Key Points
Anticoagulants and IVIg reduce thrombosis in vivo to varying degrees.
Anticoagulants do not ameliorate thrombocytopenia.
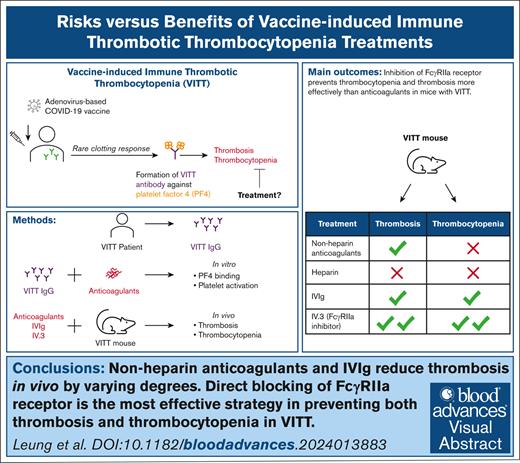
Visual Abstract
Current guidelines for treating vaccine-induced immune thrombotic thrombocytopenia (VITT) recommend nonheparin anticoagulants and IV immunoglobulin (IVIg). However, the efficacy of these treatments remains uncertain due to case studies involving small patient numbers, confounding factors (eg, concurrent treatments), and a lack of animal studies. A recent study proposed danaparoid and heparin as potential VITT therapies because of their ability to disrupt VITT IgG-platelet factor 4 (PF4) binding. Here, we examined the effects of various anticoagulants (including unfractionated [UF] heparin, danaparoid, bivalirudin, fondaparinux, and argatroban), IVIg, and the FcγRIIa receptor-blocking antibody, IV.3. Our investigation focused on VITT IgG-PF4 binding, platelet activation, thrombocytopenia, and thrombosis. Danaparoid, at therapeutic doses, was the sole anticoagulant that reduced VITT IgG-PF4 binding, verified by affinity-purified anti-PF4 VITT IgG. Although danaparoid and high-dose UF heparin (10 U/mL) inhibited platelet activation, none of the anticoagulants significantly affected thrombocytopenia in our VITT animal model and all prolonged bleeding time. IVIg and all anticoagulants except UF heparin protected the VITT mice from thrombosis. Direct FcγRIIa receptor inhibition with IV.3 antibody is an effective approach for managing both thrombosis and thrombocytopenia in the VITT mouse model. Our results underscore the necessity of animal model investigations to inform and better guide clinicians on treatment choices. This study provides compelling evidence for the development of FcγRIIa receptor blockers to prevent thrombosis in VITT and other FcγRIIa-related inflammatory disorders.
Introduction
Adenovirus vector vaccines against severe acute respiratory syndrome coronavirus 2 (ChAdOx1 nCoV-19 [Vaxzevria, AstraZeneca] and Ad26.COV2.S [Janssen/Johnson & Johnson]) have garnered clinical and public interest due to a rare yet serious complication after vaccination, termed vaccine-induced immune thrombotic thrombocytopenia (VITT). Although the use of severe acute respiratory syndrome coronavirus 2 adenovirus-based vaccines has substantially decreased in high-income countries, it is still widely used in low-medium income countries and has relevance in informing adenovirus-based vaccine development. Additionally, since the advent of VITT, VITT-like immune thromboses after viral infections are increasingly recognized.1-3
The pathogenesis of VITT involves the generation of autoantibodies against platelet factor 4 (PF4), with the onset of symptoms appearing 5 to 30 days after vaccination. The antigen/antibody immune complex induces platelet and neutrophil activation via FcγIIa,4,5 resulting in thrombosis. We have previously shown using a humanized VITT animal model that the anti-FcγIIa receptor monoclonal antibody, IV.3, the NETosis inhibitor GSK484 (a reversible peptidylarginine deiminase 4 inhibitor), and genetic ablation of peptidylarginine deiminase 4 in VITT mice led to a marked reduction in thrombosis.4 However, currently, no inhibitors are available in clinical settings that specifically target NETosis or the FcγIIa receptor.
