi@uams.edu. Data will be shared once all relevant parties approve and sign the Data Sharing Agreement. Data sharing requests are available for 5 years via the Southampton Clinical Trials Unit website.
The full-text version of this article contains a data supplement.