Due to the clinical and pathophysiological similarities between VITT and autoimmune heparin-induced thrombocytopenia (HIT), their treatment consists primarily of nonheparin anticoagulants (eg, direct oral anticoagulants, fondaparinux, danaparoid, and argatroban), IV immunoglobulin (IVIg), or less frequently, immune modulatory agents such as steroids or rituximab.6 In some cases, patients with VITT still develop new major thromboembolic events despite IVIg treatment.7 Unfractionated (UF) heparin and low-molecular-weight heparin may potentially contribute to disease progression.8 Nonetheless, a recent study showed that negatively charged anticoagulants such as UF heparin and danaparoid disrupt VITT IgG binding to PF4 and inhibit in vitro thrombus formation, suggesting their potential effectiveness as VITT treatments.9 However, in vitro findings require validation by in vivo studies. Furthermore, the World Health Organization (WHO) Guideline Development Group acknowledged the very low certainty of evidence on treatments for VITT.10,11 The WHO guidelines initially advised against the use of heparin in VITT and later recommended its use when nonheparin anticoagulants were unavailable.10 There is a critical need to evaluate and compare the in vivo antithrombotic effects of anticoagulants in a relevant mouse model of VITT. These results will assist physicians in selecting the most suitable anticoagulant therapy for VITT and VITT-like conditions.
In this study, we evaluated the impact of anticoagulants on blocking VITT IgG binding to PF4, platelet activation, thrombocytopenia, and thrombosis. We also compared the efficacy of these anticoagulants with IVIg using IV.3 as a control. The findings showed that anticoagulants inhibited VITT antibody-induced thrombosis to varying degrees, with bivalirudin showing the greatest inhibition. IVIg exhibited comparable efficacy to that of bivalirudin. However, all anticoagulants prolonged bleeding. Although addressing thrombosis is the primary concern for clinicians, it does not diminish the significance and relevance of bleeding complications associated with anticoagulant therapies.12 Hence, there is an unmet need to develop more targeted therapies with fewer side effects and complications.
Methods
Blood samples
Blood was collected with informed consent from patients clinically diagnosed with VITT,13 HIT,14 and healthy donors. Patient samples with VITT and HIT were positive in the laboratory tests (enzyme-linked immunosorbent assay [ELISA] and 14C-serotonin release assay [14C-SRA]). Three patients with HIT and 6 with VITT were recruited. This study was approved by the Human Research Ethics Committee of South Eastern Sydney Local Health District (17/211 LNR/17/POWH/501).
Antibody purification
KKO hybridoma cells were kindly provided by Gowthami Arepally (Duke University, Durham, NC). KKO and total IgG from healthy donors' and patients’ plasma were purified by Protein G Agarose affinity chromatography.15 Anti-PF4 specific IgG was affinity purified using biotin-conjugated PF4 coupled to streptavidin magnetic beads, as previously described.4 IV.3 monoclonal antibody was produced from hybridoma cells (American Type Culture Collection clone HB-217) and purified using protein G sepharose affinity chromatography. Effector deficient (aglycosylated) IV.3 was used for in vivo studies and was prepared as previously described.16
PF4 ELISA
ELISA polystyrene plates (Thermo Scientific) were coated with PF4 (15 μg/mL) with or without heparin (0.1-16 U/mL), danaparoid (0.2-16 U/mL), bivalirudin (2-16 μg/mL), fondaparinux (0.8-16 μg/mL), or argatroban (0.4-16 μg/mL). Concentrations up to 1 U/mL heparin, 0.8 U/mL danaparoid, or 1 μg/mL argatroban and fondaparinux were used for affinity purified PF4 specific VITT IgG experiments. ELISA buffer consisted of phosphate-buffered saline (PBS) with 0.05% Tween 20. Fluorescently labeled Alexa Fluor 488 (AF488) (AnaSpec, Fremont, CA)-PF4 was used to quantify the amount of PF4 remaining on the plate after the addition or precoating of the plates with PF4 and various doses of anticoagulants. Serum from controls or patients with VITT or HIT (diluted 1:8000) or anti-PF4 specific VITT IgG (5 μg/mL) was added to the ELISA wells. Optical density at 450 nm and fluorescence were measured using a plate reader (Tecan Infinite Pro, Männedorf, Zurich, Switzerland).
SRA
14C-SRA was performed as previously described.17 Briefly, washed donor platelets were incubated with radiolabelled 14C, heat-inactivated patient sera, PF4 (10 μg/mL), and various doses of anticoagulants for 60 minutes at room temperature while stirring. The reaction was stopped using PBS-EDTA buffer and centrifuged. The radioactivity (counts per minute) of the supernatant was measured using a beta counter.
Flow cytometry
Platelets were incubated in the absence or presence of heparin (0.1-10 U/mL), PF4 (10 μg/mL), and control, VITT, or HIT IgG (30 μg/mL) at 37°C for 30 minutes, stained with anti-CD62p antibody, and analyzed by flow cytometry (Fortessa X-20, BD Biosciences, San Jose, CA).
Mouse model
Double transgenic mice expressing the R131 isoform of human FcγRIIa and human PF4 in the C57BL/6 background18 were IV injected with VITT or HIT IgG (250 μg/g), or KKO (400 μg/mouse). Mouse platelets were labeled in vivo using an anti-CD42c DyLight 649 antibody (1 μg/g, Emfret, Würzburg, Germany). Therapeutic concentrations of anticoagulants followed the 2021 WHO interim guidelines11 and South Eastern Sydney Local Health District Medicine Guidelines (heparin [18 U/kg per hour], danaparoid [400 U/h], argatroban [120 μg/kg per hour], and bivalirudin [0.75 mg/kg]). Anticoagulants were administered via an osmotic pump (Alzet, Cupertino, CA) implanted the day before the bleeding assay or administration of VITT IgG. IVIg (1 g/kg) and IV.3 (1 mg/kg) were administered along with the VITT IgG. Mice were bled before IgG administration and at 1 hour and 4 hours after IgG injection. For thrombus analysis, the mice were culled after 4 hours, and the lungs were perfused with PBS followed by formalin, extracted, and imaged using the IVIS Spectrum scanner (Perkin Elmer), as previously described.4,16,19 Platelet counts were analyzed using flow cytometry. Bleeding assays in C57/Bl6 mice were conducted as previously described.20 Briefly, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Mouse tails were amputated 10 mm from the tip and then immersed in a 50 mL tube containing warmed (37°C) saline. Bleeding time was monitored for 20 minutes. All animal experiments were approved by the University of New South Wales Animal Care and Ethics Committee.
Statistics
Statistical analysis was performed using GraphPad Prism, Version 9 (GraphPad, La Jolla, CA). Data with normal distribution were analyzed using 1-way analysis of variance with Dunnet test for multiple comparisons. P values < .05 were considered statistically significant.
Results
Characteristics of patient cohort with VITT
Clinically, all patients with VITT presented with severe thrombotic events, thrombocytopenia, and elevated D-dimer and fibrinogen levels (Table 1), consistent with previously reported VITT cases.21 The IgG profile of the patient cohort with VITT was characterized using ELISA and SRA. ELISA data revealed elevated levels of anti-PF4 and anti-PF4/heparin IgG antibodies in all patients with VITT. The anti-PF4 ELISA pattern for VITT differed from that of HIT IgG (supplemental Figure 1A). Positive SRA findings also confirmed the functional activity of VITT antibodies (supplemental Figure 1B). The presence of low-dose heparin (0.1 U/mL) did not enhance platelet activation, whereas the addition of low-dose PF4 (10 μg/mL) resulted in a slight increase in VITT IgG-induced platelet activation. These data suggest that our VITT cohort had type 3 antibodies22 that are heparin-independent and anti-PF4 platelet-activating antibodies.
Stability of immobilized PF4 in the presence of anticoagulants
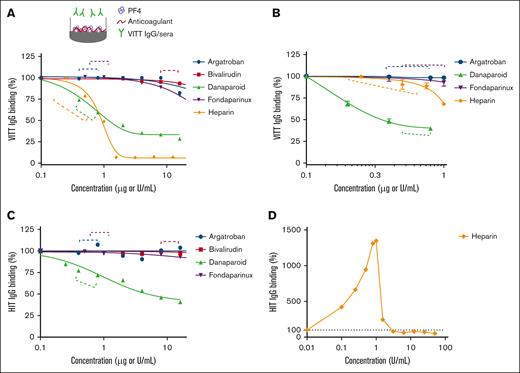
To determine whether anticoagulants interfere with VITT IgG binding to PF4, anticoagulants were added to PF4-coated plates in which the anticoagulant concentrations tested encompassed the therapeutic plasma levels (indicated by the dotted brackets in Figure 1; supplemental Figure 2). We first tested the stability of microtiter plate-immobilized PF4 in the presence of anticoagulants and found that the addition of heparin and danaparoid stripped PF4 from the ELISA plates (supplemental Figure 2A), whereas argatroban, bivalirudin, and fondaparinux had no such effect on plate-bound/immobilized PF4. To minimize PF4 stripping, the plates were co-coated with PF4 and various doses of anticoagulants. This approach resolved the stripping effect of danaparoid at all concentrations and of UF heparin at low doses (up to 0.5 U/mL) (supplemental Figure 2B). Caution is required when interpreting ELISA data using heparin at concentrations >0.5 U/mL, as well as the effects of danaparoid on binding to PF4 when these anticoagulants are added to the microtiter plates after PF4 coating. This effect is most likely due to the negative charge of UF heparin and danaparoid.
Effect of anticoagulants on VITT IgG binding to microtiter plate-bound PF4. Binding of (A) total VITT IgG and (B) anti-PF4 specific VITT IgG to PF4 (10 μg/mL) in the absence or presence of argatroban, bivalirudin, danaparoid, fondaparinux, or UF heparin. The data are representative of 3 patient samples with VITT. Binding of total HIT IgG to PF4 (10 μg/mL) and heparin (0.1 U/mL) in the absence or presence of (C) argatroban, bivalirudin, danaparoid, fondaparinux, or (D) UF heparin. IgG binding to PF4 calculated relative to VITT IgG alone (A, B), HIT IgG with 0.1 U/mL heparin (C), or HIT IgG alone (D). Dashed lines indicate the therapeutic plasma range.
Effect of anticoagulants on VITT IgG binding to microtiter plate-bound PF4. Binding of (A) total VITT IgG and (B) anti-PF4 specific VITT IgG to PF4 (10 μg/mL) in the absence or presence of argatroban, bivalirudin, danaparoid, fondaparinux, or UF heparin. The data are representative of 3 patient samples with VITT. Binding of total HIT IgG to PF4 (10 μg/mL) and heparin (0.1 U/mL) in the absence or presence of (C) argatroban, bivalirudin, danaparoid, fondaparinux, or (D) UF heparin. IgG binding to PF4 calculated relative to VITT IgG alone (A, B), HIT IgG with 0.1 U/mL heparin (C), or HIT IgG alone (D). Dashed lines indicate the therapeutic plasma range.
Danaparoid and UF heparin “inhibit” binding of VITT IgG to PF4
Applying the relevant coating steps for the respective anticoagulant, we tested their effect on VITT IgG binding to PF4. At therapeutic doses, danaparoid and UF heparin interfered with VITT IgG binding to PF4 (Figure 1A). This result corroborates the findings of Singh et al.9 However, the effect of UF heparin on total VITT IgG binding to PF4 was evident only at the higher end of the therapeutic range (Figure 1A). To confirm that this inhibitory activity was directed at specific anti-PF4 antibodies against VITT IgG, we assessed binding using affinity-purified anti-PF4 VITT IgG (supplemental Figure 3). These data support the finding that danaparoid has a prominent inhibitory effect on specific VITT IgG binding to PF4 at therapeutic concentrations. Notably, UF heparin did not inhibit the binding of anti-PF4 specific VITT IgG to PF4 at low doses (Figure 1B). The reduction in VITT IgG/PF4 binding observed at UF heparin concentrations of 1 U/mL and higher (Figure 1A) could be due to the stripping effect of PF4 at these concentrations (supplemental Figure 2B), even when UF heparin was added together with PF4 during coating. The binding properties of HIT IgG in the presence of anticoagulants were similar to those observed for VITT, with the exception of heparin (Figure 1D). In this case, HIT IgG binding increased at low-dose heparin, reaching a peak at 1 U/mL heparin before a marked decrease.
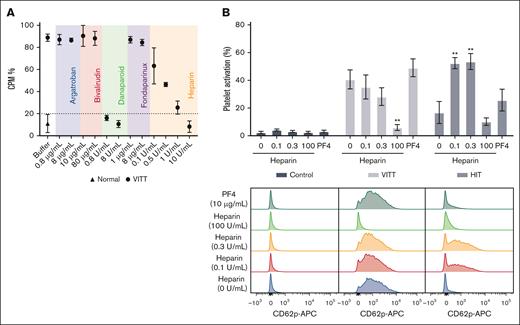
Danaparoid and heparin inhibit VITT IgG-induced platelet activation
Next, we investigated the effect of anticoagulants on platelet activation using a SRA, with a cutoff set at 20% (for negative results).17 Our findings indicated that only danaparoid completely blocked platelet activation, whereas increasing concentrations of UF heparin resulted in reduced activation (Figure 2A). Platelet activation was fully inhibited by UF heparin only at a concentration of 10 U/mL, which exceeded the therapeutic range. To further explore the effect of heparin on VITT IgG-induced platelet activation, CD62p expression on platelets was analyzed by flow cytometry. Although not statistically significant, our data show a trend that low doses of heparin (0.1 and 0.3 U/mL) reduced VITT IgG-induced platelet activation (Figure 2B). Conversely, low-dose heparin treatment significantly increased HIT-induced platelet activation. This contrasting pattern highlights the different activities and specificities of VITT and HIT IgG. The addition of low-dose exogenous PF4 resulted in a slight, but not statistically significant, increase in both VITT and HIT IgG-induced platelet activation. These data supported our SRA findings (supplemental Figure 1B). A low dose (10 μg/mL) of PF4 was used for in vitro studies, because higher doses, specifically 30 to 50 μg/mL, may lead to PF4-induced platelet activation (supplemental Figure 4).
Danaparoid and UF heparin block platelet activation by VITT IgG. (A) SRA shows that VITT-induced platelet activation is inhibited in the presence of therapeutic (0.8 μg/mL) and high-dose danaparoid (8 μg/mL) and high-dose UF heparin (10 U/mL) but not argatroban (0.8 and 8 μg/mL), bivalirudin (10 and 80 μg/mL), fondaparinux (1 and 8 μg/mL), or low-dose UF heparin (0.1, 0.5, and 1 U/mL). Data are shown as the mean ± standard deviation (SD). The data are representative of 3 patient samples with VITT. (B) IgG-induced platelet activation in the absence or presence of heparin (0.1, 0.3, and 100 U/mL) or PF4 (10 μg/mL). The platelets were treated with control (normal, n = 4), VITT (n = 6), or HIT IgG (n = 3). Data are shown as the mean ± standard error of the mean. ∗∗P < .01, relative to VITT or HIT IgG without heparin or PF4. Representative flow cytometry histogram plots showing platelet activation (anti-CD62p APC) following treatment with control, VITT or HIT IgG in the absence or presence of heparin or PF4. APC, allophycocyanin; CPM, counts per minute.
Danaparoid and UF heparin block platelet activation by VITT IgG. (A) SRA shows that VITT-induced platelet activation is inhibited in the presence of therapeutic (0.8 μg/mL) and high-dose danaparoid (8 μg/mL) and high-dose UF heparin (10 U/mL) but not argatroban (0.8 and 8 μg/mL), bivalirudin (10 and 80 μg/mL), fondaparinux (1 and 8 μg/mL), or low-dose UF heparin (0.1, 0.5, and 1 U/mL). Data are shown as the mean ± standard deviation (SD). The data are representative of 3 patient samples with VITT. (B) IgG-induced platelet activation in the absence or presence of heparin (0.1, 0.3, and 100 U/mL) or PF4 (10 μg/mL). The platelets were treated with control (normal, n = 4), VITT (n = 6), or HIT IgG (n = 3). Data are shown as the mean ± standard error of the mean. ∗∗P < .01, relative to VITT or HIT IgG without heparin or PF4. Representative flow cytometry histogram plots showing platelet activation (anti-CD62p APC) following treatment with control, VITT or HIT IgG in the absence or presence of heparin or PF4. APC, allophycocyanin; CPM, counts per minute.
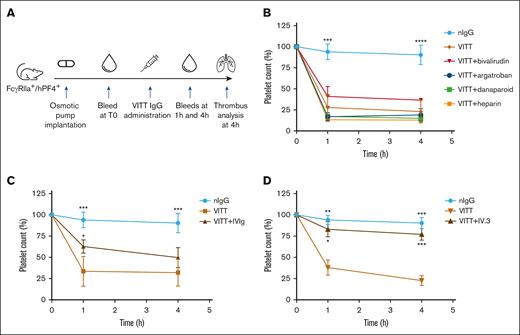
VITT IgG-induced thrombocytopenia is not improved by anticoagulants
To assess how these findings translate to the clinical manifestations of thrombosis and thrombocytopenia in VITT, anticoagulants were administered to VITT mice at human equivalent doses. The protocol is summarized in Figure 3A. Mice treated with bivalirudin, argatroban, danaparoid, and UF heparin were not protected from thrombocytopenia (Figure 3B), whereas IVIg (Figure 3C) and IV.3 (Figure 3D) treatments provided moderate and substantial platelet protection, respectively. Overall, these results are consistent with the mechanisms of anticoagulants, that is, inhibition of the coagulation cascade rather than blockage FcγRIIa-mediated cell activation. Nevertheless, these findings underscore the value of in vivo observations, as anticoagulants that affect VITT antibody binding and antibody-induced platelet activation in vitro (danaparoid and UF heparin; Figures 1 and 2) did not prevent thrombocytopenia in our mouse model. Conversely, IVIg and IV.3, which interfere with FcγRIIa cell signaling via a nonspecific and specific blockade of FcγRIIa, respectively, protected platelets from clearance. These results suggest that thrombocytopenia in VITT is due to the clearance of platelets coated with VITT IgG immune complexes rather than peripheral consumption of activated platelets by extensive thrombosis.
IVIg and IV.3 provide partial and full protection, respectively, from platelet destruction in vivo. (A) Study timeline of animal experiment. The VITT condition was recreated in FcγRIIa+/hPF4+ transgenic mice by the administration of VITT IgG. Anticoagulants, IVIg and IV.3 were administered via an osmotic pump and/or IV injection, as described in “Methods.” Platelet counts were assessed in mice after treatment with (B) anticoagulants (argatroban, bivalirudin, danaparoid, or UF heparin), (C) IVIg, and (D) aglycosylated IV.3. Data are shown as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, relative to VITT IgG. nIgG, normal IgG.
IVIg and IV.3 provide partial and full protection, respectively, from platelet destruction in vivo. (A) Study timeline of animal experiment. The VITT condition was recreated in FcγRIIa+/hPF4+ transgenic mice by the administration of VITT IgG. Anticoagulants, IVIg and IV.3 were administered via an osmotic pump and/or IV injection, as described in “Methods.” Platelet counts were assessed in mice after treatment with (B) anticoagulants (argatroban, bivalirudin, danaparoid, or UF heparin), (C) IVIg, and (D) aglycosylated IV.3. Data are shown as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, relative to VITT IgG. nIgG, normal IgG.
Anticoagulants inhibit VITT IgG-induced thrombosis
Despite the lack of protection against thrombocytopenia, treatment with bivalirudin, argatroban, or danaparoid led to significant but varying degrees of reduction in clot formation, as assessed by thrombosis in VITT mouse lungs (Figure 4A-B). Bivalirudin demonstrated the strongest inhibition, comparable with IVIg. The blockage of FcγRIIa with aglycosylated IV.3 provided the greatest reduction in thrombosis. Heparin was the least effective at reducing clots in VITT. In HIT mice, continuous infusion of heparin at therapeutic levels led to more severe thrombocytopenia and hypothermia within 1 hour (supplemental Figure 5A-C). Although there was a reduction in clots in the lungs (supplemental Figure 5B), the mice exhibited a severe reaction to heparin infusion, including shock, reduced physical activity and shallow breathing. Further studies are needed to validate these findings, using additional patient samples with HIT and imaging at multiple time points.
Effect of nonheparin anticoagulants, heparin, IVIg, and IV.3 in VITT mice. (A) Mouse lungs from FcγRIIa+/hPF4+ mice treated with VITT IgG, with or without anticoagulants, were extracted and analyzed for thrombosis by fluorescence using the IVIS SpectrumCT. (B) Representative images of hematoxylin and eosin-stained and fluorescence-stained lung sections. Images were acquired with a 10× objective using an inverted Olympus CKX53 microscope. Platelets (anti-CD42c) and DNA (4′,6-diamidino-2-phenylindole [DAPI]) are shown as magenta and cyan, respectively. Arrows indicate clots. Scale bar, 50 μm. (C) Mice treated with argatroban, bivalirudin, danaparoid or UF heparin at therapeutic concentrations had prolonged bleeding times compared with the saline control. The bleeding time was measured over 20 minutes. Data are shown as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, relative to VITT IgG (A) or saline (C).
Effect of nonheparin anticoagulants, heparin, IVIg, and IV.3 in VITT mice. (A) Mouse lungs from FcγRIIa+/hPF4+ mice treated with VITT IgG, with or without anticoagulants, were extracted and analyzed for thrombosis by fluorescence using the IVIS SpectrumCT. (B) Representative images of hematoxylin and eosin-stained and fluorescence-stained lung sections. Images were acquired with a 10× objective using an inverted Olympus CKX53 microscope. Platelets (anti-CD42c) and DNA (4′,6-diamidino-2-phenylindole [DAPI]) are shown as magenta and cyan, respectively. Arrows indicate clots. Scale bar, 50 μm. (C) Mice treated with argatroban, bivalirudin, danaparoid or UF heparin at therapeutic concentrations had prolonged bleeding times compared with the saline control. The bleeding time was measured over 20 minutes. Data are shown as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, relative to VITT IgG (A) or saline (C).
A well-known limitation of anticoagulant treatment is the risk of serious bleeding.23 To examine the impact of anticoagulants on hemostasis, tail bleeding assays were conducted on mice after treatment with clinically relevant doses. Mice treated with UF heparin, bivalirudin, argatroban, or danaparoid all experienced a significant prolongation of bleeding times (Figure 4C). Our in vivo data collectively highlight the advantages of the development of specific anti-FcγRIIa receptor inhibitors, as such inhibitors could offer more targeted and effective treatment options to prevent both thrombocytopenia and thrombosis without the bleeding side effects associated with traditional anticoagulants.
Discussion
Anticoagulants are administered to patients with thrombotic disorders primarily to halt or prevent the progression of thrombosis, thereby preventing further deterioration of the patient’s condition. With a wide range of anticoagulants available, selecting an appropriate anticoagulant for clinical use can be challenging. Furthermore, they have different mechanisms of action. For example, argatroban and bivalirudin are direct thrombin inhibitors. In contrast, fondaparinux primarily inhibits factor Xa, a key enzyme responsible for the conversion of prothrombin to thrombin. Danaparoid, a mixture of heparinoid glycosaminoglycans, primarily inhibits factor Xa but also has weak antithrombin activity. UF heparin enhances the activity of antithrombin III, which inactivates both thrombin and factor Xa. Unlike anticoagulants, high-dose IVIg nonspecifically blocks the FcγRIIa receptor.24
Treatment guidelines for VITT have been developed based on its clinical and pathophysiological similarities to HIT. Experts recognize that many questions remain unanswered, including whether patients with VITT should avoid heparin and which treatments yield the best clinical outcomes.8 This study evaluated the effect of anticoagulants in vitro and in a VITT mouse model, with the latter also compared with IVIg.
Our findings indicate that current treatments, such as nonheparin anticoagulants and IVIg effectively block thrombosis in our mouse model, albeit at the cost of prolonged bleeding time in the case of anticoagulants. Several VITT studies have suggested accelerated disease progression after treatment with UF heparin or low-molecular-weight heparin.13,25 This phenomenon may be attributable to the presence of antibodies in patients with VITT that bind to both PF4-heparin and PF4.26,27 Moreover, a large prospective study reported a higher mortality rate (20%) among patients with VITT treated with heparin compared with those receiving nonheparin anticoagulants (16%).21 Although the sample size of our study is limited, the findings are likely applicable to VITT in general due to the clonotypic nature of VITT antibodies.28
Consistent with recent findings,9 we show that only danaparoid and high-dose UF heparin effectively inhibited platelet activation in vitro. We also observed that strong negatively charged anticoagulants, such as UF heparin, not only blocked the binding of VITT IgG to immobilized PF4 but also interfered with the binding stability of positively charged PF4 to the ELISA plate surface. This observation underscores the importance of exercising caution when interpreting results involving high supra-therapeutic doses of UF heparin. The interaction between heparin and PF4 may introduce potential confounding factors in the analysis, highlighting the necessity for careful consideration of the experimental design and result interpretation.
We expanded our investigation to assess the efficacy of IVIg and various anticoagulants in blocking thrombosis in vivo, thereby enhancing the clinical relevance of our study. We found that IVIg was moderately effective in improving platelet counts and highly effective in reducing thrombosis. Unlike anticoagulants, IVIg does not prolong bleeding time. Although IVIg is recommended to be used in conjunction with anticoagulant therapy in severe cases of VITT or HIT, the potential side effects of IVIg should be considered.29 Very high concentrations are required to achieve the desired effect of nonspecific inhibition of FcγRIIa. Aggregates of IVIg can be found in batches of IVIg and can cause paradoxical prothrombotic effects, platelet aggregation,30 and potential thromboembolism.31,32 In contrast, IV.3 requires doses 1000 times lower than IVIg to achieve specific FcγRIIa inhibition to a greater extent. Nevertheless, high-dose IVIg in patients with VITT has been shown to increase platelet counts and inhibit the generation of procoagulant platelets,33 thus making it a recommended treatment option for patients with VITT, considering that the potential clinical benefits outweigh the uncommon but serious complications. Another promising treatment avenue for VITT being explored is a deglycosylated form of an anti-PF4 antibody (1E12) that recognizes overlapping epitopes on PF4 as VITT IgG.34 This approach has shown potential in inhibiting VITT-induced cell activation and thrombosis in vitro.
Although our data show that IVIg and bivalirudin were the most effective treatments for suppressing thrombosis, alternative therapies should be considered, particularly in settings in which expertise in managing HIT or VITT is limited and access to expensive treatments such as IVIg is difficult. Although current evidence suggests that heparin-based anticoagulants may be safe for managing VITT, more conclusive data are necessary.
Our in vivo data provide new insights and clinically valuable information on preventing or halting thrombosis progression induced by VITT IgG, as well as the comparative antithrombotic effects of various anticoagulants used to treat VITT. However, this model has limitations, as anticoagulants were administered before VITT IgG. Although our mouse model closely recapitulates the VITT condition, in which mice not only develop thrombosis and thrombocytopenia but also systemic reactions, anticoagulant infusion (via minipump implantation) was not performed after the development of the VITT condition due to the animals’ unstable condition and ethical considerations. Future studies will benefit from a VITT mouse model that replicates the clinical course of patients with VITT. Another limitation is the restricted availability of patient samples, which limited the drug treatment groups assessed. Recombinant or monoclonal VITT antibodies will enable further research and provide a more comprehensive evaluation of treatment options, including combination therapies and novel agents. Despite these limitations, our model effectively identified treatments that can mitigate the initiation and progression of thrombosis in VITT.
In summary, our findings highlight the need for more targeted therapies to mitigate the bleeding side effects of the current treatments. Although existing therapies effectively block thrombosis in VITT, more specific therapies are needed to minimize unwanted bleeding and replace large, costly doses of IVIg with lower-dose agents that offer greater specificity, such as FcγRIIa inhibitors. Our study serves as a valuable guide for anticoagulant selection in the treatment of VITT, VITT-like thromboses, HIT (particularly autoimmune HIT), and potentially other immune thromboses.35 Our study provides a comprehensive understanding of how these therapeutic agents affect thrombotic events in a pathophysiological context and offers important insights into treatment strategies and patient care for VITT and related conditions.
Acknowledgments
The authors thank Shiying Zheng, Rose Wong, and Feng Yan for assistance with patient samples, and Gowthami Arepally (Duke University) for providing KKO hybridoma cells. The authors acknowledge the facilities and assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy capability. The in vivo imaging data presented in this study were acquired at the Biological Resources Imaging Laboratory, a unit of the Mark Wainwright Analytical Centre of the University of New South Wales (UNSW) Sydney, which is funded in part by the Research Infrastructure Program of UNSW.
This study was funded by National Health and Medical Research Council grants APP1052616 (B.H.C.) and APP2030328 (H.H.L.L. and B.H.C.) and New South Wales Health Government Office for Health and Medical Research Senior Researcher grant RG201677 (B.H.C.) and Cardiovascular Collaborative grant RG233165 (H.H.L.L. and B.H.C.).
Authorship
Contribution: H.H.L.L. conceived the study, designed and performed the experiments, analyzed and interpreted the data, and wrote the manuscript; Z.A. and B.L. performed the experiments and analyzed the data; S.E.M. provided FcγRIIa+/hPF4+ transgenic mice and critically reviewed the manuscript; S.R. and J.C. provided clinical input and critically reviewed the manuscript; J.P. designed and performed the experiments, supervised the study, provided intellectual input, interpreted the data, and critically reviewed the manuscript; and B.H.C. conceived and supervised the study, provided intellectual input, interpreted the data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: S.E.M. is on the scientific advisory board for Veralox Therapeutics and holds an intellectual property interest in heparin-induced thrombocytopenia therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Beng H. Chong, Department of Haematology, Level 3, 4-10 South St, Research & Education Centre, Kogarah, NSW 2217, Australia; email: beng.chong@unsw.edu.au.
References
Author notes
Data are available on request from the corresponding author, Beng H. Chong (beng.chong@unsw.edu.au).
The full-text version of this article contains a data supplement.

![Effect of nonheparin anticoagulants, heparin, IVIg, and IV.3 in VITT mice. (A) Mouse lungs from FcγRIIa+/hPF4+ mice treated with VITT IgG, with or without anticoagulants, were extracted and analyzed for thrombosis by fluorescence using the IVIS SpectrumCT. (B) Representative images of hematoxylin and eosin-stained and fluorescence-stained lung sections. Images were acquired with a 10× objective using an inverted Olympus CKX53 microscope. Platelets (anti-CD42c) and DNA (4′,6-diamidino-2-phenylindole [DAPI]) are shown as magenta and cyan, respectively. Arrows indicate clots. Scale bar, 50 μm. (C) Mice treated with argatroban, bivalirudin, danaparoid or UF heparin at therapeutic concentrations had prolonged bleeding times compared with the saline control. The bleeding time was measured over 20 minutes. Data are shown as mean ± SD. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, relative to VITT IgG (A) or saline (C).](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/22/10.1182_bloodadvances.2024013883/2/m_blooda_adv-2024-013883-gr4.jpeg?Expires=1766112344&Signature=lmTR85chRRUrNV4~YmJNbosImN3bara3-xAYj-D3fuuw2Ltd0~vqoCIMlirKWhnFM630DbcA4jLRRGLeydo5NGRpVAt63q8qDNreh7QvfOsWqjF9SnwAlOb8rjWNBDy0jRLY3ZRqJKIS2QoacMvyPzckuLpUCi41N5jLW~gGwS1BXLMilamDAOTix6JGR5BPk3jeS5Qw5w21Z751IBfEqmfXDL6jWjrMA46Zo3aIykdO-7CC1kRNhoQE1US4ncFzrzcytPZPrPQjtaOOR0D7QhJzdIv1NperdD75SlCaHw-clymJKFSyNaPFe2m~DkDT2vcVe~A41uG8gSDu-jwgJA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
